Abstract
Diterpenoids are important compounds for plant survival and have beneficial properties for humans. Bioactive abietanic diterpenes are synthesized in roots of Salvia sclarea (e.g. aethiopinone, 1-oxoaethiopinone, salvipisone, and ferruginol), but at a very low level (about 1 % of root dry weight). To enhance the biosynthesis of this interesting class of compounds, heterologous AtDXS (d-xylulose 5-phosphate synthase) or AtDXR (1-deoxy-d-xylulose 5 phosphate reductoisomerase) genes, encoding the up-stream enzymes of the plastidial 2-C-methyl-D-erythritol 4-phosphate (MEP)-dependent terpenoid pathway, were ectopically expressed in S. sclarea hairy roots. Quantitative targeted metabolic analysis (HPLC–DAD) revealed that three independent root lines, expressing different levels of DXS or DXR transcripts and proteins, synthesized a significant higher content of abietanic diterpenes, compared to the control hairy root line transformed with the empty vector. The increase was gene-dependent, since the overexpression of the AtDXR triggered a 4.4-fold increase in aethiopinone, an abietane quinone-type tricyclic diterpene. In addition, aethiopinone was proved to be cytotoxic to different solid tumor cell lines, with the highest effect on human melanoma A375 cell line (IC50 11.4 µM). Overall these results show that it is possible to boost the metabolic flow towards the synthesis of abietanic diterpenes in S. sclarea hairy roots by overexpressing genes involved in the first steps of the MEP-pathway and provide new insights for the large-scale production of this class of compounds, with potential application in cancer treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plant diterpenes, with more than 10,000 different chemical structures, are one of the largest and most diverse classes of plant metabolites. Beyond their industrial use (fragrances, resins, sweeteners and others), plant diterpenes are also important for their pharmological activities, e.g. the antitumor taxol derived from Taxus species (Jennewein and Croteau 2001), and ingenol-3-angelate from Euphorbia peplus (Li et al. 2010), the cAMP-regulating and vasodilator drug forskolin from Coleus forskohlii (Alasbahi and Melzig 2010), and the analgesic and antidiabetic drug candidate marrubiin from Marrubium species (Mnonopi et al. 2012).
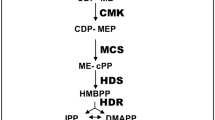
Salvia sclarea (clary sage) is a well-known cash crop for the extraction of the diterpene sclareol, which is synthesized in the aerial parts of the plants. However, diterpenes produced in S. sclarea roots have recently attracted attention for their biological activities as anti-bacterial (Kuźma et al. 2007) and for their promising antitumoral activities (Rózalski et al. 2006). Diterpenes, isolated in S. sclarea roots (Ulubelen et al. 1997) and hairy roots (Kuźma et al. 2006), belong to the class of tri-cyclic diterpenes, either of the abietane-phenolic or abietane-quinone-type. Since several plant-derived compounds with the quinone moiety present some anti-proliferation and anti-metastasis effects in various cancer types both in vitro and in vivo (Fronza et al. 2012), abietane-quinone diterpenes would appear to deserve greater attention. However, the potential translation of these and several other promising bioactive diterpenes into effective new plant-derived drugs is prevented by the low yields and purity of diterpenes extracted from natural sources, which are often insufficient to carry out pre-clinical studies. In absence of reliable chemical synthesis procedures, metabolic engineering strategies aimed at enhancing biosynthesis of specific secondary metabolites is an attracting alternative (see Yoon and Nodwell 2014 for a recent review). Genes encoding enzymes acting either up- or down-stream in the plastidial methylerythritol 4-phosphate (MEP) derived terpenoid biosynthetic pathway, from which plant diterpenes are derived, have been targeted to improve the biosynthesis of different isoprenoid compounds (Dudareva et al. 2013; Moses et al. 2013, for a recent reviews). As for other terpenoids, cyclic diterpenes originate from the plastidial 1-deoxy-d-xylulose 5-phosphate (DXP) pathway and are synthesized from dimethylallyl diphosphate (DMAPP) and three molecule of isopentenyl diphosphate (IPP), yielding the C20 metabolite geranylgeranlyl diphosphate (GGPP), which constitutes the backbone for the synthesis of diverse plant diterpenes. Genes and encoded proteins involved in the up-stream reactions from DMAPP and IPP to GGPP have been widely studied in model crop and medicinal plants (Cordoba et al. 2009; Rodríguez-Concepción et al. 2001; Carretero-Paulet et al. 2006). The first step is a transketolase-like decarboxylation reaction, catalyzed by the 1-deoxy-d-xylulose 5-phosphate synthase (DXS) using pyruvate and glyceraldehyde-3-phosphate (GAP) as substrates to yield the first intermediate, 1-deoxy-d-xylulose-5-phosphate (DPP) (Rohmer et al. 1996; Lange et al. 1998; Lois et al. 1998). The DPP is then converted to 2-C-methyl-d-erythritol 4-phosphate (MEP) by the NADPH-dependent reaction catalyzed by the 1-deoxy-d-xylulose-5-phosphate reductoisomerase (DXR). Through the action of six additional consecutive enzymatic reactions, MEP is converted into a 5:1 mixture of IPP and DMAPP from which plant terpenes originate (Lange et al. 2000; Rodríguez-Concepción and Boronat 2002).
Since their identification, the first two enzymes of the isoprenoid pathway (DXS and DXR) have both been proposed as potential control flux points for the synthesis of several terpenoid end-products in different plant species (Chappell 1995; Wu et al. 2006; Phillips et al. 2008). Therefore, up-regulation of the genes encoding these two enzymes has been applied to enhance the synthesis of different classes of isoprenoids in different plants. Besides carotenoids in crop plants (Enfissi et al. 2005; Morris et al. 2006), overepression of the DXS gene has been successful in boosting the synthesis of different terpenoids in several medicinal plants, such as Lavandula latifolia (Miñoz-Bertomeu et al. 2006), Catharanthus roseus (Peebles et al. 2011) and Salvia milthiorrhiza (Kai et al. 2011). In addition, overexpression of the DXR gene was efficient at enhancing the synthesis of monoterpene essential oil in Mentha piperita (Mahmoud and Croteau 2001), terpenoid-indol alkaloid in C. roseus (Veau et al. 2000), artemisinin in Artemisia annua (Xiang et al. 2012) and taxadiene in transgenic Arabidopsis (Carretero-Paulet et al. 2006).
In the present study we report that the content of bioactive abietane-type diterpenoids in hairy roots of Salvia sclarea can be enhanced through increased precursor availability in the MEP-dependent isoprenoid pathway, by modifying the level of expression of the first two committed enzymes of this pathway, i.e. DXS or DXR. In addition, aethiopinone was proved to be cytotoxic to different solid tumor cell lines, with a major effect on the A375 human melanoma cell line.
Materials and methods
Plant material and growth conditions
Salvia sclarea plants (B&T World Seeds, France) were surface sterilized with 70 % (v/v) ethanol for 1 min and then in 2 % (v/v) sodium hypochlorite solution for 10 min, thoroughly washed and sown in Murashige and Skoog (MS) (Murashige and Skoog 1962) pH 5.8 medium containing 30 g l−1 sucrose and 9 g l−1 agar. The plants were grown at 23 °C under a photoperiod of 8 h dark and 16 h light (110 μmol m−2 s−1) in a controlled growth chamber.
