Abstract
The exogenous application of salicylic acid (SA) not only protects plants against stress, but also enhances their growth and productivity. In this study, proliferating shoots of Cistus heterophyllus subsp. carthaginensis, an endangered plant species, were incubated in the presence of 0, 10, 100, and 1,000 μM SA for a period of 2 months. Overall growth, phenylpropanoid metabolism and antioxidant capacity were then determined. At low SA concentration, the efficiency of photosystem II (PSII) and shoot growth remained stable, while chlorophyll and carotenoid levels increased. Furthermore, there were no major changes in the levels of H2O2 in the different treatments (less than 10 % compared with the control), but an increase in lipid peroxidation, proline content and free and bound SA concentrations was observed in 100 μM SA-treated shoots. SA treatments resulted in increased activities of phenylalanine ammonia lyase (EC 4.3.1.24) and soluble peroxidases (EC 1.11.1.7), which strongly correlated with the decrease in soluble flavanols and the increase of proanthocyanidins, whereas cell wall-bound peroxidases exhibited a SA-concentration-dependent down-regulation. The results provided evidence that the differences in SA-induced changes in phenolic metabolism, especially the oxidation of flavanols by soluble peroxidases, could serve as a backup defence system contributing to a reduction in oxidative cellular damage, as suggested by the high anti-lipid oxidation activity displayed by Cistus extracts.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cistus heterophyllus subsp. carthaginensis is an Iberian-North African endemism that appears on the Spanish Red List of Threatened Plants as critically endangered (Sánchez-Gómez et al. 2002). The only known European populations of C. heterophyllus, comprising only 3 and 22 known individuals, respectively, are in the provinces of Valencia and Murcia (Spain). Due to their naturally high phenolic antioxidant content, several Cistus species have been used in folk medicine as antiinflammatory, antiulcerogenic, antimicrobial and antispasmodic agents (Barrajón-Catalán et al. 2010; and references herein). However, no previous studies have specifically addressed the phytochemistry and biological activity of C. heterophyllus.
As well as being essential for the conservation of biodiversity, in vitro culture has been shown to be effective in stimulating the accumulation of phytochemicals in plant cells by elicitation with biotic and abiotic elicitors as well as with stress-related mediators such as methyl jasmonate or salicylic acid (SA) (Matkowski 2008).
SA is a multifaceted hormone that plays an important role in the induction of stress tolerance in plants (Horváth et al. 2007; Vlot et al. 2009; Gadzovska et al. 2012). The exogenous application of SA not only provides protection against various biotic and abiotic stresses, but also enhances the growth and productivity of plants (reviewed by Hayat et al. 2010; Rivas-San Vicente and Plasencia 2011). In particular, several authors (Chen et al. 2006; Kováčik et al. 2009a) have revealed that SA provokes changes in the gene expression related with the biosynthesis and production of phenylpropanoids, such as those of phenylalanine ammonia-lyase (PAL), the first enzyme of the overall phenylpropanoid biosynthetic pathway, and class III plant peroxidases (EC 1.11.1.7; hydrogen donor: H2O2 oxidoreductase, Prxs), a key polymorphic group of heme-containing glycosylated enzymes that can oxidize a wide variety of phenolic compounds using hydrogen peroxide as the electron acceptor (Passardi et al. 2005; Almagro et al. 2009), resulting in changes in plant metabolome.
Since phenylpropanoids are required for plant growth, development and adaptation (Vogt 2010), and since, furthermore, many of these compounds, especially polyphenolics, are powerful antioxidants whose positive health-related effects in many oxidative-stress related diseases have been repeatedly reported (Soobrattee et al. 2005; Valko et al. 2007), an increase in the production of antioxidative polyphenols could be a promising approach for improving both plant vigour and their content of medicinally valuable compounds (Amoo et al. 2012, ; Pérez-Tortosa et al. 2012).
With this in mind, the purpose of this study was to analyze the influence of different SA doses (10, 100 and 1,000 μM) on C. heterophyllus plantlet growth performance, and to determine whether SA treatment improves the antioxidant properties of Cistus shoots by modifying the activity of some of the key enzymes involved in phenylpropanoid metabolism such as PAL and Prx. From a practical point of view, this work looks at the possibility of SA application in conservation programmes of rare and threatened plants in order to improve both their growth and antioxidant capacity.
