Abstract
Cereal plants take up iron from the soil via a phytosiderophore-mediated chelation system. Following root absorption, iron is transported through the xylem and phloem of the plant with the help of a variety of efflux and influx transporters belonging to the Zrt Irt-like protein (ZIP) and yellow stripe-like (YSL) protein families. Iron-regulated transporter1, a member of the ZIP family, mobilises ferrous [Fe(II)] ions, while several YSL family members such as YSL2, YSL15 and YSL18 can transport both ferric [Fe(II)] and ferrous [F`III)] ions into developing grains via chelation with mugineic acid or its derivatives. The iron is accumulated largely in the outer aleurone layer and embryo of the grains, which are removed during milling, leaving behind consumable endosperm that contains a very low amount of iron. This review highlights the uptake, transport and loading mechanisms for iron in cereal grains and provides an overview of strategies adopted for developing highly iron-enriched grains.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Iron is an essential nutritional mineral and its deficiency can lead to the development of Iron Deficiency Anaemia (IDA) along with impaired brain development due to the malfunctioning of the central nervous system (Lozoff and Georgieff 2006). It also affects cognitive functions such as learning and memory as well as the body’s immune system (Gordon 2003). Worldwide, 2 billion people are anaemic, the major cause being iron deficiency (WHO 2008). WHO estimates that IDA affects 88 % of pregnant and 74 % of non-pregnant women and 60 % of children, especially in Africa and Southeast Asia (WHO 2002). In India, approximately 14 million men, 28 million pregnant women and 85 million children suffer from anaemia every year (King 2002; Stein et al. 2008). The major contributing factor to iron deficiency is the low amount of bioavailable iron found in dietary foods (Glahn et al. 2002).
Iron bioavailability can be increased by the use of many available supplementary additives, but most of these are as expensive as are commercially available iron supplement capsules (Hurell 2002). Some additives also exhibit undesirable colour reactions in the food and promote fat oxidation in stored cereals, which are disadvantageous (Bovell-benjamin et al. 1999; Hurell 2002). Although the FAO/WHO Expert Committee on Food Additives approved these components for iron fortification strategies, still they have not implemented at the country level (FAO/WHO 1999). Hence, it is better to develop iron-biofortified foods naturally by the application of technology rather than add iron supplementary chemicals to food or use iron supplement capsules (Datta and Khush 2002).
In India and other Asian countries, most people consume cereal products as their staple foods, and coincidently, the people of these countries have the highest IDA percentages in the world (Gillespie and Haddad 2001). Therefore, increasing the levels of bioavailable iron in their staple food crops is highly desirable. However, edible cereal grains such as rice contain very low amounts of iron due to the removal of embryo and aleurone layers during milling (Ozturk et al. 2006). Genetic engineering has been established as a promising strategy to improve the iron content in edible tissues and found the new platform of biotechnological research in mineral nutrition.
This review mainly focuses on the different biotechnological approaches that have been employed to overcome the probable barriers to iron loading in cereal grains as well as possible approaches to be applied to future iron-biofortification programmes.
The important criteria for iron loading are the soil iron status and the sensitivity of higher plants towards it. Most of the higher plants grow in aerobic conditions, and approximately 30 % of cultivated land is alkaline in nature. In this calcareous or alkaline condition, Fe is present in the insoluble ferric form, which cannot be absorbed by the plants (Kim and Guerinot 2007). Apart from the soil iron status, iron insolubility is another reason for plant iron deficiency (Guerinot 2001). In order to overcome iron deficiency, higher plants have evolved several adaptive mechanisms to increase iron solubility and uptake from soil through their roots. Plants induce several biochemical responses that increase the solubility of iron in the soil and make it easily accessible through the roots. Higher plants possess 2 different types of iron uptake mechanisms (Marschner et al. 1986). The reduction-based Strategy I is employed by non-graminaceous monocots. In this strategy, a plasma membrane-coupled reductase protein, namely, Ferric Reduction Oxidase 2 (FRO2) converts insoluble soil ferric ions [Fe(III)] into more-soluble ferrous ions [Fe(II)] through the oxidation of a reducing agent such as NADPH. Along with the reductase, the plasma membrane H+ATPases actively take part in this uptake mechanism by lowering the pH of the soil and creating an electrochemical gradient between soil particles and the plasma membranes of the root hairs. Thus, the energy-driven Proton Motive Force (PMF) facilitates the uptake of different solutes through their respective carriers and channels in the root epidermis. Hence, under iron-limiting conditions, the reduction of Fe(III) to Fe(II) is an obligatory step for iron uptake after which the plant automatically switches off its reduction mechanism (Kim and Guerinot 2007).
Iron nutrition in cereal plants
Iron uptake
Graminaceous plants possess a different type of iron acquisition mechanism referred to as the Strategy II mechanism. Cereals including rice, wheat, and maize acquire iron through secretion from their roots of low molecular weight compounds called phytosiderophores that contain Mugineic Acid (MA) and its derivatives. MA binds to iron mainly in the ferric form and solubilises it in the rhizosphere for reabsorption through the roots (Kawai et al. 2001). MAs are amino acid derivatives, their precursor being l-methionine. Intermediates in the MA biosynthesis pathway, for instance the Deoxymugineic Acid (DMA), 3-Hydroxymugineic Acid (HMA) and 3-epihydroxymugineic acid, can also chelate soil iron particles. During iron deficiency, there is a positive correlation between the amount of secreted MAs and the iron sensitivity of the secreting plant (Negishi et al. 2002). Nicotianamine, an intermediate in the MA biosynthetic pathway, plays a crucial role in long-distance transport of iron in plants (Takahashi et al. 2003). Hence, MAs efficiently mobilise Fe and serve an important role in Fe acquisition for grasses growing on calcareous soils.