Plasmid construction
Plasmids containing the full-length cDNA of A. thaliana DXS (Accession no. U27099) or DXR (Accession no. AY91405) were obtained from the Arabidopsis Biological Resource Center. The coding sequence of the two genes was amplified by polymerase chain reaction (PCR) using a High Fidelity DNA Polymerase (Pfx, Invitrogen, Carlsbad, CA, USA) with the AtDXSHindIII forward primer and AtDXSSacI reverse primer or AtDXRHindIII forward primer and AtDXRSacI reverse primer (Primers sequence are reported in Supplemetary Table 1). The resulting amplicons were cloned into the HindIII/SacI sites of the binary PKYLX71:35S2 vector, under the control of a constitutive strong promoter, cauliflower mosaic virus (35S2) and containing the neomycin phosphotransferase II (nptII) selectable marker (Schardl et al. 1987). The resulting binary vector was sequenced, to verify the absence of mutations, and shuttled into the A. rhizogenes ATCC 15834, by the standard freeze thaw and CaCl2 method (Weigel and Glazebrook 2006).
S. sclarea hairy root culture
The AtDXS, AtDXR and the empty vector (EV) recombinant A. rhizogenes ATCC 15834 were grown (OD600 = 0.3–0.5) in Yeast Extract Broth liquid medium (0.1 % Yeast extract, 0.5 % Beef extract, 0.5 % Peptone, 0.5 % Sucrose, 0.04 % MgSO4), containing 50 mg l−1 kanamycin, at 28 °C. S. sclarea sterile leaf sections, from twenty day-old plants, were submerged in the bacterial suspension for 30 min, co-cultivated at 28 °C for 3 days in MS medium solidified with 9 g l−1 and then transferred to fresh MS medium with 50 mg l−1 kanamycin and cefotaxime 100 mg l−1. After three weeks of several transfers to medium containing 50 mg l−1 kanamycin and decreasing concentrations of cefotaxime (100 down to 50 mg l−1), hairy roots developing from the infected areas were individually excised, sub-cultured several times on hormone-free medium and maintained at 23 °C in the dark. Kanamycin-resistant hairy root independent lines, with no bacterial contamination, were selected, and sub-cultured into 250 ml flasks, containing a hormone-free liquid MS medium supplemented with 50 mg l−1 kanamycin and kept on a gyratory shaker at 120 rpm at 23 °C in the dark. The cultures were sub-cultured every week.
DNA isolation and PCR analysis
Genomic DNA was extracted from hairy roots by the cetryl trimethyl ammonium bromide (CTAB) method as described in Doyle and Doyle (1990). The DNA was used as the template in PCR analysis to detecting the presence of AtDXS or AtDXR genes in transgenic hairy roots using a forward primer located at the promoter of the PKYLX71:35S2 vector (PKY1) and a reverse primer located at the 3′ end of the rbscS-E9 3′ terminator (CH11). The reactions were performed using 2.5 units of Taq polymerase (Invitrogen, Carlsbad, CA, USA), the conditions for amplification were as follows: 94 °C for 5 min, followed by 30 cycles at 94 °C 30 s, annealing 30 s at the different Tm, according to the primers melting temperatures, 72 °C 1 min, and at final extension 72 °C for 5 min. To detect the absence of contaminating agrobacteria, the hairy root genomic DNA was also amplified with virD2 specific primers (Haas et al. 1995). Primer sequences are reported in Supplementary Table 1.
RNA extraction and RT-PCR analysis
Total RNA was extracted using the plant RNA/DNA Purification kit (Norgen Biotek Corporation Ontario, Canada), according to the manufacturer’s protocol. For semi-quantitative RT-PCR, complementary DNA was synthesized from 1 μg total RNA, previously treated with RNase-free DNAse I (Invitrogen, Carlsbad, CA, USA), using an oligo-dT primer and the Superscript III RT (Invitrogen, Carlsbad, CA, USA) at 50 °C for 50 min. In the PCR reactions one microliter of cDNA was used as template with specific primers (Supplementary Table 1) and 2.5 units of Taq polymerase (Invitrogen, Carlsbad, CA, USA), using the following condition: initial denaturation at 94 °C 1 min, followed by 30 cycles denaturation at 94 °C 30 s, annealing 1 min at the different Tm, according to the primers melting temperatures, extension at 72 °C 1 min and final extension at 72 °C for 5 min. The S. sclarea 18S gene-specific primers were used to normalize equal amounts of RNA for each samples as a internal standard.
Quantitative real-time reverse transcription-PCR
Complementary DNA was synthesized from 1 μg total RNA and reverse-transcribed by Superscript III Reverse transcriptase (Invitrogen). Quantitative RT-PCR was performed using a Light Cycler rapid thermal cycler system (Roche Diagnostics Ltd, Lewes, UK) according to the manufacturer’s instructions. Reactions were performed in a 20 µl volume with 0.5 µM primers and Light Cycler-DNA Master SYBR Green I mix (Roche Diagnostics Ltd, Lewes, UK). The sequences of specific primers are listed in Supplementary Table 1. Transcript levels of AtDXS and AtDXR genes in independent transgenic hairy roots was quantified using SYBR green detection and the CT value of S. sclarea 18S rRNA was subtracted from that of the gene of interest to obtain the ΔCT value. Standard deviations were calculated from three different replica for each independent hairy root lines.
Production of the recombinat AtDXS protein and polyclonal antibodies
The coding sequence of the AtDXS gene was amplified using the specific primers (Supplementary Table 1). The resulting amplicons were cloned behind an N-terminal 6X-His-Tag into the BamHI/HindIII sites of the pQE30 expression vector and the resulting vector transformed into the E. coli M15 pREP4 strain. The recombinant protein was extracted and purified from 500 ml of bacterial culture induced with 1 mM IPTG at 37 °C overnight. The bacterial pellet was dissolved in lysis buffer with 50 mM TrisHCl, pH 8, NaCl 1 mM, EDTA 50 mM, PMSF 0,5 mM, Triton X-100 0.8 %, supplemented with lysozyme (1 mg ml−1), DNAse I 1 mg ml−1 and sonicated for 5 min at 10 s. After centrifugation, the pellet was resuspended in buffer 8 M Urea (pH 8) and the recombinant protein purified on a Ni-Sepharose High Performance agarose column, according to the manufacture’s instructions (GE Healthcare Bio-Science, Uppsala, Sweden). Western blot analysis with an anti-His antibody was performed during amplification and purification of His-fusion protein. Approximately 1.5 mg of purified recombinant protein was used for the production of a polyclonal antiserum in rabbits (Primm Biotech, Milan, Italy).
