Abstract
Many hazelnut (Corylus avellana L.) cultivars fail to thrive in vitro on standard growth medium and the reasons for poor growth are not well understood. Our initial study of five C. avellana cultivars showed that changes in the mineral nutrients of Driver and Kuniyuki walnut (DKW) medium, including doubling the minor nutrients, produced improved growth and shoot quality. The objectives of this study were to determine the effects of the individual minor mineral nutrients from DKW medium and if added nickel was required for optimal growth. Five factors were tested at 0.5 × to 4× DKW medium concentrations, [H3BO3, CuSO4·5H2O, MnSO4·H2O, Na2MoO4·2H2O and Zn(NO3)2·6H20], in a response surface design with 39 treatment combinations. Ni was not present in the DKW medium formulation so NiSO4·6H2O was varied from 0 to 6 µM. There were many significant interactions among the minor nutrients. Higher concentrations (4×) of B, Mo, and Zn increased overall shoot quality, length, and multiplication. Increased Mo improved some responses for each cultivar, and it interacted significantly with Cu and Zn. The addition of Ni greatly improved the shoot quality and length of ‘Sacajawea.’ Ni interactions were significant for the other cultivars as well, and altered the requirements for the other minor nutrients, but did not necessarily improve the overall shoot response. Improved growth and shoot quality for most cultivars required increased amounts of B, Mo, and Zn and less Mn and Cu. ‘Sacajawea’ required increased B, Cu, Zn, and Ni. All of the cultivars required minor nutrient formulations that differed greatly from DKW medium or other published minor nutrient formulations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Micropropagation can produce large quantities of clonal hazelnut (C. avellana) cultivars with more rapid multiplication and ease of rooting than standard propagation. Although commercial nurseries produce hazelnuts through layering and grafting, the process is not as efficient as for other more easily propagated plants. Some cultivars are more difficult to micropropagate and may be difficult to establish, multiply, or elongate in culture. Commonly used protocols often only modify the plant growth regulators without changing the mineral nutrients (Bassil et al. 1992; Yu and Reed 1993, 1995; Nas and Read 2001; Andres et al. 2002; Damiano et al. 2005). The chemical composition of growth media can be essential for successful micropropagation and new methods are being used to determine improved nutrient combinations (Niedz and Evens 2007; Adelberg et al. 2010; Reed et al. 2013).
Plants require 17 essential elements to sustain healthy growth and development. Of these, the minor nutrients are components of enzymes and needed only in very small amounts (Marschner 1995). The minor nutrients required for plant growth are B, Cl, Co, Cu, Mn, Mo, Ni, Na, Si and Zn. For most plants the tissue content of minor nutrients usually exceeds the physiological requirements, but minor nutrient deficiencies are common in certain soil types (Bennett 1993). Analysis of Oregon-grown hazelnut leaf tissues found a wide range of minor nutrients in healthy leaves: 26–650 ppm Mn, 51–400 ppm Fe, 5–15 ppm Cu, 31–75 ppm B, and 16–60 ppm Zn (Olsen 2013).
Most plant tissue culture media contain only some of the essential minor nutrients, and often lack Co, Ni and Si, because they are required in such small amounts that the agar or gellan gum gelling agents contain an adequate supply (Singha et al. 1985; Williams 1993). Although Ni is not used in Driver and Kuniyuki walnut (DKW) medium (Driver and Kuniyuki 1984), woody plant medium (WPM) (Lloyd and McCown 1980), or Murashige and Skoog (MS) (Murashige and Skoog 1962), it may improve the culture of many plants.
Witte et al. (2002) found that addition of 100–200 nM (1–2 µM) NiSO4 to MS medium was sufficient to increase urease activity in cultured potato leaves. They noted that the amount of urease activity depended on the amount of added Ni, and also depended on the chemical make-up of the agar. Addition of Ni also increased shoot regeneration of Jatropha curcas L. leaf explants (Sarkar et al. 2010).