Iron transport through roots
After absorption through the roots, reduced ferrous ions are transported across the root epidermal membrane by specific carriers or channel proteins such as the ZRT IRT-like Protein (ZIP) family transporters (Kim and Guerinot 2007). Alternatively, different types of epidermal and endodermal efflux transporters mainly transport Fe-phytosiderophore (Fe-PS) complexes into the vascular bundles of the plants (Conte and walker 2011). Fe and other metals are transported both symplastically and apoplastically within the root system. In rice and barley, Efflux Transporters (ETs) such as Transporter of Mugineic Acid 1 (OsTOM1), OsTOM2 and HvTOM1 bind to DMA and export it from the root to the soil. DMA chelates the soil Fe(III) and is further absorbed as the DMA-Fe(III) complex through the Yellow Stripe-Like (YSL) type of Influx Transporters (ITs) present in the root (Nozoye et al. 2011; Fig. 1). Along with the TOM proteins, another group of rice efflux transporters, viz., Phenolics Efflux Zero1 (PEZ1), helps to solubilise the apoplasmic precipitated iron by secretion of phenolics and caffeic acid (Ishimaru et al. 2011).
Dual strategy of iron transport in rice
Most importantly, rice plants possess a dual uptake system for iron acquisition. Under waterlogged conditions, Fe is present in the Fe(II) form and is easily accessible through the Iron-regulated Transporter (IRT); this occurs in paddy fields where the Fe(II) form is more prevalent due to the low availability of oxygen in the soil (Kim and Guerinot 2007). To acclimatise to the conditions of lower Fe(III) concentrations in the soil, rice plants secrete low quantities of DMA and thus exhibit a combination of the Strategy I and Strategy II iron uptake mechanisms in which both TOM and IRT transporter proteins are used.
Long distance transport through xylem and phloem
Metals are mainly transported apoplastically to the older leaves of plants through the xylem. Different ETs are present in the root endodermis and pericycle membrane to transport the metals. In rice and barley plants, TOM1 helps to transport Fe(III) into the xylem as well as into the phloem in the DMA-chelated form (Nozoye et al. 2011). In rice, Fe(III) is also transported as a citrate-chelated form through the Ferric Reductase Defective-Like (FRDL) channel transporter, whereas Fe(II) is mainly bound to Nicotianamic Acid (NA) and translocated as NA–Fe(II) in the xylem (Yokosho et al. 2009; Kakei et al. 2009). In the nodal region of graminaceous plants, nutrients are transferred to the phloem from the xylem and then translocated to the flag leaf and spike regions (Yamaji and Ma 2009).
The most important transport is phloem-mediated, through which Fe and Zn are transported symplastically to the youngest leaves, floral parts and developing seeds (Wang et al. 2011). In the phloem, several ZIP and YSL family transporters play an important role in Fe and Zn transport. In Arabidopsis, barley and rice plants, different divalent cations are transported selectively through particular ZIP family members (Waters and Sankaran 2011). In rice, among the ZIP family members, OsZIP1, 3, 5 and 7 are only associated with Zn2+ transport, while OsZip4 and 8 transport both Zn2+ and Fe2+ and are expressed in the vascular tissues, leaf mesophyll cells and apical meristems of plants (Lee et al. 2010). One of the important members of the ZIP family, Iron-Regulated Transporter 1 (IRT1), facilitates the transport of NA-Fe(II) into the phloem, whereas Fe(III) is transported via chelation with DMA or NA through the TOM1 efflux channel and several YSL family members, respectively (Nozoye et al. 2011; Curie et al. 2009). Different OsYSL members are expressed in different tissues of the rice plant, as are other transporter group members (Bashir et al. 2010; Table 1).
Loading and accumulation of iron in seeds
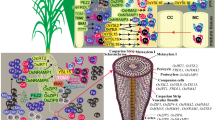
In cereal plants, senescent leaves and roots actually transport Fe through their phloem into developing seeds, thus making the grains or seeds the principal area of exploration for iron and other mineral nutrition. In the castor bean, a novel Fe(III)-binding 17 kDa protein, viz., Iron Transporter Protein (ITP), has been found in the phloem (Kruger et al. 2002), which indicates that, while NA mobilises Fe(III) in and out of the phloem, the actual transport occurs via ITP within the phloem (Fig. 2). Wheat remobilises 77 % of iron in the shoots to the seeds, whereas rice transports only 4 % (Morrissey and Guerinot 2009). Generally, in grain, nutrients and iron are transported into the maternal seed coat region through the phloem and then translocated into the apoplastic region between the maternal seed coat and the aleurone or endosperm through different types of efflux and influx transporters such as ZIP, Nramp and the YSLs (Tauris et al. 2009; Fig. 2).
Iron transport and loading from a roots to d seeds via c xylem and b phloem (modified from Morrissey and Guerinot 2009 and Waters and Sankaran 2011, IRT Iron Regulated Transporter, ZIP ZRT IRT-like protein, PS Phytosiderophore, TOM Transporter of Mugineic acid, NA Nicotianamine Acid, DMA Deoxymugineic Acid, YSL Yellow Stripe Like, PD Plasmodesmata, ITP Iron Transporter Protein, MTP Metal Transporter Protein, VIT Vacuolar Iron Transporter, FRDL Ferric Reductase Defective-Like)
What are the barriers to iron loading in the grain endosperm?
In developing countries, cereals are the staple food that provides more than 50 % of calories, but unfortunately, the consumable endosperm of the grain helps very little in iron nutrition due to its low iron content. Several iron-loading barriers are as follows: Firstly, a variety of transporters and chelating molecules such as YSL, IRT, NA and DMA are localised in the outer tissue region of the resting seed (Fig. 2) and transport iron into the inner endosperm only during germination, a process that clearly indicates low Fe-bioavailability in the endosperm (Walker and Waters 2011). Secondly, the presence of very low amounts of ferritin protein moieties in the endosperm tissue of the mature grain (Stein et al. 2009) is a major drawback to iron bioavailability. In most cereal grains, the presence of phytic acid is another large concern for iron nutrition, as iron and zinc are localised in the phytate-chelated globoid form, mainly within the vacuole of the seed coat, peripheral aleurone and embryo (Ozturk et al. 2006; Cakmak et al. 2010), and thus cannot be mobilised into the endosperm. Ferric ions move into the vacuole of the aleurone layer through several types of Vacuolar Iron Transporter (VIT) and Nramp types of transporter families (Fig. 2). Nanoscale secondary ion mass spectrometry has confirmed the presence of phytate-chelated forms of metals such as Mn, Zn and Fe in the vacuole, further indicating the low quantities of minerals in the seeds (Ravet et al. 2009; Smart et al. 2010).