Western blot analysis
Hairy roots (100 mg) frozen in liquid nitrogen were homogenized and lysed in buffer containing 50 mM Tris–HCl (pH 7.5), 400 mM NaCl, 1 mM EDTA, 20 mM DTT, 5 % glycerol, 1 % Triton X-100 and 1 × Protease Cocktail Inhibitor (Sigma Aldrich, Saint Louis, MO, USA). Total proteins (50 µg), separated on SDS-PAGE gel, were transferred to PVDF membranes and incubated for 3 h with a blocking solution (3 % BSA in 50 mM Tris, 200 mM NaCl, 0,1 % Tween 20), before the overnight incubation at 4 °C with the primary anti-ATDXS (1:5000); anti-ZmDXR (diluition 1:2000, kindly donated by Prof. M.H. Walter, Plant Biochemical Institute, Halle, Germany) or anti-actin 1:1000 (Agrisera AB, Sweden) antibodies. After washing, the membranes were incubated with an appropriate peroxidase-conjugate secondary antibodies and the immune-bands were detected by enhanced chemiluminescence reagent (ECL), according to the manufacture’s instructions (Pierce ECL, Thermo Scientific, Rockford, IL, USA). Bands densitometry was analyzed using the Gel Doc 2000 system (Biorad Laboratories, CA, USA).
Hairy roots growth
Equal amounts (0.5 g) of control line (EV) and overexpressing AtDXS or AtDXR gene hairy root lines were inoculated into MS hormone-free liquid medium and dry weight was monitored for one month at 1-week intervals.
Qualitative and quantitative determination of diterpenoids
Lyophilized and powdered hairy roots (0.5 g) were extracted with acetone for 72 h at room temperature. The extract was filtered through a Millipore filter (0.45 µm) and evaporated under reduced pressure. The residue was dissolved in methanol and subjected to HPLC–DAD analysis (Agilent1200 Series, G1312A binary pump, G1329A automatic sample injector, G1315D diode array detector). The HPLC fingerprint was carried out on a C8 column (Agilent, Zorbax eclips C8 250 × 4.6 mm) with a sample injection volume of 50 µl. The mobile phase was a gradient elution of water acidified with 0.1 % formic acid (solvent A) and acetonitrile (solvent B), starting with 35 % B and rising to 100 % B after 30 min, at a flow rate of 1.0 ml min−1.
The different diterpenes were detected at 280 nm and concentration calculated by the interpolation of the peak areas with calibration curves, constructed with standard purified compounds over the range 10–200 µg ml−1. Content of diterpenoids in roots was expressed as mg g−1 of root dry weight.
Purification of aethiopinone from hairy roots
Hairy roots (200 g) were lyophilized and then extracted with acetone at room temperature for 48 h. The evaporated residue (4 g) was dissolved in acetone and subjected to silica gel column chromatography (4 × 60 cm, 50 g of silica gel for 1 g of crude extract) and the column eluted with CH2Cl2, followed by increasing concentrations of AcOEt (between 1 and 100 %) and then by increasing concentrations of MeOH (between 10 and 100 %). Fractions of 15 ml were collected, analyzed by TLC (silica gel plates, in CH2Cl2 or mixtures CH2Cl2-CHCl3 97:3, 95:5, 9:1, 8:2), CHCl3–MeOH 99:1, 98:2, 97:3, 9:1, 8:2), and grouped into 20 fractions. Fraction 3 was identified as pure aethiopinone.
Human cell culture and treatment
Breast adenocarcinoma (MCF7), uterin cervix epithelial carcinoma (HeLa), prostate adenocarcinoma PC3, human melanoma A375 and human normal skin keratinocyte (HaCat), cells obtained from Cell Bank in GMP-IST (Genova, Italy) were maintained in Dulbecco’s modified Eagle medium (DMEM), supplemented with 10 % (v/v) FBS, 2 mM l-glutamine and antibiotics (100 U/mL penicillin, 100 µg ml−1 streptomycin) from Invitrogen (Carlsbad, CA, USA), at 37 °C in humidified atmosphere with 5 % CO2. To ensure logarithmic growth, cells were sub-cultured every 2 days. Stock solution (50 mM) of purified aethiopinone in DMSO was stored a −20 °C in the dark and diluted just before use in the sterile culture medium. In all experiments, final concentrations of DMSO did not exceed 0.10 % (v/v).
Cell proliferation and viability
MCF7, PC3, A375 and HaCat (1 × 104/well) and Hela (5000/well) cells were seeded in triplicate in 96 well-plates and incubated for 24 h with DMSO or aethiopinone at a concentration between 5 µM and 50 µM). The number of viable cells was quantified by [3-4,5-dimethyldiazol-2-yl]-2,5-diphenyl tetrazolium bromide (MTT) conversion assay (Sigma-Aldrich, St. Louis, MO, USA), according to Mosmann (1983). Briefly, after the indicated treatment, 25 µl MTT (5 mg/mL in PBS) were added and cells were incubated for an additional 3 h at 37 °C. Thereafter, cells were lysed and solubilized with 100 µl of buffer containing 50 % (v:v) N,N-dimethylformamide, 20 % SDS, with an adjusted pH 4.5 (Opipari et al. 1992). The absorbance was measured with a microplate reader (Titertek multiskan MCC7340, LabSystems, Vienna, VA, USA) equipped with a 620 nm filter. IC50 values were calculated from cell viability dose–response curves and defined as the concentration resulting in 50 % inhibition of cell survival at 24 h, compared to control cells treated with 0.10 % DMSO.
Analysis of cell cycle and hypodiploidy by flow cytometry
Cell DNA content was measured by propidium iodide (PI) incorporation into permeabilized cells, as described by Nicoletti et al. (1991). Briefly, the cells harvested after aethiopinone treatment were washed with cold PBS and incubated with a PI solution (0.1 % sodium citrate, 0.1 % Triton X-100 and 50 μg ml−1 of PI (Sigma-Aldrich, St. Louis, USA), 10 μg ml−1 RNAse A for 30 min at room temperature. Data from 10.000 events for each sample were collected by a FACScalibur Flow Cytometr (Becton–Dickinson, San Josè, CA). Cellular debris was excluded from the analysis by raising the forward scatter threshold. The percentage of cells in the sub G0/G1 phase was quantified using CellQuest Software (Becton–Dickinson). Results were expressed as a mean ± SD of three experiments performed in triplicate.
Statistical analysis
All reported data represent the mean ± SD of at least three independent experiments performed in triplicate. The statistical significance of transcript and protein levels was analyzed by one-way analysis of variance (ANOVA), with Tukey’s post-test, using GraphPad Prism 5 software. The statistical significance of hairy root growth rate and diterpene content was examined in the two-way analysis of variance (ANOVA) with Bonferroni post-test analysis using GraphPad Prism 5 software.
Results
Overexpression of AtDXS or AtDXR genes in S. sclarea hairy roots
Heterologous AtDXS or AtDXR genes, with their own chloroplast transit peptides, were overexpressed in S. sclarea hairy roots, by A. rhizogenes (ATCC 15834) transformation, under the control of the constitutive 35S Cauliflower Mosaic Virus (CaMV) promoter. A total of ten kanamycin-resistant independent hairy root lines were generated for each gene, with a stable insertion of the transgene in the genome and absence of bacterial contamination (Supplementary Fig. 1). The control hairy root lines generated from empty vector transformation were denoted as EV.