There are only a few studies on the effects of minor nutrients on in vitro culture of hazelnut and they produced very different results. Nas and Read (2004) made many modifications to common media for use with hybrid hazelnuts to produce NRM, including a 10× increase in the minor elements Cu and Mo. However, Italian hazelnut cultivars had improved growth with an altered MS medium minor nutrient content: Mn was increased to 4×, Zn decreased to 0.34×, and Mo was eliminated from the medium (Bacchetta et al. 2008). Our earlier study of the mineral nutrients required for optimal hazelnut micropropagation determined that all of the five cultivars studied grew best with twice the normal DKW medium level of minor nutrients stock, the highest concentration tested (Hand 2013; Hand et al. 2014).
The objective of this study was to determine the effect of the five individual DKW medium minor nutrient mineral factors on the growth and development of three hazelnut cultivars. A second objective was to determine if the minor nutrient Ni was required or would promote hazelnut growth. A response surface design was used for modeling shoot responses.
Materials and methods
Plant material and culture conditions
Shoot cultures of hazelnut cultivars Dorris (PI 657898), Jefferson (PI 657902), and Sacajawea (PI 654984) from the germplasm collections were grown on NCGR-COR medium (Yu and Reed 1995) composed of DKW medium mineral salts, with 30 g l−1 glucose, 200 mg sequestrene 138 Fe (Fe EDDHA), 2 mg l−1 thiamine, 2 mg l−1 nicotinic acid, 2 mg l−1 glycine, 1 g l−1 myo-inositol, 22.2 μM N6 benzyladenine (BA), and 0.5 % (w/v) agar (PhytoTechnology Laboratories, A1111). Medium (40 ml) in tissue culture vessels (Magenta GA7, Magenta, Chicago, IL), was autoclaved at 121 °C for 20 min. Cultures were transferred to fresh medium at 3-week intervals. Growth room conditions were 25 °C with a 16-h photoperiod of half warm-white and half cool-white fluorescent tubes. The average illumination measured at the top of the vessels was 80 µmol m2s−1.
Mineral nutrition
Experimental design for modeling the responses was conducted with the software program Design-Expert® 8 (Design-Expert 2010). The three cultivars were tested using a multi-factor response surface design. The minor nutrients were separated into six independent factors, creating a six-dimensional experimental design space: H3BO3, CuSO4·5H2O, MnSO4·H2O, Na2MoO4·2H2O, Zn(NO3)2·6H20, and NiSO4·6H2O (Table 1). The minor element concentrations ranged from 0.5 to 4× the standard DKW medium amounts, and Ni was set at zero to 6 µM based on the literature (Witte et al. 2002). Design points were selected algorithmically to sample the design space. Treatments were developed from the design points. There were 39 treatments run in two sets with DKW medium controls in each set and internal replications (Table 2). All treatments had the standard DKW medium macro nutrients and vitamins, 30 gL−1 glucose, 200 mgL−1 sequestrene Fe 138, and 8 μM BA.
For the initial experimental culture, shoots were cut to 2.5 cm with shoot tips removed. For each transfer, shoots were cut above the basal zone, the lower leaves removed and each piece cut to about 2.5 cm. Each treatment consisted of two culture vessels with five shoots for each genotype. Culture vessels were randomized on the growth room shelf. Shoots were grown on each treatment medium with transfers to fresh medium at 3-week intervals, and the last growth period for 4 weeks.
Data collection
Three shoots in each culture vessel (at a diagonal from the corner label) were examined for eight responses (n = 6) and the remaining shoots were photographed. Shoot quality was a subjective visual assessment of shoot appearance (Niedz et al. 2007): 1 = poor, 2 = moderate, and 3 = good. Shoots longer than 5 mm were counted, and the longest shoot of each original shoot was measured in millimeters. Leaf color was rated 1 = yellow, 2 = light green, and 3 = dark green. A portable Soil–Plant Analysis Development (SPAD) 502 chlorophyll meter (Minolta Camera Co. Ltd., Tokyo, Japan) was used to measure chlorophyll content of the second leaf from the top of the shoot. Callus size was rated: 1 = callus > 2 mm, 2 = callus ≤ 2 mm, and 3 = absent. Leaf size was rated: 1 = small, 2 = medium, 3 = large.
Statistical analysis
Response surface modeling was based on analysis of the mean shoot responses from the six shoots of each genotype grown in the same treatment or 12 shoots in the case of internally replicated treatments. Design-Expert 8 software was used with the highest order polynomial predictor models to model the shoot responses in the six-dimensional design space. Stepwise backward elimination regression was used to remove factors that were not significant from the full model. p values ≤0.05 of models and factors were considered significant.