To overcome these barriers, several promising biotechnological approaches have been studied to provide better iron nutrition to malnourished people (Fig. 3).
Biotechnological interventions
Tissue-specific overexpression of the ferric reductase gene in rice plants
In rice plants, the activity of FRO2 is very low, even under Fe-deficient conditions. The expression of Osfro1 is observed under Cu-, Zn- and Mn-deficient conditions, whereas Osfro2 is expressed only under Fe-deficient conditions and restricted to the shoots (Ishimaru et al. 2007). Therefore, to increase root ferric reductase activity, Atfro2 was introduced into rice plants, but the transgenic plant did not show any transgene expression due to the weak activity of the native promoter (Vasconcelos et al. 2004). The failure was circumvented by the introduction of the yeast ferric reductase gene fre1, under the control of the root-specific irt1 promoter, which enhanced iron uptake in Fe-deficient rice plants due to the resulting higher ferric reductase activity. However, the amount of iron in the resulting transgenic rice grains was similar to that in non-transgenic brown rice (Ishimaru et al. 2007). Therefore, it is evident that fre1 plays a role in increasing the adaptability of plants to iron-deficient conditions, but it is not effective for iron enrichment in the grain.
Gene manipulation in a phytosiderophore-mediated chelation strategy
In cereal plants, MAs are the most important members of the transporter families, among which nicotianamine plays the central role because it chelates both ferrous and ferric ions (von Wiren et al. 1999). Most of the genes involved in the MA biosynthesis pathway have been characterised (Nozoye et al. 2007). In the biosynthetic pathway for mugineic acids, the nas gene produces the nicotianamine acid synthase (NAS) enzyme that catalyses the transformation of l-methionine to nicotianamine, which further produces an intermediate ‘oxo’ acid through the nicotianamine amino acid transferase (NAAT) enzyme encoded by the naat gene. Deoxymugineic acid (DMA), the first product of the mugineic acid family, is then synthesised by the dmas gene product. DMA subsequently produces epi-hydroxymugineic acid and MA with the help of the ids3 and ids2 gene products. In rice plants, variant nas, naat and dmas genes have been isolated and functionally characterised (Nozoye et al. 2007). Rice seed-specific overexpression of the Osnas1 gene under the control of the GlutelinB1 promoter increased grain iron bioavailability along with zinc enrichment (Zheng et al. 2010). Furthermore, large amounts of nicotianamine production in rice plants have been reported through the overexpression of three Osnas genes, namely, Osnas1, Osnas2 and Osnas3, under the control of a double CaMV35S promoter that ultimately increased grain iron content up to 2.4-, 3.5- and 2.7-fold, respectively (Johnson et al. 2011). Prior reports had suggested that incorporation of the Hvnas1 gene under the control of the rice actin1 promoter was a successful attempt at iron enrichment of seeds (Masuda et al. 2009). Another strategy, the activation tagging of the Osnas3 gene, yielded a twofold enrichment of the iron content in the seeds (Lee et al. 2009a). A similar approach was applied to the Osnas2 gene, which ultimately showed a 2.7-fold zinc enhancement in rice seeds (Lee et al. 2011). Introduction of other MA biosynthesis genes such as Hvnaat1 and Hvids3 led to better tolerance of the transgenic rice plants to calcareous soils as well as higher amounts of iron and zinc transported into the seeds compared with non-transgenic plants (Takahashi et al. 2001; Suzuki et al. 2008). A loss-of-function mutation in the Osnaat1 gene also resulted in 1.8- and 3.8-fold higher iron accumulation in brown and white rice, respectively, and may be used as a potent tool in future iron biofortification programmes (Cheng et al. 2007).
Overexpression of different groups of transporters
Overexpression of transporters has had a positive impact on grain iron concentration, as reflected in several biotechnological applications. IRT is a very common member of the ZIP family of transporters, which are mainly responsible for transporting ferrous ions (Vert et al. 2002). In iron-deficient conditions, the expression of Atirt1 is increased in the external root cell layers, and the protein’s function has been identified as participating in monoubiquitin-dependent endocytosis (Barberon et al. 2011). The expression of the Atirt2 gene is similar to that of irt1 but is localised to the subapical cell layers of the root (Vert et al. 2001). Recently, the AtIRT3 transporter, which mainly transports zinc and iron rather than other heavy metals, has been identified (Lin et al. 2009). Hence, use of Atirt3 has an advantage over use of irt1 because no other heavy metal loading occurs except for Fe and Zn. A rice cDNA library screened with rice Expression Sequence Tag (EST, D49213), having high homology to the Atirt1 gene. Further functional analysis of the homologous region called Osirt1 demonstrated higher expression under iron-starvation conditions in the roots and shoots. The Osirt2 gene has also been identified, but its expression was very weak under Fe-deficient conditions (Ishimaru et al. 2007). Overexpression of Osirt1 under the control of the maize ubiquitin-1 promoter led to high iron accumulation in the grains (Lee and An 2009), but morphological parameters such as height, tiller number and grain yield of the transgenic plants were altered. The negative impact on the plant’s agronomic performance may have been due to the constitutive expression of the IRT1 transporter along with toxicity due to excess Zn and Cd. The overproduction of another transporter protein in Brassica juncea, such as the ABC Transporter of Mitochondrion 3 (AtATM3), which helps to transport Fe–S clusters into the cytoplasm from the mitochondria, significantly maintained iron homeostasis in the plants. However, accumulation of heavy metals such as Pb, Cd is a major drawback for its use in an iron biofortification programme, similar to Yeast Cadmium Factor 1 (YCF1) protein-mediated heavy metal acquisition (Bhuiyan et al. 2011a, b).