Expression analysis of the AtDXS (a) or AtDXR (b) genes in transgenic S. sclarea hairy root lines, by semi-quantitative RT-PCR of ten hairy root lines for each gene and qRT-PCR amplification of three transgenic hairy root lines for each gene, used for further characterization. Relative transcript levels with the largest ΔCT were arbitrarily set as 1. For each amplification, 18S rRNA gene was amplified as internal standard to normalize equal amounts of RNA. Quantitative values represent mean ± SD of three experiment performed in triplicate for each transgenic hairy root line (**P < 0.01; ***P < 0.001)
Semi-quantitative reverse transcription PCR confirmed that eight out of ten independently AtDXS transformed hairy root lines expressed the AtDXS gene, while the AtDXR gene was expressed in all transformed root lines (Fig. 1a, b). A subset of these lines was, then, analyzed by quantitative real-time PCR, using specific set of primers, which did not amplify endogenous DXS or DXR gene. Transcript levels of AtDXS or AtDXR gene were normalized against the level detected in the respective DXS or DXR lines with the lowest expression of the transgene. Three different transformed root lines, with a high, moderate, and low expression level of each gene were selected and analyzed by Western blot analysis, using polyclonal antibodies against the AtDXS or ZmDXR protein (Fig. 2a, b). In the S. sclarea transgenic root lines, either AtDXS or AtDXR proteins, with an expected size of 68 and 52 kDa, respectively, were highly expressed, above the endogenous protein level recognized by the two Abs (Fig. 2b). These transgenic hairy root lines, with varying amounts of AtDXS or AtDXR proteins, were further analyzed for biomass and metabolic changes.
Expression level of DXS (a) and DXR (b) proteins in three different transgenic S. sclarea hairy root lines. Total protein extracts from selected hairy root lines were separated by SDS-PAGE and analyzed by western blot analysis with polyclonal Ab against AtDXS or ZmDXR. Actin level was used for normalization. Densitometric values represent mean values ± SD of three different experiment of each transgenic hairy root line. (*P < 0.05; **P < 0.01; ***P < 0.001)
Phenotype and biomass growth of the overexpressing hairy root lines
After A. rhizogenes mediated leaf-disk transformation with binary vectors carrying either the AtDXS or AtDXR gene, hairy root primordia emerged from wound sites of leaf disks within 20–30 days after inoculation. About 12 weeks after co-cultivation with A. rhizogenes, putative transgenic hairy roots of S. sclarea began to grow more rapidly and were subcultured in selective liquid medium. The three different hairy root lines overexpressing the AtDXS or AtDXR gene had typical yellowish or light reddish abundant roots, which were highly branched, with a phenotype indistinguishable from that of the control EV line.
It is frequently reported that perturbation of the metabolic pathway through overexpression of biosynthetic genes might have a detrimental growth effect and hence may adversely affect the final production of secondary metabolites. Therefore, after isolation, equal amounts of hairy roots of each overexpressing lines were inoculated into MS hormone-free liquid medium and their growth monitored at one-week intervals (Fig. 3a, b). The hairy root lines expressing a low (DXS10) or medium level (DXS7) of the exogenous AtDXS protein had on average a five-fold increase in dry weight, very similar to the final dry weight (DW) achieved by the control line (EV) generate from empty vector transformation. In contrast, the DW of the DXS3 line only doubled after 1 month, probably due to some inhibitory effects of the high level of the heterologous plant DXS protein (Fig. 3a). Overexpression of the AtDXR protein was compatible with active growth of the transgenic hairy root lines. After 4 weeks, the DW of the DXR overexpressing hairy root lines increased on average six-fold, not statistically differing from the final DW achieved by the control line (EV). Interestingly, the DXR4 root line, with the highest level of AtDXR protein, grew faster and, after 4 weeks in hormone-free liquid medium, had a significantly (P < 0.001) higher DW than the blank EV line (Fig. 3b).
Biomass production, expressed as dry weight, of three independent hairy root lines overexpressing different levels of the AtDXS (a) or AtDXR (b) proteins, compared to EV (empty vector) hairy root line, during one month of culture. Data represent mean values ± SD of three experimental replicates for each transgenic hairy root. (*P < 0.05; **P < 0.01; ***P < 0.001)
Accumulation of abietanic diterpenoids in transgenic hairy root lines
To evaluate whether or not the overexpression of the heterologous AtDXS or AtDXR protein was a valuable strategy to boost the synthesis of abietanic diterpenes in S. sclarea hairy roots, acetone extracts from three independent samples of each transgenic hairy root line were analyzed by HPLC–DAD. The typical chromatogram of diterpenes of the control EV hairy root line is reported in Fig. 4a, where peak 1 is related to carnosic acid (Rt 16.1), peak 2 to salvipisone (Rt 21.2), peak 3 to aethiopinone (Rt 24.8), peak 4 to 1-oxo-aethiopinone (Rt 25.6), peak 5 to 1-oxo-ferruginol (Rt 26.1) and peak 6 to Ferruginol (Rt 26.9). Overexpression of either AtDXS or AtDXR protein enhanced the synthesis of the different diterpenoids in the transgenic hairy roots (Fig. 4a). Structures of single HPLC purified compounds were resolved and confirmed by NMR analysis (Fig. 4b). Quantitative analysis, with standard curves of the purified compounds, evidenced that the total content in the blank EV root line (calculated as a sum of the identified diterpenes) was 1.29 mg g−1 DW (Table 1). Total diterpene content was significantly enhanced in all three AtDXS transgenic hairy root lines. The maximum accumulation, corresponding to a 2.2-fold increase compared to the amount in the blank EV line, was detected in the DXS3 line (2.43 ± 0.40 mg g−1 root DW, P < 0.001), characterized by the highest expression level of the AtDXS protein. A general increase in the content of different abietanic diterpenes was also achieved by AtDXR overexpression. A 3.3-fold increase (P < 0.001) in total abietanic diterpenes was detected in the line DXR7 (4.50 ± 0.53 mg g−1 root DW), compared to the blank EV line (1.29 ± 0.14 mg g−1 root DW). When the main different abietanic diterpenoids synthesized in S. sclarea roots were analyzed separately, it was found that overexpression of the AtDXS or AtDXR gene enhanced the synthesis of all the five identified abietanic diterpenes, albeit at variable levels. The most significant effect of AtDXS overexpression was the increase in the amount of the abietane-quinone type diterpene aethiopinone, whose content increased from 0.40 ± 0.03 mg g−1 DW of the control untransformed hairy roots line up to 1.01 ± 0.2 mg g−1 DW in the DXS3 line, which overexpresses the highest level of the AtDXS protein. This amount corresponds to a 2.5-fold increase above the content of EV line. Aethiopinone was also the diterpene whose synthesis was preferentially enhanced (P < 0.001) by AtDXR overexpression. The highest content in aethiopinone was found in the DXR7 line (1.90 ± 0.33 mg g−1 root DW), corresponding to a 4.6-fold increase above the basal level detected in the blank EV line (0.40 ± 0.03 mg g−1 DW).