Results
Responses to the mineral nutrient factors at each design point treatment were used to produce the graphical models. The statistical analysis and graphs present the factors with the greatest influence on each response. For each genotype, the two most significant factors were used as the axes in the design graphs. Red dots indicate actual data from a design point when it was present in the optimal section of the graph. Graphs with and without Ni were presented when Ni was a significant factor.
Quality
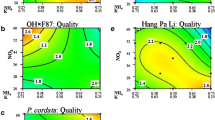
There were significant models (p ≤ 0.03) for quality for the three genotypes (Table 3). Many treatments produced shoots with better quality than the controls (Fig. 1). Overall quality of ‘Dorris’ was the result of many significant interactions (Table 3). High B, Zn, and Mo combined with low Mn, and Cu, without Ni, produced the best quality (Fig. 2a). Several interactions with Ni were highly significant. A high concentration of Ni reduced the need for B and Zn and produced equivalent shoot quality to those with high B and no Ni (Fig. 2b). Only B significantly improved ‘Jefferson’ quality (Table 3). Increasing B increased overall quality, and none of the other factors were significant (Fig. 2c). Significant interactions of Cu × Zn, and Mn × Ni were seen for ‘Sacajawea’ (Table 3). Without Ni in the medium, high concentrations of Cu, Zn, B, and Mo with low Mn significantly improved shoot quality (Fig. 2d). When high concentrations of Ni were present, high B, Cu, Mn, and Zn with low Mo significantly increased quality even more (Fig. 2e).
Response surface graphs of minor mineral nutrient effects on quality for three hazelnuts a ‘Dorris’, Treatment 8 design point shown as a red dot, b ‘Dorris’ with nickel, c ‘Jefferson’ with boron as the only significant factor, d ‘Sacajawaea’, and e ‘Sacajawea’ with nickel. The quality ratings were low = 1, high = 3 and highest (red–yellow) to lowest quality (green–blue). (Color figure online)
Shoot length
All three genotypes had significant models (p < 0.006) for shoot length (Table 3). The ideal shoot length for hazelnut was 40–60 mm in a 4-week period. Boron was significant for shoot length for all genotypes and two of the cultivars had multiple interactions (Table 3). The smallest shoots of the three genotypes were produced by ‘Dorris’ (≤40 mm). High levels of B, Cu, moderate Mo, and low Zn produced increased shoot length for ‘Dorris’ (Fig. 3a). High Ni and Mo combined with low Cu, Mn and Zn also produced long shoots over all B concentrations (Table 3; Fig. 3b). For ‘Jefferson,’ high B and low Zn concentrations resulted in increased (50 mm) shoot length compared to the control treatment (44 mm) (Fig. 3c, Table 3). For ‘Sacajawea’ shoot length was influenced by several significant interactions (Table 3). High B and Mo with all other minor nutrients low (0.5–1×) produced the longest shoots (Fig. 3d). High Ni produced equally long shoots when B and Mn were high and all other factors low (Fig. 3e).
Response surface graphs of minor nutrient effects on shoot length (mm) for three hazelnuts a ‘Dorris,’ b ‘Dorris’ with nickel, Treatment 17 design point shown as a red dot, c ‘Jefferson’, control design point shown as a red dot, d ‘Sacajawaea’, and e ‘Sacajawea’ with nickel. The shoot lengths were color coordinated from longest (red–yellow) to shortest (green–blue). (Color figure online)
Shoot number
Models were significant for ‘Dorris’ (p < 0.003) and ‘Jefferson’ (p < 0.009), but not for ‘Sacajawea’ (Table 3). For ‘Dorris’ high levels of B with low Zn and Cu produced the most shoots (2.6) (Fig. 4a). For ‘Jefferson’ shoot number was influenced by Ni interactions with Mn and Mo (Table 3). Without Ni the shoot number increased (>2.5 shoots) with high B and Mo combined with low Mn (Fig. 4b). High Ni and Mn with low Mo and B also resulted in increased shoots (>2.8 shoots) (Fig. 4c).