In cereal plants, the YSL family of transporters is crucial for iron transport. The family has been divided into four groups, but the functional properties of all members are yet to be characterised except for group I, OsYSL18 (group IV) and HvYSL5 (group II; Zheng et al. 2011). In rice, 18 YSL family transporters have been reported to date, among which the three YSL transporters OsYSL2, OsYSL15 and OsYSL18 are very promising for iron biofortification purposes (Curie et al. 2009) due to their iron loading capacities in different plant parts (Table 1). OsYSL2 transports both Fe(II) and Fe(III)-NA into the leaf primordial and scutellum tissues of the grain (Nozoye et al. 2007; Aoyama et al. 2009), whereas OsYSL15 and OsYSL18 transport only Fe(III)-DMA into developing seeds and floral parts, respectively (Inoue et al. 2009; Ishimaru et al. 2010). Introduction of these transporters into rice plants redirects higher iron concentrations to the rice grains. In spite of a large amount of iron accumulation, agronomic performance was compromised in the transgenic Osysl15 overexpressor plants (Lee et al. 2009b), as in the Osirt1 overexpressor plants. Tissue-specific activity of the transporter has been suggested as a suitable strategy for iron loading into grains without disturbing the morphology of the plants, as exemplified by the overexpression of Osysl2 under the control of the phloem-specific sut1 promoter. Polished grain from these transgenic rice plants revealed a 4.4-fold enhancement of seed iron content compared with non-transgenic grain (Ishimaru et al. 2010). An RNAi-YSL2 line of transgenic rice plants also confirmed the involvement of the YSL2 transporter in the iron translocation mechanism in the seeds (Ishimaru et al. 2010) and supports the proposition that RNAi is a potent tool for the development of Genetically Modified (GM) crops (Ali et al. 2010).
Another important YSL family member, Osysl18, may be a promising biotechnological tool for future use, but no Osysl18 overexpressor or knockdown (antisense/RNAi) line has yet been reported. Recently developed transgenic rice plants expressing Hvysl1 showed increased sensitivity towards alkaline soil and can accumulate more iron in leaves compared to seeds, which indicates that Hvysl1 mobilises iron into the plant’s vegetative parts (Gomez-Galera et al. 2012).
Different bHLH (111 types in rice) transcriptional factors greatly influence metal transport in plants through a complex signalling mechanism (Hindt and Guerinot 2012). In rice, one of the Fe-deficiency-inducible bHLH transcription factors, called OsIRO2, has been identified, and its promoter region was found to contain a sequence similar to Iron Deficiency responsive Elements (IDE). Under Fe-deficiency conditions, IDE binding proteins activate the expression of the OsIRO2 protein, which helps to synthesise Fe-regulated gene products through a signal transduction pathway and ultimately facilitates iron translocation to the shoots (Ogo et al. 2007). Overexpression of Osiro2 under the CaMV35S promoter resulted in enhancement of iron uptake from the soil and translocation to the grain (Ogo et al. 2007).
In wheat plants, an NAC transcription factor, namely, No Apical Meristem (NAM) protein, helps to mobilise iron and zinc into the developing seeds, as confirmed by an NAM-RNAi knockdown line (Walker and Waters 2011). The Osnac5 gene has been functionally characterised as an ABA-dependent abiotic stress-related regulator and its maximum expression was demonstrated in the flag leaves of high iron and zinc rice cultivars (Sperotto et al. 2009; Takasaki et al. 2010; Song et al. 2011), which indicates its novelty for future transgenic development. A new class of NA transporter, Zinc-Induced Facilitator1 (ZIF1), identified in Arabidopsis, helps to translocate zinc to the vacuoles from the cytoplasm as well as increase the iron trafficking phenomenon through the cytoplasm (Haydon et al. 2012). Cereal plants such as rice and maize also contain several types of Zinc-Induced Facilitator Like (ZIFL) transporters, which may play a crucial role in iron biofortification due to their iron transport activities (Ricachenevsky et al. 2011).
Tissue-specific overexpression of ferritin
Ferritin (ca. 450 kDa), the most common source of non-haem iron, can accumulate approximately 4500 ferric ions in its central cavity. The outer protein coat consists of 24 oligopolypeptides (ca. 20 kDa) that are folded together with each other through hydrogen and ionic bonds to form a large cage-like protein shell (Masuda et al. 2010). Ferritin has a central mineral cavity and 12 mineral attachment sites on its inner surface along with 8 entry and exit channels on the outer surface (Theil 2003). Ferritin is mainly localised in the plastidial stroma but is also found in plant mitochondria (Zancani et al. 2004). The de novo synthesis of ferritin is regulated in a very controlled way because Fe-overloading results in the production of Reactive Oxygen Species (ROS), particularly OH*, through the Fenton reaction, which can damage the cellular metabolism (Theil 2003). Recently, it has been noted that Fe binds to NO to form an iron-nitrosyl compound, thus alleviating the cellular iron through upregulation of a variety of transporters and the ferritin gene. In contrast, it has been observed that levels of frataxin, a mitochondrial protein, are inversely related to ferritin gene expression, which was evident from a frataxin mutant of Arabidopsis that showed higher iron compartmentalisation in the root (Ramirez et al. 2011). Apart from iron concentration, H2O2 and Abscisic acid (ABA) also upregulate the expression of the different classes of ferritin genes, as reported for Arabidopsis (Briat et al. 2010). Hence, through a signal cascade mechanism, ferritin plays an important role in iron homeostasis to prevent the cell from iron toxicity or deficiency and acts as a sink for ferric ion, which ultimately helps to alleviate iron uptake problems due to Fe insolubility in the soil.