Identification of abietane type diterpenes in S. sclarea hairy roots overexpressing AtDXS or AtDXR proteins by HPLC–DAD. Comparison of the chromatograms of the best line (DXS3 and DXR7) with the control EV line (a) and chemical structure of the most representative abietane diterpenes confirmed by NMR (b) (1) carnosic acid; (2) salvipisone; (3) aethiopinone; (4) 1-oxo-aethiopinone; (5) 1-oxo-ferruginol; (6) ferruginol
Taken together, the metabolic data indicate that increased pools of precursors of the MEP-dependent isoprenoid pathway, achieved by overexpressing heterologous AtDXS or AtDXR enzymes, acting up-stream of GGPP, the universal diterpenoid precursor, are sufficient to boost significantly the synthesis of abietane diterpenes in clary sage hairy roots.
Cytotoxicity of aethiopinone on solid tumor cell lines
Aethiopinone has recently attracted attention due to its high cytotoxic activity against HL-60 and NALM-6 leukemia cells (Rózalski et al. 2006). To test its cytotoxicity against solid tumor lines, breast adenocarcinoma (MCF7), uterin cervix epithelial carcinoma (HeLa), prostate adenocarcinoma (PC3) and human melanoma (A375) cell lines were incubated for 24 h with increasing concentrations of aethiopinone, purified from S. sclarea hairy roots (0.5–100 µM). Cell viability was determined by MTT proliferation assay. As reported in Table 2, IC50 after aethiopinone treatment varied in the different tumor cell lines, with the lowest IC50 value (11.4 ± 0.5 µM) found against the A375 human melanoma cell line. Interestingly, in control HaCat cells, derived from normal human skin, cytotoxic effects were observed only at about nine-fold higher doses of aethiopinone. To ascertain whether loss of cell viability due to aethiopinone treatment in A375 cells was mediated by apoptosis, apoptotic cells were identified by flow cytometric analysis of DNA fragmentation after nuclei staining with propidium iodide. Cells were incubated for 24 h with 5 µM and 10 µM aethiopinone, and then the population of cells with sub G0/G1 DNA content, indicative of apoptotic cell death, was determined. Aethiopinione treatment induced apoptosis of A375 cells with 28.5 and 42.5 % cell death at 5 and 10 µM respectively (Fig. 5). These results encourage further studies on this abietanic diterpene as a promising anti-tumoral active compound, especially against resistant melanoma cells.
Percentage of apoptotic hypodiploid (Sub G0/G1) A375 cells after treatment with DMSO or different concentration (5 or 10 μM) of aethiopinone for 24 h. DNA fragmentation was evaluated by flow cytometry (PI staining of permeabilized cells) and results are expressed as the percentage of the total cell population analyzed (10,000 events). Data represent mean values ± SD of three experiment performed in duplicate. (**P < 0.01; ***P < 0.001)
Discussion
Root diterpenes of S. sclarea plants belong to the class of tri-cyclic diterpenes either of the abietane-phenolic type (e.g. carnosic acid and ferruginol) or abietane-quinone-type (aethiopinone, salvipisone and 1-oxoaethiopinone) (Ulubelen et al. 1994; Kuźma et al. 2006). It has been demonstrated that aethiopinone has a relatively high cytotoxicity against HL-60 and NALM-6 leukemia cells (Rózalski et al. 2006). Also sclareol has been found to induce apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53-independent mechanism of action (Noori et al. 2010; Mahaira et al. 2011).
Despite their known pharmacological importance, abietanic diterpenoids are synthesized in the roots of S. sclarea, and in other Salvia spp (e.g. S. aethiopis and others) at very low levels (<1 % dry weight). Such levels are often inadequate for large-scale production, and pose a serious problem to carry out in vitro and in vivo assays required for their potential use as novel antitumor molecules. One possibility is to extract plant bioactive secondary metabolites normally biosynthesized in roots of differentiated plants from hairy root cultures, in which biosynthesis of the specific metabolites might be further optimized by metabolic engineering approaches (Georgiev et al. 2012; Talano et al. 2012).
This approach was used to establish S. sclarea hairy root culture, in which the plastidial MEP-dependent isoprenoid pathway was modified by enhancing the availability of the DXS or DXR proteins, the first two enzymes of this pathway, in order to obtain a high amount of common precursors that might converge towards a higher accumulation of diterpenes. DXS and DXR genes have been extensively studied and overexpressed in plants to enhance the synthesis of different classes of terpenoids (Yoon and Nodwell 2014). Overexpression of the DXS protein was effective in boosting the synthesis of diterpenes in S. sclarea hairy roots, increasing preferentially the accumulation of aethiopinone, an abietane-quinone-type diterpene. Similar results have been reported in transgenic Arabidopsis and tomato plants in which DXS activity had been altered, confirming the regulatory role of DXS in controlling flux through the MEP pathway (Estévez et al. 2001; Enfissi et al. 2005; Morris et al. 2006). Less successful examples are available for medicinal plants: enhanced synthesis of tanshinone, an antitumoral labdane-type diterpene, has been obtained in S. miltiorrhiza hairy roots by overexpressing the SmDXS gene (Kai et al. 2011) and other medicinal plants, such as L. latifolia (Miñoz-Bertomeu et al. 2006), C. roseus (Peebles et al. 2011) and Artemisia annua (Xiang et al. 2012). Our data indicate that DXS overexpression also contributes, at least partially, to direct the metabolic flux towards the synthesis of abietanic diterpenoids in S. sclarea hairy roots. However, it is worth emphasizing that the increased level of the exogenous DXS protein should not exceed a threshold value that might be detrimental to active growth, as shown in the S. sclarea DXR3 hairy root line. The undesirable side-effects of an excessive level of DXS protein on hairy root growth might be associated to a general competition with the basal protein synthesis machinery or to the accumulation of other MEP-derived secondary metabolites, such as phytohormones (cytokines, gibberellin or ABA), known to regulate plant growth and development The DXR enzyme has also been the target of intensive research aimed at understanding its regulatory control on the isoprenoid biosynthesis and improving ultimately the biosynthesis of different class of isoprenoids, although the results are more controversial than those reported for DXS up-regulation (Rodríguez-Concepción et al. 2001; Dudareva et al. 2005).
In the case of DXR overexpression, although the three overexpressing hairy root lines analyzed had different levels of the exogenous proteins, no detrimental effects associated to high levels of the DXR protein on transgenic hairy root growth were observed. The final dry weight of all the DXR overexpressing lines tested did not significantly differ from that of the EV control line. As already reported, this is a tremendous benefit for large-scale production of hairy root biomass as a source for extracting purified bioactive secondary metabolites. In addition, overexpression of the AtDXR gene proved to be a more efficient strategy in boosting the synthesis of abietane-type diterpenes in transgenic hairy roots. The increase in total diterpenoids was, on average, higher than the increase triggered by overexpressing the DXS protein. The highest increase was detected in the line DXR7, with a content in total abietane diterpenoids about three times higher than that of the EV hairy root line and higher than the two-fold increase detected in the best line overexpressing the plant DXS gene (DXS3).