Response surface graphs of minor mineral nutrient effects on the number of shoots produced by hazelnuts a ‘Dorris’, b ‘Jefferson,’ c ‘Jefferson’ with nickel. Callus ratings (1 = large callus, 3 = no callus) for three hazelnuts d ‘Dorris,’ e ‘Dorris’ with nickel, f ‘Jefferson,’ g ‘Jefferson’ with nickel. Red indicates more shoots or less callus; green or blue indicates fewer shoots or more callus. (Color figure online)
Callus
Both ‘Dorris’ and ‘Jefferson’ responses were significant for callus (p < 0.002), but the ‘Sacajawea’ response was not significant (Table 3). There were significant interactions for ‘Dorris’, but Ni was not significant (Table 3; Fig. 4d, e). The least amount of callus (rating of 3) for ‘Dorris’ was projected with high Zn and low Cu, B, and Mo. There were many significant interactions for callus production for ‘Jefferson’ (Table 3). Without Ni, high Zn, Mo, and Mn, and moderate to high levels of Cu were projected to produce the least callus (Fig. 4f). There were Ni × Zn and Ni × B interactions for ‘Jefferson’, but callus reduction was not as dramatic as without Ni (Fig. 4g). There were no significant effects of minor nutrients on callus production for ‘Sacajawea’, and it produced a large amount of callus.
Leaf responses
Leaf size models were significant for ‘Dorris’ and ‘Jefferson.’ Shoots with a leaf size rating of two were considered the best size. There was a significant interaction of Cu × Ni for leaf size for ‘Dorris’ such that treatments with low Ni and Cu produced larger leaves (Fig. 5a). The leaf size of ‘Jefferson’ increased with increasing Ni at low concentrations of the other nutrients, and a moderate leaf size was produced with high Cu and without Ni (Fig. 5b). Leaf size was not significant for any of the minor nutrients for ‘Sacajawea’. SPAD models were all significant with many interactions (Table 4). Leaves with a SPAD reading of 20 were yellow, 25–30 were green, and at 35 leaves were dark green. SPAD for ‘Dorris’ was in the green range with high B, Cu, Mn, and Zn, but with low Mo (Fig. 5c). Adding Ni increased SPAD readings with low Mn and high levels of the other nutrients (Fig. 5d). SPAD ratings for ‘Jefferson’ were 30–32 for the best treatments and were mostly influenced by high Cu and Mn, or in treatments with Ni by high Cu, Mn, and Zn (Fig. 5e, f). ‘Sacajawea’ SPAD responses were high with increased Cu and Mn and low concentrations of other minor nutrients, but also with Ni and high Cu and Mn (Fig. 5e–h).
Response surface graphs of minor mineral nutrient effects on leaf size ratings (1 = small, 3 = large) for hazelnuts a ‘Dorris’, b ‘Jefferson.’ SPAD measurements (20 = yellow, 35 = dark green) for three hazelnuts c ‘Dorris,’ d ‘Dorris’ with nickel, e ‘Jefferson,’ f ‘Jefferson’ with nickel,’ g ‘Sacajawaea,’ and h ‘Sacajawea’ with nickel. Colors indicate the highest number or reading (red–yellow) to lowest (green–blue). (Color figure online)
Discussion
In vitro studies of minor nutrients are not common, although these chemicals play an important role in the growth of all plants. The likelihood of important effects in vitro should not be overlooked. However, this type of study is very complex because minor nutrients interact greatly with other nutrients. Media used in the culture of hazelnuts vary widely in the concentrations and types of minor nutrients (Table 5). The use of modeling software in this study allowed for visualization of the many interactions of minor nutrients in plant growth media and the resulting effects on shoot growth.