Due to the high iron storage capacity of the ferritin protein, iron-biofortified rice grain has been generated through the overexpression of soybean and bean ferritin genes under the control of the GlutelinB1 promoter (Goto et al. 1999; Vasconcelos et al. 2003). The milled seeds of the transgenic rice plants exhibited approximately two to threefold enhancement of their iron content along with ~1.6-fold enhancement of zinc. A constitutive promoter such as ubiquitin-1 contributed to twofold higher iron accumulation in transgenic rice leaves compared to non-transgenic seeds, regardless of total iron content (Drakakai et al. 2000). Hence, use of seed-specific promoters is an important criterion for development of high-iron grain. Globulin1, a seed-specific promoter, also showed tenfold higher ferritin gene expression in the central part of the endosperm (Qu and Takaiwa 2004). Subsequently, double transformant rice seeds carrying two constructs, namely, glutelinb1-soyferH1 and globulin1-soyferH1 and a single transformant of globulin1-soyferH1 were established as effective for an iron biofortification programme due to their 5.8 and 11.4-fold enhancement of ferritin protein accumulation in the seeds, respectively (Qu et al. 2005). However, 30 % increment of the iron in brown rice was not consistent with the level of ferritin proteins expressed in the seeds (Qu et al. 2005). The endogenous ferritin genes of different cereals may emerge as an alternative tool for iron biofortification. Recently, overexpression of the rice endogenous ferritin (Osfer2) gene under the control of the OsglutelinA2 promoter revealed a 2.1-fold enhancement of iron content along with a 1.36-fold zinc enhancement in the transgenic aromatic milled rice grain (Paul et al. 2012). In addition to the iron sequestration property, overexpression of the alfalfa ferritin protein also caused higher abiotic stress tolerance in grapevine, which indicates its dual role in transgenic plant development (Zok et al. 2010).
Downregulation of phytic acid biosynthesis
In cereal crops, phytic acid (myo-inositol-hexakis phosphate), the major antinutrient, chelates essential nutritional minerals such as Fe, Zn and Ca and makes them indigestible by the human intestine due to its lack of a phytase enzyme (Brinch-Pedersen et al. 2007; Persson et al. 2009; Coulibaly et al. 2011). Synchrotron soft X-ray microscopy and high-resolution secondary ion mass spectrometry clearly revealed the presence of the Fe-phytate chelated form in the aleurone layer globoids of wheat and barley grains (Regvar et al. 2011; Lombi et al. 2011; Moore et al. 2012).
To reduce the phytic acid content in grain, several low phytate crops have already been developed (Raboy 2007), but unfortunately, their growth and seed germination rates are very poor. The biosynthesis of phytic acid is a complex metabolic pathway and is regulated by metabolic enzymes such as Myo Inositol-Phosphate-Synthase (MIPS) and multiple groups of Inositol Phosphate Kinases (IPKs), which are the main metabolic enzymes involved in the phytic acid biosynthetic pathway (Suzuki et al. 2007). Hence, the reduction using antisense technology of RINO1 protein, which is involved in a specific step of the phytic acid biosynthetic pathway, is a promising biotechnological approach to increase iron bioavailability in seeds (Fig. 3; Kuwano et al. 2009). In Arabidopsis, downregulation of ipk1 and ipk2 resulted in 93 % less phytate in seeds (Stevenson-Paulik et al. 2005), while in maize and soybean, low phytate seeds have been produced through tissue-specific silencing of an ABC transporter (Shi et al. 2007).
Combined approaches
The expression of the different iron and metal transporter, carrier and storage proteins are highly dependent on the external as well as the internal iron status and other metal concentrations in the plant. Hence, biotechnological approaches incorporating two machineries such as combining transporters and iron storage molecules are most promising for an iron biofortification programme (Fig. 3). Transgenic maize seeds contained 20–70 %-enhanced iron levels when the plants were transformed with Aspergillus phytase and soybean ferritin genes together (Drakakai et al. 2005). Another successful combination with a Phaseolus ferritin, rice metallothionenin (rgmt) and the Aspergillus phytase (phyA) gene showed enrichment of the iron content in rice grain (Lucca et al. 2001). Approximately sevenfold higher levels of cysteine residues in soluble seed proteins along with 130-fold more phytase helped to improve the iron bioavailability of rice grain. Recently, transgenic rice plants harbouring both the Atnas1 gene under the control of the CaMV35S promoter and Phaseolus ferritin under the control of the endosperm-specific globulin promoter provided five to sixfold enhancement of iron accumulation in grain (Writh et al. 2009).
Future gateway to iron bioavailability
To maintain cellular iron homeostasis, plants can control the entire pathway of iron uptake, transport and loading through upregulation and downregulation of a variety of transporters, cell signalling, and iron storage molecules. Nevertheless, cell signalling mechanisms and the role of signalling molecules such as frataxin, calcein and Fe–S cluster protein molecules will be emphasised more in the future to increase cellular iron levels without hampering iron homeostasis. Even now, more iron and zinc transporters and their upregulation by particular transcriptional factors are yet to be discovered. Therefore, more research needs to be focused mainly on the function and localisation of different transporters, efflux and influx proteins, their signal transduction mechanisms and their different biotechnological implementations in grains for enhanced bioavailability (Table 2). Furthermore, it is important to analyse the bioavailability of the enhanced iron levels and to perform detailed studies of the agronomic performances of the transgenic crops.