These data indicate that DXR protein might exert a tighter control over the entire isoprenoid metabolic flux than the DXS protein and are consistent with previous results reporting a positive correlation between enhanced plastid isoprenoid biosynthesis and DXR transcript accumulation in different plant species (Walter et al. 2000; Veau et al. 2000; Carretero-Paulet et al. 2002; Hans et al. 2004; Hsieh and Goodman 2005; Mayrhofer et al. 2005; Bede et al. 2006; Hasunuma et al. 2008, 2010). Transgene-mediated alteration of DXR protein levels in peppermint plants has been shown to lead to concomitant changes in the production of monoterpene essential oils in leucoplasts (Mahmoud and Croteau 2001). Similar results have been reported in A. thaliana plants overexpressing DXR, demonstrating that the production of MEP from DXP catalyzed by DXR also limits the biosynthesis of isoprenoids in chloroplasts (Carretero-Paulet et al. 2006) and in transplastomic tobacco plants that overproduced DXR from Synechocystis sp. strain PCC6803 (Hasunuma et al. 2008).
Although our findings and results reported elsewhere point to DXR as one flux control enzymatic step to IPP and DMAPP availability, its contribution to the precursor supply of the MEP-pathway is not a general rule. DXR levels do not correlate with the accumulation of carotenoids in the chromoplasts of ripening tomato fruit (Rodríguez-Concepción et al. 2001) or the diurnal oscillation of the biosynthesis and emission of MEP-derived isoprenoid volatiles in snapdragon flowers (Dudareva et al. 2005). In general, these conflicting findings support the notion that the final outcome of modifying DXR gene expression, may vary according to the plant species and the presence of other potential post-translational modifications that might regulate enzymatic activity (Cordoba et al. 2009; Pulido et al. 2013).
Abietane diterpenes, especially those containing quinone moieties, are often reported to have cytotoxic effects on cancer cell lines (Fronza et al. 2012). The roots of S. sclarea are rich in abietane diterpenoids (e.g. aethiopinone, 1-oxoaethiopinone, salvipisone, and ferruginol), with known antibacterial, antifungal, and several others pharmacological properties (Topcu and Goren 2007). Aethiopinone, salvipisone, 1-oxoaethiopinone and ferruginol have been proved to have bacteriostatic as well as bactericidal effects in Staphylococcus aureus and Staphylococcus epidermidis strains (Kuźma et al. 2007). More recently, this class of tricycle diterpenoids has raised considerable interest due to its cytotoxic activity against human leukemic cell lines (Rózalski et al. 2006). In the present study, aethiopinone was proved to be cytotoxic also to different solid tumor cell lines, i.e. MCF7 (breast adenocarcinoma, IC50 30 µM), HeLa (epithelial carcinoma, IC50 33 µM), PC3 (prostate adenocarcinoma, IC50 48 µM), and A375 (human melanoma, IC50 11.4 µM). Preliminary results have also shown that aethiopinone treatment induced apoptosis of A375 cells with 28.5 and 42.5 % cell death at 5 and 10 µM respectively.
An increasing number of quinone diterpenoids are being found in Salvia spp. One of the most extensively studied species is S. miltiorrhiza, which is used in many therapeutic remedies in Chinese traditional medicine. Tanshinones are the major bioactive compounds of this plant and a recent studies have shown their anticancer activity (Dat et al. 2007; Zhou et al. 2008; Lu et al. 2009; Pan et al. 2010). These detailed studies on the anti-proliferative activity of tanshinone, which shares structural similarity in the quinone moiety with aethiopinone, suggest the need for additional investigation of the molecular mechanisms underlying the cytotoxicity that we found primarily in human melanoma cells. The incidence of malignant melanoma is increasing faster than any other cancer, and successful systemic chemotherapy is rare (Rigel and Carucci 2000). Therefore, further studies are necessary for deep understanding of the molecular targets of this promising abietane diterpenoid and for further optimization towards the development of an antiproliferative agent to treat tumors, such as melanomas.
In conclusion, ectopic overexpression of both AtDXS and AtDXR genes was effective at enhancing the synthesis of abietane-type diterpenoids, and expecially, aethiopinone. Overexpression of the AtDXR gene was a more efficient strategy in boosting the synthesis of abietane-type diterpenes in transgenic hairy roots, since the increase was on average higher than the increase triggered by overexpressing the DXS protein, indicating that the DXR enzyme might exert a tighter control than the DXS enzyme over the entire isoprenoid metabolic flux.
Further optimization of the biosynthesis of this interesting class of diterpenes might be achieved by modifying the level of expression of enzymes other than DXS or DXR. These include genes encoding enzymes acting down-stream of the IPP and DMAPP precursors, such as geranylgeranyl diphosphate synthase (GGPPS) involved in the synthesis of GGPP, the universal precursors of plastidial isoprenoids, or encoding more specific diterpene synthases, as demonstrated for increased content of tanshinone in S. miltiorrhiza hairy roots (Kai et al. 2011). Such an approach will undoubtedly benefit from the discover of new genes involved in the down-stream enzymatic steps of the diterpene biosythtetic pathway through the highthroughput deep sequencing projects of Salvia spp (Caniard et al. 2012; Yang et al. 2013) and other medicinal plants (Zerbe et al. 2013).