One difficulty, not readily apparent in working with minor nutrients, was that sources of agar contain widely variable amounts of minor nutrients (Williams 1993; Scholten and Pierik 1998). This was likely a major source of the variation in plant response seen among laboratories. Although more highly purified agars are now available, agar composition analysis should be performed before adjusting minor nutrients. Seven agar brands and Gelrite were analyzed and found to contain a wide range of concentrations of the required minor nutrients (Scholten and Pierik 1998). Williams (1993) analyzed several agars and also found that each source varied greatly in mineral content. In this study we used 0.5 % agar from PhytoTechnology (A111) containing: 24.8 ppm B, 9.3 ppm Cu, 86.5 ppm Fe, 53.4 ppm Mn, 11.2 ppm Mo and 21.1 ppm Zn with <0.1 ppm Ni (unpublished data). The composition of our agar was different from those of gelling agents tested by Scholten and Pierik (1998) and Williams (1993). For example, B was 1.4 ppm in Gelrite, 34 ppm in BiTek agar, and 109 ppm in Bacto agar (Williams 1993); Mn varied from 0.3 ppm in Bacto agar, 0.5 ppm in BiTek agar, and 5.3 ppm in Gelrite. Nickel ranged from not detected in Difco Bacto agar to 0.037 ppm in Merck 1614 agar (Scholten and Pierik 1998). The nutrient levels of our gelling agents were likely different from those of previous hazelnut media formulation studies. Bacchetta et al. (2008) used 0.7 % ‘Plant Agar’ (Duchefa, Haarlem, Netherlands) while Nas and Read (2004) used 0.6 % Sigma agar. Depending on the agar brand and batch number, the amount of nutrients available to the cultures could vary because some nutrients were mobile and others were bound to the agar (Scholten and Pierik 1998). When changing minor nutrient formulations, the agar analysis should be considered to avoid any possible toxicity problems.
Plants use B in the metabolism of phenolic acids, and lignin biosynthesis. Boron was directly related to cell wall maintenance (Hu et al. 1996) and B deficiency also affected auxin transport (Bairu et al. 2009). We found that B significantly influenced many aspects of hazelnut growth because of multiple interactions with other minor nutrients. Boron concentrations at 4 × DKW medium (310 µM) produced the best growth (Table 3; Figs. 1, 2, 3). High B concentrations significantly improved quality for ‘Jefferson’, shoot quality, length and number for ‘Dorris’, and shoot length and number for ‘Sacajawea’ (Fig. 2). Lopez-Lefebre et al. (2002) found that increasing B concentrations from 1 to 30 µM in tobacco plants increased leaf and root biomass. They noted that B positively increased uptake of N, P, K, Na, Fe, Mn and decreased uptake of Zn, Mg, and Cu; B and Ca had a synergistic effect because as B increased there was increasing uptake of Ca. We also found multiple interactions of B with Cu, Zn, and Ni (Tables 3, 4). Increased B (from 0.1 to 6 mM) in MS medium for apple tissue culture resulted in increased P, Ca, and Mg in shoots while K, Fe, Mn, and Zn decreased (Mouhtaridou et al. 2004). High B decreased apple shoot chlorophyll content as indicated by SPAD measurement. The SPAD readings of hazelnuts indicated that a high chlorophyll content (SPAD > 30) was observed at all B concentrations for two of the three genotypes; ‘Dorris’ with high B had the highest SPAD (Table 4). By comparison, B in leaves from field trees ranged from 31 to 75 ppm for normal trees and <25 ppm in deficient plants (Olsen 2013).
DKW medium and WPM contain 1 µM Cu, while NRM contains 10 µM and MS and HM are ≤0.1 µM Cu (Table 5). There were Cu interactions for most of the responses for ‘Dorris’ and for quality for ‘Sacajawea.’ In our study, the concentration of Cu for quality, shoot number, and shoot length was projected to be best for ‘Dorris’ and ‘Jefferson’ at moderate to low levels (0.5 × to 2×), but with much higher amounts for ‘Sacajawea’ (Figs. 2, 3, 4). When Cu was increased for ‘Sacajawea’ quality increased (Fig. 2), but shoot length decreased (Fig. 3). The opposite was seen for ‘Dorris’ where less Cu improved quality and more Cu with high Mo improved shoot length, unless Ni was present. Oregon hazelnut cultivars grown on standard DKW medium with 1 µM Cu had shoot lengths of 34–43 mm, but treatments with 0.5 × Cu were often taller (Fig. 3). In a similar study, four hybrid C. americana × C. avellana hazelnuts grown with 10 µM CuSO4·5H2O and 10 µM Mo produced 35–50 mm shoots (Nas and Read 2004). We only observed a Cu × Mo interaction in ‘Dorris’, indicating a likely genotype-dependent reaction that may also apply to the hybrid hazelnuts in the Nas and Read (2004) study. Several other studies found improved shoot or embryo production with increased Cu concentrations (Joshi and Kothari 2007; Kothari-Chajer et al. 2008; Jain et al. 2009, 2012). The opposite effect was found for daylilies (Hemerocallis) where the relatively low concentration of Cu in MS medium (0.1 µM) was too high for optimal growth (Adelberg et al. 2010).