Materials and methods
Plant material and medium composition
Explants were obtained from in vitro cultures of C. heterophyllus subsp. carthaginensis previously established in our laboratory and maintained by bimonthly subculture (at least seven subcultures before starting the assays). In vitro shoots were grown on half-strength MS medium (Murashige and Skoog 1962) (MS/2) without growth regulators and supplemented with casein hydrolysate (250 mg l−1), sucrose (3 % w/v) and 0.8 % Difco Bacto agar. All cultures were kept at 25 °C with a 16 h-light/8 h-dark cycle, and 100 μmol m−2 s−1 photon flux density (Sanyo, versatile environmental test chamber, MLR-351H, Japan).
SA treatments
Eighty 40-day-old shoots (five per vessel) were placed onto fresh basal medium, buffered to pH 6.0 with 3 mM MES-KOH, a concentration commonly used to supplement media for in vitro cultures (Gratão et al. 2008; Yuan et al. 2012). Hydroalcoholic SA solutions were sterilized by filtration through 0.22 μm filters (Millex GV, Millipore) and added aseptically to autoclaved media at the desired concentrations (0, 10, 100, and 1,000 μM). Equal volumes of ethanol were added to the control vessels. After autoclaving, the pH of the media were in the range of 5.7 ± 0.2. After 8 weeks, shoot tissues were immediately frozen in liquid nitrogen, pulverized in a liquid nitrogen-cooled analytical mill (IKA, Labortechnik, Germany) for 3 × 30 s intervals and stored at −80 °C until used.
Measurement of chlorophyll fluorescence and chlorophyll and carotenoid contents
Chlorophyll fluorescence measurements were made on the upper (adaxial) surface of leaflets using a PAM-210 chlorophyll fluorometer system (Heinz Waltz, Effeltrich, Germany). Plantlets were kept for 25 min in darkness to determine the minimal fluorescence level in the dark-adapted state (F O) and the maximal fluorescence in this state (F m). Calculations for maximum quantum efficiency of PSII photochemistry [(F m−F O )/F m and (F m−F O )/F O ] were carried as recommended by Maxwell and Johnson (2000) and Lichtenthaler et al. (2005).
Chlorophyll a (Chl a) and b (Chl b) and total carotenoids x + c (xanthophylls and carotenes) were extracted with 100 % methanol several times (×3) until the extract became colourless. Their levels were determined using the extinction coefficients and the equations reported by Lichtenthaler and Wellburn (1983).
Determination of stress markers
Endogenous hydrogen peroxide contents were determined by the ferric-xylenol orange (FOX1) method as described by Cheeseman (2006). Liquid nitrogen-powdered shoots (0.25 g) were homogenized using an Ultra-Turrax disperser (IKA, Germany) in 1 ml of 5 % trichloroacetic acid (TCA), and centrifuged (15,000g, 10 min, 4 °C). 25 μl of supernatant was mixed with 500 μL of FOX1 medium (0.5 mM Fe(NH4)2(SO4)2, 50 mM H2SO4, 0.2 mM xylenol orange and 200 mM sorbitol). After 30 min of incubation in the dark, the H2O2 concentration was determined based on the difference in absorption at 560 nm using a seven-point standard curve covering the range of 0.5–10 μM.
The degree of lipid peroxidation was determined by measuring thiobarbituric acid-reacting substances (TBARS) at 532 nm, with a correction for non-specific absorbance at 440 and 600 nm (Hodges et al. 1999), using the same supernatants as in the FOX1 assay. The MDA concentration was calculated using an extinction coefficient of 155 mM−1 cm−1.
The proline content was determined spectrophotometrically in a sulphosalicylic acid extract, using acid-ninhydrin reagent after Bates et al. (1973).
Extraction and quantification of free and bound salicylic acid
Extraction and analysis of free and bound SA (SAG, 2-O-β-D-glucosylsalicylic acid) were performed according to Verberne et al. (2002). Bound SA was quantified following acidic hydrolysis with 8 M hydrochloric acid. Quantification was performed using an HPLC system (Waters Alliance 2695) equipped with a Waters 2475 multi-wavelength fluorescence detector and a Phenomenex column (Torrance, CA, USA), type LUNA C18 (250 × 4.60 mm i.d., 5 μm) with a Phenomenex SecurityGuard pre-column. An internal standard, 2,3-dihydroxybenzoic acid (2,3-DHBA), was used to estimate SA recovery. The excitation and emission wavelengths used were 294 and 426 nm for SA, and 305 and 437 nm for 2,3-DHBA, respectively (Zawoznik et al. 2007).