Abbreviations
- AT:
-
Arabidopsis thaliana
- DXS:
-
d-xylulose 5-phosphate synthase
- DXR:
-
1-Deoxy-d-xylulose 5 phosphate reductoisomerase
- MEP:
-
2-C-methyl-D-erythritol 4-phosphate
- CAMV:
-
Cauliflower mosaic virus
References
Alasbahi RH, Melzig MF (2010) Plectranthus barbatus: a review of phytochemistry, ethnobotanical uses and pharmacology—Part 2. Planta Med 76:753–765
Bede JC, Musser RO, Felton GW, Korth KL (2006) Caterpillar herbivory and salivary enzymes decrease transcript levels of Medicago truncatula genes encoding early enzymes in terpenoid biosynthesis. Plant Mol Biol 60:519–531
Caniard A, Zerbe P, Legrand S, Cohade A, Valot N, Magnard JL, Bohlmann J, Legendre L (2012) Discovery and functional characterization of two diterpene synthase for sclareol biosynthesis in Salvia sclarea (L.) and their relevance for perfume manufacture. BMC Plant Biol 12:119–132
Carretero-Paulet L, Ahumada I, Cunillera N, Rodríguez-Concepción M, Ferrer A, Boronat A, Campos N (2002) Expression and molecular analysis of the Arabidopsis DXR gene enconding 1-deoxy-D-xylulose 5-phosphate reductoisomerase, the first committed enzyme of the 2-C-methyl-D-erythritol 4-phosphate pathway. Plant Physiol 129:1581–1591
Carretero-Paulet L, Cairó A, Botella-Pavía P, Besumbes O, Campos N, Boronat A, Rodríguez-Concepción M (2006) Enhanced flux through the methylerythritol 4-phosphate pathway in Arabidopsis plants overexpressing deoxylulose 5-phosphate reductoisomerase. Plant Mol Biol 62:683–695
Chappell J (1995) Biochemistry and molecular biology of the isoprenoid pathway in plants. Annu Rev Plant Mol Biol 46:521–547
Cordoba E, Salmi M, León P (2009) Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J Exp Bot 60:2933–2943
Dat NT, Jin X, Lee JH, Lee D, Hong YS, Lee K, Kim YH, Lee JJ (2007) Abietane diterpenes from Salvia miltiorrhiza inhibit the activation of hypoxia-inducible factor-1. J Nat Prod 70:1093–1097
Doyle JJ, Doyle JL (1990) A rapid total DNA preparation procedure for fresh plant tissue. Focus 12:13–15
Dudareva N, Andersson S, Orlova I, Gatto N, Reichelt M, Rhodes D, Boland W, Gerchenzon J (2005) The nonmevalonate pathway supportos both monoterpene and sesquiterpenes formation in snapdragon flowers. Proc Natl Acad Sci USA 102:933–938
Dudareva N, Klempien A, Muhlemann JK, Kaplan I (2013) Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol 198:16–32
Enfissi EM, Fraser PD, Lois LM, Boronat A, Schuch W, Bramley PM (2005) Metabolic engineering of the mevalonate and non mevalonate isopentenyl diphosphate-forming pathways for the production of health-promoting isoprenoids in tomato. Plant Biotech J 3:17–27
Estévez JM, Cantero A, Reindl A, Reichler S, León P (2001) 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276:22901–22909
Fronza M, Lamy E, Günther S, Heinzmann B, Laufer S, Merfort I (2012) Abietane diterpenes induce cytotoxic effects in human pancreatic cancer cell line MIA PaCa-2 through different modes of action. Phytochemistry 78:107–119
Georgiev M, Agostini E, Ludwig-Muller J, Xu J (2012) Genetically transformed roots: from plant disease to biotechnological resource. Trends Biotech 30:528–537
Haas JH, Moore LW, Ream W, Manulis S (1995) Universal PCR primers for detection of phytopathogenic Agrobacterium strains. Appl Environ Microbiol 61:2879–2884
Hans J, Hause B, Strack D, Walter MH (2004) Cloning, characterization, and immunolocalization of a mycorrhiza-inducible 1-deoxy-D-xylulose 5-phosphate reductoisomerase in arbuscule-containing cells of maize. Plant Physiol 134:614–624
Hasunuma T, Takeno S, Hayashi S, Sendai M, Bamba T, Yoshimura S, Tomizawa K, Fukusaki E, Miyake C (2008) Overexpression of 1-deoxy-D-xylulose-5-phosphate reductoisomerase gene in chloroplast contributes to increment of isoprenoid production. J Biosci Bioeng 105:518–526
Hasunuma T, Kondo A, Miyake C (2010) Metabolic engineering by plastid transformation as a strategy to modulate isoprenoid yield in plants. Methods Mol Biol 643:213–227
Hsieh MH, Goodman HM (2005) The Arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol 138:641–653
Jennewein S, Croteau R (2001) Taxol: biosynthesis, molecular genetics, and biotechnological applications. Appl Microbiol Biotechnol 57:13–19
Kai G, Xu H, Zhou C, Liao P, Xiao J, Luo X, You L, Zhang L (2011) Metabolic engineering tanshinone biosynthetic pathway in Salvia miltiorrhiza hairy root cultures. Metab Engin 13:319–327
Kuźma Ł, Skrzypek Z, Wysokińska H (2006) Diterpenoids and triterpenoids in hairy roots of Salvia sclarea. Plant Cell Tissue Org Cult 84:171–179
Kuźma L, Rozalsk M, Walencka E, Rozalska B, Wysokinska H (2007) Antimicrobial activity of diterpenoids from hairy roots of Salvia sclarea L: salvipisone as a potential antibiofilm agent active against antibiotic resistant staphylococci. Phytomedicine 14:31–35
Lange BM, Wildung MR, McCaskill D, Croteau R (1998) A family of transketolases that directs isoprenoid biosynthesis via a mevalonate-independent pathway. Proc Nat Acad Sci USA 95:2100–2104
Lange BM, Rujan T, Martin W, Croteau R (2000) Isoprenoid biosynthesis: the evolution of two ancient and distinct pathways across genomes. Proc Natl Acad Sci USA 97:13172–13177
Li L, Shukla S, Lee A, Garfield SH, Maloney DJ, Ambudkar SV, Yuspa SH (2010) The skin cancer chemotherapeutic agent ingenol-3-angelate (PEP005) is a substrate for the epidermal multidrug transporter (ABCB1)and targets tumor vasculature. Cancer Res 70:4509–4519
Lois LM, Campos N, Putra SR, Danielsen K, Rohmer M, Boronat A (1998) Cloning and characterization of a gene from Escherichia coli encoding a tranketolase-like enzyme that catalyzes the synthesis of D-1-deoxyxylulose 5 –phosphate, a common precursor for isoprenoid, thiamin, and pyridoxol biosynthesis. Proc Natl Acad Sci USA 95:2105–2110
Lu Q, Zhang P, Zhang X, Chen J (2009) Experimental study of the anti-cancer mechanism of tanshinone IIA against human breast cancer. J Int Mol Med 24:773–780
Mahaira LG, Tsimplouli C, Sakellaridis N, Alevizopoulos K, Demetzos C, Han Z, Pantazis P, Dimas K (2011) The labdane diterpene sclareol (labd-14-ene-8, 13-diol) induces apoptosis in human tumor cell lines and suppression of tumor growth in vivo via a p53-independent mechanism of action. Eur J Pharmacol 666:173–182
Mahmoud SS, Croteau RB (2001) Metabolic engineering of essential oil yield and composition in mint by altering expression of deoxy-xylulose phosphate reductoisomerase and menthofuran synthase. Proc Nat Acad Sci USA 98:8915–8920
Mayrhofer S, Teuber M, Zimmer I, Louis S, Fischbach RJ, Schnitzler JP (2005) Diurnal and seasonal variation of isoprene biosynthesis-related genes in grey poplar leaves. Plant Physiol 139:474–484
Miñoz-Bertomeu J, Arrillaga I, Ros R, Segura J (2006) Up-regulation of 1-deoxy-D-xylulose-5-phosphate synthase enhances production of essential oils in transgenic spike lavender. Plant Physiol 142:890–900
Mnonopi N, Levendal RA, Mzilikazi N, Frost CL (2012) Marrubiin, a constituent of Leonotis leonurus, alleviates diabetic symptoms. Phytomedicine 19:488–493