The concentrations of Zn in tissue culture media range from 0.1 to 70 µM (George 2008). Kothari-Chajer et al. (2008) found that excluding Zn in MS medium reduced regeneration from callus in millet, indicating a role in organogenesis. Increasing Zn to 4× the DKW medium concentration resulted in high quality ratings for ‘Dorris’ and ‘Jefferson,’ but was not significant for ‘Sacajawea’ quality, although 0.5 × to 1 × Zn were projected to produce the best shoot length and numbers. Zn in healthy field-grown hazelnut trees varied from 16 to 60 ppm with deficiencies noted at <10 ppm (Olsen 2013). This large range may indicate that hazelnuts trees were tolerant of high Zn concentrations.
Molybdenum is required in lower amounts for most plants than all the other minor nutrients except Ni (Marschner 1995). We found that Mo affected many responses and was involved in interactions with other nutrients (Tables 3, 4). Optimal Mo concentrations were at 4 × (6.4 µM) for the best quality ratings (Fig. 2), while requirements for shoot length varied as a result of interactions with Ni in some genotypes (Fig. 3). Increasing Mo was in agreement with Nas and Read (2004) where 10.3 µM Mo was deemed optimal when combined with high Cu, and was 10× more than MS medium and WPM (Table 5). Bacchetta et al. (2008) found no Mo in their leaf samples and did not include it in the medium; possibly the agar used contained enough Mo for normal growth.
Low (100 µM) or normal (200 µM) DKW medium Mn concentrations resulted in improved quality, shoot length and shoot number for the hazelnuts in this study (Figs. 2, 3, 4). Interactions of Mn × Ni resulted in an increased Mn requirement for ‘Dorris’ and ‘Sacajawea’ for the best quality (Fig. 2). Increasing Mn to 4 × (800 µm) improved SPAD for two of the three cultivars. Standard DKW medium Mn concentrations (200 µM) were double those of the other commonly used hazelnut media (Table 5). The concentrations of Mn in many published tissue culture media range from 25 to 150 µM (George 2008). Millet callus grown on 400 µM Mn had improved fresh weight compared to MS medium (Kothari-Chajer et al. 2008). Jain et al. (2012) tested MS medium minor nutrient concentrations on Stevia rebaudiana and found that increasing Mn to 400 µM doubled the shoot number and resulted in increased chlorophyll content.
Nickel was not included in most plant tissue culture media, possibly because it was present in most types of agar (Witte et al. 2002). In our study, interactions of Ni were significant and altered the requirements for other nutrients, but did not necessarily improve the overall shoot response (Fig. 2; Table 3). Adding Ni at 6 µM provided higher quality ratings for ‘Dorris’ and ‘Sacajawea’ (Fig. 2) with low levels of B. Shoot length for ‘Dorris’ could be increased with added Ni (6 µM) along with higher Mo and lower Cu. Increased shoot length for ‘Sacajawea’ resulted from added Ni (6 µM) and reduced Mo (Fig. 2). The addition of 6 µM Ni, interacting with high concentrations of Mn, greatly improved shoot quality and length in ‘Sacajawea’. Kropat et al. (2011) found that 25 µM Ni was suitable for growing Chlamydomonas, but if chelating agents were added more Ni was required. Moraes et al. (2009) grew rice plants in a nutrient solution with a range of concentrations of Ni and Mo, and after 21 days, leaves exposed to 10 µM Ni had more shoot regeneration than those grown without Ni. Sarkar et al. (2010) found 1,000 µM Ni to have toxic effects. Rout et al. (1998) increased the number of somatic embryos per culture for Setaria italica by >4 times on medium with 6.5 µM Ni, but embryogenesis declined at higher concentrations. Overall, addition of 6 µM Ni to DKW medium minor nutrients in our study produced significant interactions with the other minor nutrients and in a few cases produced better shoot responses.