References
Ali N, Datta K, Datta SK (2010) RNA interference in designing transgenic crops. GM Crops 1:207–213
Aoyama T, Kobyashi T, Takahashi M, Nagasaka S, Usuda K et al (2009) Osysl18 is a rice iron (III)-deoxymugienic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol Biol 70:681–692
Barberon M, Zelazny E, Robert S, Friml J, Conejero G, Curie C, Friml J, Vert G (2011) Monoubiquitin-dependent endocytosis of the IRON REGULATED TRANSPORTER1(IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA 108:12985–12986
Bashir K, Ishimaru Y, Nishizawa NK (2010) Iron uptake and loading into rice grains. Rice 3:122–130
Bhuiyan MSU, Min SR, Jeong JW, Sultana S, Choi KS, Lee Y, Liu JR (2011a) Overexpression of AtATM3 in Brassica juncea confers enhanced heavy metal tolerance and accumulation. Plant Cell Tiss Organ Cult 107:69–77
Bhuiyan MSU, Min SR, Jeong JW, Sultana S, Choi KS, Song YW, Lee Y, Lim YP, Liu JR (2011b) Overexpression of a yeast cadmium factor I (YCF1) enhances heavy metal tolerance and accumulation in Brassica juncea. Plant Cell Tiss Organ Cult 105:85–91
Bovell-Benjamin AC, Allen LH, Frankel EN, Guinard JX (1999) Sensor quality and lipid oxidation of maize porridge as affected by iron amino acid chelates and EDTA. J Food Sci 64:371–376
Briat JF, Ravet K, Arnaud N, Duc C, Boucherez J, Touraiine B, Cellier F, Gaymard F (2010) New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann Bot 105:811–822
Brinch-Pedersen H, Borg S, Tauris B, Holm PB (2007) Molecular genetic approaches to increasing mineral availability and vitamin content of cereals. J Cereal Sci 46:308–326
Cakmak I, Pfeiffer WH, McClafferty B (2010) Biofortification of durum wheat with zinc and iron. Cereal Chem 87:10–20
Cheng L, Wang F, Shou H, Huang F, Zheng L et al (2007) Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiol 145:1647–1657
Conte S, Walker EL (2011) Transporter contributing to iron trafficking in plants. Mol Plant. doi:10.1093/mp/ssr015
Coulibaly A, Kouakou B, Chen J (2011) Phytic acid in cereal grains: structure, healthy or harmful ways to reduce phytic acid in cereal grains and their effects on nutritional quality. Am J Plant Nutr Fertil Tech 1:1–22
Curie C, Cassin G, Couch D, Divol F, Higuchi K et al (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103:1–11
Datta SK, Khush GS (2002) Improving rice to meet food and nutrient needs: biotechnological approaches. J Crop Prod 6:229–247
Drakakai G, Christou P, Stoger S (2000) Constitutive expression of soybean ferritin cDNA in transgenic wheat and rice results in increased iron levels in vegetative tissues not in seeds. Transgenic Res 9:445–452
Drakakai G, Marcel S, Glahn RP, Lund EK, Pariagh S, Fischer R, Christou P, Stoger E (2005) Endosperm specific coexpression of recombinant soybean ferritin and Aspergillus phytase in maize results in significant increases in the levels of bioavailable iron. Plant Mol Biol 59:869–880
FAO/WHO (1999) Joint FAO/WHO expert committee on food additives. 53rd meeting. WHO, Geneva, Switzerland
Gillespie S and Haddad L (2001) Malnutrition in Asia and the Pacific: nutrition and development series, attacking the double burden of malnutrition in Asia and Pacific. Asian Development Bank, International Food Policy Research Institute, pp 5–14
Glahn RP, Chen SQ, Welch RM, Gregorio GB (2002) Comparison of iron bioavailability from 15 rice genotypes: studies using an invitro digestion/caco 2 cell culture model. J Agr Food Chem 50:3586–3591
Gomez-Galera S, Sudhakar D, Pelacho AM, Capell T, Christou P (2012) Constitutive expression of a barley Fe phytosiderophore transporter increases alkaline soil tolerance and results in iron partitioning between vegetative and storage tissues under stress. Plant Physiol Biochem 53:46–53
Gordon N (2003) Iron deficiency and the intellect. Brain Dev 25:3–8
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286
Guerinot ML (2001) Improving rice yields—ironing out the details. Nat Biotechnol 19:417–418
Haydon MJ, Kawachi M, Wirtz M, Hillmer S, Hell R, Kramer U (2012) Vacuolar Nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. Plant Cell. doi:10.1105/tpc.111.095042
Hindt MN, Guerinot ML (2012) Getting a sense for signals: regulation of the plant iron deficiency response. Biochim Biophys Acta. doi:10.1016/j.bbamcr.2012.03.010
Hurell RF (2002) Fortification: overcoming technical and practical barriers, forging effective strategies to combat iron deficiency. J Nutr 132:806–812
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y et al (2009) Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem 284:3470–3479
Ishimaru Y, Kim SA, Tsukamoto T, Oki HTK, Watanabe S et al (2007) Mutational reconstructed ferric chelate reductase confers enhanced tolerance in rice to iron deficiency in calcareous soil. Proc Natl Acad Sci USA 104:7373–7378
Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T et al (2010) Rice metal–nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J 62:379–390
Ishimaru Y, Kakei Y, Shimo H, Bashir K, Sato Y et al (2011) A rice phenolics efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J Biol Chem 286:24649–24655
Johnson AAT, Kyriacou B, Damien L, Callahan DL, Carruthers L, Stangoulis J, Lombi E, Tester M (2011) Constitutive overexpression of the Osnas gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS ONE 6:e24476
Kakei Y, Yamaguchi I, Kobyashi T, Takahashi M, Nakanishi H, Yamakawa T, Nishizawa NK (2009) A highly sensitive, quick and simple quantification method for nicotianamine and 2’-deoxymugienic acid from minimum samples using LC/ESI-TOF-MS achieves functional analysis of these components in plants. Plant Cell Physiol 50:1988–1993
Kawai S, Kamei S, Matsuda Y, Ando R, Kondo S, Ishizawa A, Alam S (2001) Concentrations of iron and phytosiderophores in xylem sap of iron-deficient barley plants. Soil Sci Plant Nutr 47:265–272
Kim SA, Guerinot ML (2007) Mining iron: iron uptake and transport in plants. FEBS Lett 581:273–2280
King JC (2002) Evaluation the impact of plant biofortification on human biofortication, symposium: plant Breeding: a new tool for fighting micronutrient malnutrition. J Nutr 132:511–513
Kruger C, Berkowitz O, StephanUdo W, Hell R (2002) A metal-binding member of the late embryogenesis abundant protein family transports iron in the phloem of Ricinus communis L. J Biol Chem 277:25062–25069
Kuwano M, Mimura T, Takaiwa F, Yoshida KT (2009) Generation of stable ‘low phytic acid’ transgenic rice through antisense repression of the 1D-myo-inositol 3-phosphate synthase gene (RINO1) using the 18-kDa oleosin promoter. Plant Biotech J 7:96–105
Lee S, An G (2009) Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ 32:408–416
Lee S, Chiecko JC, Kim SA, Walker EL, Lee Y, Guerinot ML, An G (2009a) Disruption of Osysl15 leads to iron inefficiency in rice plants. Plant Physiol 150:786–800
Lee S, Jeon US, Lee SJ, Kim YK, Persson DP et al (2009b) Iron fortification of rice seeds through activation the nicotinamine synthase gene. Proc Natl Acad Sci USA 106:22014–22019
Lee S, Kim SA, Lee J, Guerinot ML, An G (2010) Zinc deficiency-inducible OsZIP8 encodes a plasma membrane localized zinc transporter in rice. Mol Cells 29:551–558.