Morris WL, Ducreux LJ, Hedden P, Millam S, Taylor MA (2006) Overexpression of a bacterial 1-deoxy-D-xylulose 5-phosphate synthase gene in potato tubers perturbs the isoprenoid metabolic network: implications for the control of the tuber life cycle. J Exp Bot 57:3007–3018
Moses T, Pollier J, Thevelein JM, Goossens A (2013) Bioengineering of plant (tri)terpenoids: from metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytol 200:27–43
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Plant Physiol 15:473–497
Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C (1991) A rapid and simple method for measuring thymocite apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods 139:271–279
Noori S, Hassan ZM, Mohammad M, Habibi Z, Sohrabi N, Bavanolhagh S (2010) Sclareol modulates the Treg intra-tumoral infiltrated cell and inhibits tumor growth in vivo. Cell Immunol 263:148–153
Opipari AWJr, Hu HM, Yabkowitz R, Dixit VM (1992) The A20 zinc-finger protein protects cells from turmor necrosis factor cytotoxicity. J Biol Chem 297:12424–12427
Pan TL, Hung YC, Wang PW, Chen ST, Hsu TK, Sintupisut N, Cheng CS, Lyu PC (2010) Functional proteomic and structural insights into molecular targets related to the growth inhibitory effect of tanshinone IIA on HeLa cells. Proteomics 10:914–929
Peebles AM, Sander G, Hughes E, Peacok R, Shanks J, San K (2011) The expression of 1-deoxy-D-xylulose synthase and geraniol-10-hydroxylase or anthranilate synthase increases terpenoid indole alkaloid accumulation in Catharanthus roseus hairy roots. Metab Eng 13:234–240
Phillips M, León P, Boronat A, Rodríguez-Concepción M (2008) The plastidial MEP pathway unified nomenclature and resources. Trend Plant Sci 13:619–623
Pulido P, Toledo-Ortiz G, Phillips MA, Wright LP, Rodríguez-Concepción M (2013) Arabidopsis j-protein j20 delivers the first enzyme of the plastidial isoprenoid pathway to protein quality control. Plant Cell 25:4183–4194
Rigel DS, Carucci JA (2000) Malignant melanoma: prevention, early detection, and treatment in the 21st century. J CA Cancer Clin 50:215–236
Rodríguez-Concepción M, Boronat A (2002) Elucidation of the methylerythritol phosphate pathway for isoprenoid biosynthesis in bacteria and plastids. A metabolic milestone achieved through genomics. Plant Physiol 130:1079–1089
Rodríguez-Concepción M, Ahumada I, Diez-Juez E, Sauret-Gueto S, Lois LM, Gallego F, Carretero Paulet L, Campos N, Boronat A (2001) 1-Deoxy-D-xylulose 5-phosphate reductoisomerase and plastid isoprenoids biosynthesis during tomato fruit ripening. Plant J 27:213–222
Rohmer M, Seeman M, Horbach S, Bringer-Meyer S, Sahm H (1996) Glyceraldehyde 3-phosphate and pyruvate as precursors of isoprenic units in an alternative non-mevalonate pathway for terpenoid biosynthesis. J Am Chem Soc 118:2564–2566
Rózalski M, Kuźma L, Krajewska U, Wysokinska H (2006) Cytotoxic and proapoptotic activity of diterpenoids from in vitro cultivated Salvia sclarea roots. Studies on the leukemia cell lines. Z Naturforsch 61:483–488
Schardl CL, Byrd AD, Bezion G, Altschuler MA, Hildebrand DF, Hunt AG (1987) Design and construction of a versatile system for the expression of foreign genes in plants. Gene 61:1–11
Talano M, Oller A, González P, Agostini E (2012) Hairy roots their multiple applications and recents patents. Recent Plant Biotech 6:115–133
Topcu G, Goren AC (2007) Biological activity of diterpenoids isolated from Anatolian laminaceae plants. Rec Nat Prod 1:1–16
Ulubelen A, Eriş C, Sönmez U, Kartal M, Kurucu S, Bozok-Johansson C (1994) Terpenoids from Salvia sclarea. Phytochemistry 36:971–974
Ulubelen A, Sonmez U, Topcu G (1997) Diterpenoids from the roots of Salvia sclarea. Phytochemistry 344:1297–1299
Veau B, Courtois A, Oudin J, Chenieux C, Rideau M, Clastre M (2000) Cloning and expression of cDNAs enconding two enzymes of the MEP pathway in Catharanthus roseus. Biochem Biophys Acta Gene Struct Exp 1517:159–163
Walter M, Fester T, Strack D (2000) Arbuscular mycorrhizal fungi induce the non-mevalonate methylerythritol phosphate pathway of isoprenoid biosynthesis correlated with the accumulation of the yellow pigment and other apocarotenoids. Plant J 21:571–578
Weigel D, Glazebrook J (2006) Transformation of Agrobacterium using the freeze-thaw method. CSH Protoc 7:110–115
Wu S, Shalk M, Clark A, Miles RB, Coates R, Chappell J (2006) Redirection of cytosolic or plastidic isoprenoid precursors elevates terpene production in plants. Nat Biotech 24:1141–1147
Xiang L, Zeng L, Yuan Y, Chen M, Wang F, Liu X, Zeng L, Lan X, Liao Z (2012) Enanchement of artemisinin biosynthesis by overexpressing dxr, cyp71av1 and cpr in the plants of Artemisia annua L. Plant Omics J 5:503–507
Yang L, Ding G, Lin H, Cheng H, Kong Y, Wei Y, Fang X, Liu R, Wang L, Chen X, Yang C (2013) Transcriptome analysis of medicinal plant Salvia miltiorrhiza and identification of genes related to tanshinone biosynthesis. PLoS ONE 8:e80464
Yoon V, Nodwell JR (2014) Activating secondary metabolism with stress and chemicals. J Ind Microbiol Biotechnol 41:415–424
Zerbe P, Hamberger B, Yuen MM, Chiang A, Sandhu HK, Madilao LL, Nguyen A, Hamberger B, Bach SS, Bohlmann J (2013) Gene discovery of modular diterpene metabolism in non model systems. Plant Physiol 162:1073–1091
Zhou L, Chan WK, Xu N, Xiao K, Luo H, Luo KQ, Chang DC (2008) Tanshinone IIA, an isolated compound from Salvia miltiorrhiza Bunge, induces apoptosis in HeLa cells through mitotic arrest. Life Sci 83:394–403
Acknowledgments
This work was supported by funds provided to AL by the Research Project of National Interest (PRIN 2005), the Italian Ministry of University of Education (MIUR) and by a Short-Term Scientific Mission to MCV by COST Action FA1006 “Plant Metabolic Engineering for High Value Products”. We would like to acknowledge Prof. M.H. Walter, Plant Biochemical Institute, Halle, Germany, for the gift of ZmDXR antibody.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.


11240_2014_514_MOESM2_ESM.jpg
Supplementary Fig. 1 Stable insertion of the heterologous DXS or DXR genes into the genome of ten independent lines of Salvia sclarea transgenic hairy roots for each gene. PCR amplification from genomic DNA, using specific primers of A. thaliana DXS (a) or A. thaliana DXR (b). Genomic DNA was used for amplifying virD gene to confirm absence of contaminant bacteria. PC, positive control (vector carrying the respective exogenous genes), EV, empty vector (hairy root generate from empty vector transformation), NS, negative control without DNA sample (JPEG 55 kb)
Rights and permissions
About this article
Cite this article
Vaccaro, M., Malafronte, N., Alfieri, M. et al. Enhanced biosynthesis of bioactive abietane diterpenes by overexpressing AtDXS or AtDXR genes in Salvia sclarea hairy roots. Plant Cell Tiss Organ Cult 119, 65–77 (2014). https://doi.org/10.1007/s11240-014-0514-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0514-4