Conclusions
Our results indicated that minor nutrient interactions were common in DKW medium and significantly affected the growth of hazelnut shoot cultures. Changing the mineral nutrients in growth media is a tedious process, mostly as a result of these interactions. In addition, the contribution of agar to minor nutrient composition of the various growth media further complicates the issue. The diverse response of these three genotypes confirms that nutrient uptake or utilization varied based on genotype. Improved growth and shoot quality in ‘Dorris’ and ‘Jefferson’ required greatly increased B, Mo and Zn combined with low Mn and Cu; ‘Sacajawea’ required increased B, Mn, Zn and Ni with low Mo for the best growth (Table 5). These minor nutrient requirements will be incorporated into the improved hazelnut growth media that we are developing for use with diverse hazelnut cultivars.
Abbreviations
- BA:
-
N6 benzyladenine
- DE:
-
Design expert software
- DKW:
-
Driver and Kuniyuki Walnut
- Fe EDDHA:
-
Ferric ethylenediamine-N,N′-bis(2-hydroxyphenylacetic acid)
- IBA:
-
Indole-3-butyric acid
- Mesos:
-
MgSO4 and KH2PO4
- MS:
-
Murashige and Skoog
- NCGR-COR:
-
Yu and Reed Hazelnut Medium
- PI:
-
Plant Introduction number (US National Plant Germplasm System)
- WPM:
-
Woody Plant Medium
References
Adelberg JW, Delgado MP, Tomkins JT (2010) Spent medium analysis for liquid culture micropropagation of Hemerocallis on Murashige and Skoog medium. In Vitro Cell Dev Biol Plant 46:95–107
Andres H, Fernandez B, Rodriguez R, Rodriguez A (2002) Phytohormone contents in Corylus avellana and their relationship to age and other developmental processes. Plant Cell Tiss Organ Cult 70:173–180
Bacchetta L, Aramini M, Bernardini C, Rugini E (2008) In vitro propagation of traditional Italian hazelnut cultivars as a tool for the valorization and conservation of local genetic resources. HortScience 43:562–566
Bairu MW, Stirk WA, Van Staden J (2009) Factors contributing to in vitro shoot-tip necrosis and their physiological interactions. Plant Cell Tiss Organ Cult 98:239–248
Bassil N, Mok D, Mok M, Rebhuhn BJ (1992) Micropropagation of the hazelnut, Corylus avellana. Acta Hortic 300:137–140
Bennett WF (1993) Nutrient deficiencies and toxicities in crop plants. APS Press, Minneapolis, p 202
Damiano C, Catenaro E, Giovinazzi J, Frattarelli A (2005) Micropropagation of hazelnut (Corylus avellana L.). Acta Hort 686:221–225
Design-Expert (2010) Stat-Ease, Inc., Minneapolis
Driver JA, Kuniyuki AH (1984) In vitro propagation of Paradox walnut rootstock. HortScience 19:507–509
George EF (2008) The components of plant tissue culture media I: macro- and micro-nutrients. In: George EF, Puttock DJM, George HJ (eds) Plant propagation by tissue culture, 3rd edn. Springer, New York, pp 65–113
Hand CR (2013) Improving initiation and mineral nutrition for hazelnut (Corylus avellana) micropropagation. Master of Science, Horticulture, Oregon State University, Corvallis, Oregon, May, 2013, 108
Hand CR, Maki S, Reed BM (2014) Modeling optimal mineral nutrition for hazelnut (Corylus avellana) micropropagation. Plant Cell Tiss Organ Cult (in press)
Hu HN, Brown PH, Labavitch JM (1996) Species variability in boron requirement is correlated with cell wall pectin. J Exp Bot 47:227–232
Jain P, Kachhwaha S, Kothari SL (2009) Improved micropropagation protocol and enhancement in biomass and chlorophyll content in Stevia rebaudiana (Bert.) Bertoni by using high copper levels in the culture medium. Scientia Hortic 119:315–319
Jain P, Kachhwaha S, Kothari SL (2012) Optimization of micronutrients for the improvement of in vitro plant regeneration. Indian J Biotech 11:486–490
Joshi A, Kothari SL (2007) High copper levels in the medium improves shoot bud differentiation and elongation from the cultured cotyledons of Capsicum annuum L. Plant Cell Tiss Organ Cult 88:127–133