Lee S, Persson DP, Hansen TH, Husted S, Schjoerring JK et al (2011) Bio-available zinc in rice seed is increased by activation tagging of nicotianamine synthase. Plant Biotech J 9:865–873
Lin YF, Liang HM, Yang SY, Boch A, Clemens S et al (2009) Arabidopsis IRT3 is a zinc regulated and plasma membrane localized zinc/iron transporter. New Phytol 182:392–404
Lombi E, Smith E, Hansen TH, Paterson D, DeJonge MD et al (2011) Megapixel imaging of (micro) nutrients in mature barley grains. J Exp Bot 62:273–282
Lozoff B, Georgieff MK (2006) Iron deficiency and brain development. Semin Pediatr Neurol 13:158–165
Lucca P, Hurell R, Potrykus I (2001) Genetic engineering approaches to improve the bioavailability and the level of iron in rice grains. Theor Appl Genet 102:392–397
Marschner H, Romheld V, Kissel M (1986) Different strategies in higer plants in mobilization and uptake of iron. J Plant Nutr 9:695–713
Masuda H, Usuda K, Kobyashi T, Ishimaru Y, Kakei Y et al (2009) Overexpression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice 2:155–166
Masuda T, Goto F, Yoshihara T, Mikami B (2010) Crystal structure of plant ferritin reveals a novel metal binding sites that functions as a transit site for the metal transfer in ferritin. J Biol Chem 285:4049–4059
Moore KL, Zhao FJ, Gritsch CS, Tosi P, Hawkesford MJ, McGrath SP, Shewry PR, Grovenor CRM (2012) Localisation of iron in wheat grain using high resolution secondary ion mass spectroscopy. J Cereal Sci 55:183–187
Morrissey J, Guerinot ML (2009) Iron uptake and transport in plants: the good, the bad and the ionome. Chem Rev 109:4553–4567
Negishi T, Nakanishi H, Yazaki J, Kishimoto N, Fujii F et al (2002) cDNA microarray analysis of gene expression during Fe-deficiency stress in barley suggests that polar transport of vesicles is implicated in phytosiderophore secretion in Fe-deficient barley roots. Plant J 30:83–94
Nozoye T, Inoue H, Takahashi M, Ishimaru Y, Nakanishi H, Mori S, Nishizawa NK (2007) The expression of iron homeostasis-related genes during rice germination. Plant Mol Biol 64:35–47
Nozoye T, Nagasaka S, Kobyashi T, Takahashi M, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011) Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem 286:5446–5454
Ogo Y, Itai RN, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa NK (2007) The rice bHLH protein OslRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J 51:366–377
Ozturk L, Yazici MA, Yucel C, Torum A, Cekic C, Bagci A, Ozkan H, Barun HJ, Sayers Z, Cakmak I (2006) Concentration and localization of zinc during seed development and germination in wheat. Physiol Plantarum 128:144–152
Paul S, Ali N, Gayen D, Datta SK, Datta K (2012) Molecular breeding of Osfer2 gene to increase iron nutrition in rice. GM Crop Food 3:310–316
Persson DP, Hansen TH, Laursen KH, Schjoerring JK, Husted S (2009) Simultaneousiron, zinc, sulfur and phosphorus speciation analysis of barley grain tissues using SEC-ICP-MS and IP-ICP-MS. Metallomics 1:418–426
Qu LQ, Takaiwa F (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotech J 2:113–125
Qu L, Yoshihara T, Ooyama A, Goto F, Takaiwa F (2005) Iron accumulation does not parallel the high expression of ferritin in transgenic rice seeds. Planta 222:225–233
Raboy V (2007) The ABCs of low-phytate crops. Nat Biotechnol 25:874–875
Ramirez M, Simontacchi M, Murgia I, Zabaleta E, Lamattina L (2011) Nitric oxide, nitrosyl iron complexes, ferritin and frataxin: a well equipped team to preserve plant iron homeostasis. Plant Sci 181:582–592
Ravet K, Touraine B, Boucherez J, Briat JF, Gaymard F, Cellier F (2009) Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J 57:400–412
Regvar M, Eichert D, Kaulich B, Gianoncelli A, Pongrac P, Vogel-Mikus K, Kreft I (2011) New insights into globoids of protein storage vacuoles in wheat aleurone using synchrotron soft X-ray microscopy. J Exp Bot 62:3929–3939
Ricachenevsky FK, Sperotto RA, Menguer PK, Sperb ER, Lopes KL, Fett JP (2011) ZINC-INDUCED FACILITATOR-l LIKE family in plants: lineage specific expansion in monocotyledons and conserved genomic and expression features among rice (Oryza sativa) paralogs. BMC Plant Biol 11:1–22
Shi J, Wang H, Schellin K, Li B, Faller M et al (2007) Embryo-specific silencing of a transporter reduces phytic acid content of maize and soybean seeds. Nat Biotechnol 25:930–937
Smart KE, Smith JAC, Kilburn MR, Martin BGH, Hawes C, Grovenor CRM (2010) High-resolution elemental localization in vacuolate plant cells by nanoscale secondary ion mass spectrometry. Plant J 63:870–879
Song SY, Chen Y, Chen J, Dai XY, Zhang WH (2011) Physiological mechanisms underlying OsNAC5-dependent tolerance of rice plants to abiotic stress. Planta 234:331–345
Sperotto RA, Ricachenevsky FK, Duarte GL, Boff KL, Lopes KL, Sperb ER, Fett JP (2009) Identification of up-regulated genes in flag leaves during rice grain filling and characterization of OsNAC5, a new ABA-dependent transcription factor. Planta 230:985–1002