Kothari-Chajer AMS, Kachhwaha S, Kothari SL (2008) Micronutrient optimization results into highly improved in vitro plant regeneration in kodo (Paspalum scrobiculatum L.) and finger (Eleusine coracana (L.) Gaertn.) millets. Plant Cell Tiss Organ Cult 94:105–112
Kropat J, Hong-Hermesdorf A, Casero D, Ent P, Castruita M, Pellegrini M, Merchant SS, Malasarn D (2011) A revised mineral nutrient supplement increases biomass and growth rate in Chlamydomonas reinhardtii. Plant J 66:770–780
Lloyd G, McCown B (1980) Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb Proc Int Plant Prop Soc 30:421–427
Lopez-Lefebre LR, Rivero RM, Garcia PC, Sanchez E, Ruiz JM, Romero L (2002) Boron effect on mineral nutrients of tobacco. J Plant Nutr 25:509–522
Marschner H (1995) Mineral nutrition of higher plants. Academic Press, New York, pp 1–5
Moraes MF, Reis AR, Moraes LAC, Lavres J, Vivian R, Cabral CP, Malavolta E (2009) Effects of molybdenum, nickel, and nitrogen sources on the mineral nutrition and growth of rice plants. Commun Soil Sci Plant Anal 40:3238–3251
Mouhtaridou GN, Sotiropoulos TE, Dimassi KN, Therios IN (2004) Effects of boron on growth, and chlorophyll and mineral contents of shoots of the apple rootstock MM 106 cultured in vitro. Biol Plant 48(4):617–619
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15:473–497
Nas MN, Read PE (2001) Micropropagation of hybrid hazelnut: medium composition, physical state and iron source affect shoot morphogenesis, multiplication and explant vitality. Acta Hortic 556:251–258
Nas MN, Read PE (2004) A hypothesis for the development of a defined tissue culture medium of higher plants and micropropagation of hazelnuts. Sci Hortic 101:189–200
Niedz RP, Evens TJ (2007) Regulating plant tissue growth by mineral nutrition. In Vitro Cell Dev Biol Plant 43:370–381
Niedz RP, Hyndman SE, Evens TJ (2007) Using a Gestalt to measure the quality of in vitro responses. Sci Hortic 112:349–359
Olsen JL (2013) Orchard nutrition. In: Growing hazelnuts in the Pacific Northwest. Oregon State University Extension Service Corvallis, OR:1-5 http://ir.library.oregonstate.edu/xmlui/handle/1957/43813
Reed BM, Wada S, DeNoma J, Niedz RP (2013) Improving in vitro mineral nutrition for diverse pear germplasm. In Vitro Cell Dev Biol Plant 49:343–355
Rout GR, Samantaray S, Das P (1998) The role of nickel on somatic embryogenesis in Setaria italica L. in vitro. Euphytica 101:319–324
Sarkar T, Anand KGV, Reddy MP (2010) Effect of nickel on regeneration in Jatropha curcas L. and assessment of genotoxicity using RAPD markers. Biometals 23:1149–1158
Scholten HJ, Pierik RLM (1998) Agar as a gelling agent: chemical and physical analysis. Plant Cell Rep 17:230–235
Singha S, Townsend EC, Oberly GH (1985) Mineral nutrient status of crabapple and pear shoots cultured in vitro on varying concentrations of three commercial agars. J Am Soc Hort Sci 110:407–411
Williams RR (1993) Mineral nutrition in vitro—a mechanistic approach. Aust J Bot 41:237–251
Witte C-P, Tiller SA, Taylor MA, Davies HV (2002) Addition of nickel to Murashige and Skoog medium in plant tissue culture activates urease and may reduce metabolic stress. Plant Cell Tiss Organ Cult 68:103–104
Yu X, Reed BM (1993) Improved shoot multiplication of mature hazelnut (Corylus avellana L.) in vitro using glucose as a carbon source. Plant Cell Rep 12:256–259
Yu X, Reed BM (1995) A micropropagation system for hazelnuts (Corylus species). HortScience 30:120–123
Acknowledgments
This study was part of a MS thesis project by CH and was supported by the Oregon Hazelnut Commission and USDA-ARS CRIS 5358-21000-044-00D.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hand, C., Reed, B.M. Minor nutrients are critical for the improved growth of Corylus avellana shoot cultures. Plant Cell Tiss Organ Cult 119, 427–439 (2014). https://doi.org/10.1007/s11240-014-0545-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0545-x