Stein AJ, Meenakshi JV, Qaim M, Nestel P, Sachdev HPS, Bhutta AZ (2008) Potential impacts of iron biofortification in India. Soc Sci Med 66:1797–1808
Stein RJ, Ricachenevsky FK, Fett JP (2009) Differential regulation of two rice ferritin genes (OsFER1 and OsFER2). Plant Sci 177:563–569
Stevenson-Paulik J, Bastidas RJ, Chiou ST, Frye RA, York JD (2005) Generation of phytate-free seeds in Arabidopsis through disruption of inositol polyphosphate kinases. Proc Natl Acad Sci USA 102:12612–12617
Suzuki M, Tanaka K, Kuwano M, Yoshida KT (2007) Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L): implications for the phytic acid biosynthetic pathway. Gene 405:55–64
Suzuki M, Morikawa KC, Nakanishi H, Takahashi M, Saigusa M, Mori S, Nishizawa NK (2008) Transgenic rice lines that include barley genes have increased tolerance to low iron availability in a calcareous paddy soil. Soil Sci Plant Nutr 54:77–85
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa NK, Mori S (2001) Enhanced tolerance to rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol 19:466–469
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2003) Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15:1263–1280
Takasaki H, Maruyama K, Kidokoro S, Ito Y, Fujita Y, Shinozaki K, Yamaguchi-Shinozaki K, Nakashima K (2010) The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol Genet Genom 284:173–183
Tauris B, Borg S, Gregersen PL, Holm PB (2009) A roadmap for zinc trafficking in the developing barley grain based on laser capture microdissection and gene expression profiling. J Exp Bot 60:1333–1347
Theil EC (2003) Ferritin: at the crossroads of iron and oxygen metabolism. In: Metal-binding proteins and trace element metabolism. J Nutr 133:1549–1553
Vasconcelos M, Datta K, Oliva N, Khalekuzzaman M, Torrizo L, Krishnan S, Oliveira M, Goto F, Datta SK (2003) Enhanced iron and zinc accumulation in transgenic rice with the ferritin gene. Plant Sci 164:371–378
Vasconcelos M, Musetti V, Li C, Datta SK, Grusak MA (2004) Functional analysis of transgenic rice (Oryza sativa L.) transformed with Arabidopsis thaliana ferric reductase (Atfro2). Soil Sci Plant Nutr 50:1151–1157
Vert G, Briat JF, Curie C (2001) Arabidopsis IRT2 gene encodes a root-periphery iron transporter. Plant J 26:181–189
Vert G, Grotz N, Dedaldechamp F, Gaymard F, Guerinot ML, Briat JF, Curie C (2002) IRT1, an Arabidopsis transporter essential for iron uptake from the soil and plant growth. Plant Cell 14:1223–1233
von Wiren N, Klair S, Bansal S, Briat J, Khodr H, Shioiri RA, Leigh R, Hider C (1999) Nicotianamine chelates both Fe III and Fe II. Implications for metal transport in plants. Plant Physiol 119:1107–1114
Walker EL, Waters BM (2011) The role of transition metal homeostasis in plant seed development. Curr Opin Plant Biol 14:318–324
Wang YX, Specht A, Horst WJ (2011) Stable isotope labeling and zinc distribution in grains studied by laser ablation ICP-MS in an ear culture system reveals zinc transport barriers during grain filling in wheat. New Phytol 189:428–437
Waters BM, Sankaran RP (2011) Moving micronutrients from soil to seeds: genes and physiological process from a biofortification perspective. Plant Sci 180:562–574
WHO (2002) The World Health Report. Geneva, WHO publishing web. http://www.who.int/nutrition/topics/ida
WHO (2008) Micronutrient deficiencies: iron deficiency anemia. Geneva, WHO publishing web. http://www.who.int/nutrition/topics/ida
Writh J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B et al (2009) Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotech J 7:631–644
Yamaji N, Ma JF (2009) A transporter at the node responsible for intervascular transfer of silicon in rice. Plant Cell 21:2878–2883
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009) OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149:297–305
Zancani M, Peresson C, Biroccio A, Federici G, Urbani A et al (2004) Evidence for the presence of ferritin in plant mitochondria. Eur J Biochem 271:3657–3664
Zheng L, Cheng Z, Ai C, Jiang X, Bei X et al (2010) Nicotianamine, a novel enhancer of rice iron bioavailability to humans. PLoS ONE 5:e10190
Zheng L, Fuji M, Yamaji N, Sasaki A, Yamane M, Sakurai I, Sato K, Ma JF (2011) Isolation and charecterisation of barley yellow stripe-like gene Hvysl5. Plant Cell Physiol 52:765–774
Zok A, Olah R, Hideg E, Horvath VG, Kos PB, Majer P, Varadi GY, Szegedi E (2010) Effect of Medicago sativa ferritin gene on stress tolerance in transgenic grapevine. Plant Cell Tiss Organ Cult 100:339–344
Acknowledgments
We record our special thanks to the Department of Biotechnology (DBT) of Government of India and University Grants Commission (UGC) for financial support for different research projects in the laboratories.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Paul, S., Ali, N., Sarkar, S.N. et al. Loading and bioavailability of iron in cereal grains. Plant Cell Tiss Organ Cult 113, 363–373 (2013). https://doi.org/10.1007/s11240-012-0286-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0286-7