Extraction and assay for PAL (EC 4.3.1.24) and Prx (EC 1.11.1.7)
Liquid nitrogen powdered shoots (0.6 g) were homogenized at 4 °C in cold extraction buffer [50 mM Tris-HCl, pH 8.8, 5 mM ethylenediamine tetra-acetic acid (EDTA), 5 mM ascorbic acid, 1 mM phenylmethylsulphonyl fluoride (PMSF), 0.1 % (w/v) Triton X-100, 200 μM dithiothreitol; (DTT), and 0.25 % (w/v) polyvinylpolypyrrolidone]. Homogenates were centrifuged (48,000g, 30 min, 4 °C) and the supernatants (2.5 ml) were then desalted by chromatography on PD-10 Sephadex G-25 columns (GE Healthcare, Buckinghamshire, UK), equilibrated either with 25 mM Tris-HCl (pH 8.8) containing 1 mM EDTA to determine PAL activity, or with 25 mM sodium acetate, pH 5.5 to determine Prx activity. The pellets were washed twice with 10 ml of 50 mM Tris-HCl (pH 7.5) containing 2 % (w/v) Triton X-100 and three times with phosphate buffer before incubating with 1 M KCl for 30 min with continuous shaking at 4 °C. After centrifugation (1,000g, 10 min, 4 °C), the supernatants were desalted and used for determining the ionically-bound Prx activities.
PAL activity was determined spectrophotometrically by following the conversion of l-phenylalanine into trans-cinnamic acid (ε290 = 9.5 mM−1 cm−1), according to Olsen et al. (2008). Prx activiy was estimated at 30 °C (Ferrer et al. 1990) using 0.1 mg ml−1 tetramethylbenzidine-HCl (ε652 = 39 mM−1 cm−1) as electron donor.
Determination of phenolics, flavanols, condensed tannins, and lignin
Powdered shoots (0.25 g) were mixed with 2 ml of methanol, incubated in dark condition for 1 h on a shaker at minimal speed, and centrifuged (12,000g, 5 min, 4 °C).
The supernatant was used for the determination of total phenolics (Everette et al. 2010) and flavanols (López-Arnaldos et al. 2001) using the Folin-Ciocalteu and p-dimethylamino-cinnamaldehyde (DMACA) reagents with calibration curves for caffeic acid (10–1,000 μM) and (+) catechin (100–400 μM), respectively. The pellets were washed several times with methanol and then, air dried at 60 °C. The resulting residues were used for lignin determination using the thioglycolic acid method as previously described (Hatzilazarou et al. 2006).
Condensed tannin contents were estimated by measuring the absorbance at 545 nm of the aqueous phase from the second partitioning (after incubation with HCl) carried out to obtain the bound SA according to Verberne et al. (2002). Results were expressed as cyanidin equivalents by using an ε545 = 34.7 mM−1 cm−1 (Vermerris and Nicholson 2006).
HPLC analysis for identification and quantification of individual phenols, and phenolic families, were carried out according to the elution conditions described in Sharma et al. (2005) by using a Waters Alliance 2695 separation module connected to a Waters 2996 diode array detector. Separations were performed in a LiChroCART RP-18 reversed-phase column (250 × 4 mm i.d., 5 μm particle size) supplied by Merck. Calibration curves corresponding to several standard compounds (provided by Sigma) were constructed in order to identify and quantify the peaks obtained in the chromatograms.
Antioxidant activities
Antioxidant activity of Cistus methanolic extracts was determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method, the ferric reducing antioxidant power (FRAP) assay, and the conjugated diene formation test as previously described (Pérez-Tortosa et al. 2012). The hypochlorous acid (HOCl) scavenging potential of the extracts was assayed using an electrophoretic assay according to Grippa et al. (2000) with minor modification. Briefly, the reaction mixture contained 90 μl of 0.05 % (w/v) bovine serum albumin in sodium phosphate buffer (100 mM, pH 7.0), 25 μl of methanolic extracts and 10 μl of 6.72 mM NaOCl solution. Control reaction media using (+)-catechin (25 μl, 2.5 mM) and ascorbic acid (25 μL, 2.5 mM) were also prepared. After a period of incubation of 15 min at room temperature, 15 μl of these samples were analyzed by non-denaturing PAGE on a 7.5 % (w/v) acrylamide gel using a Mini-Protean II Cell (Bio-Rad, Hercules, CA). Duplicate gels were stained with Coomassie brilliant blue R-250. Band intensities were quantified using ImageJ software (version 1.34s, NIH, Bethesda, MD; [http://rbs.info.nih.gov/ij].
Statistical analysis
Data are presented as mean ± standard errors (SE) from three independent experiments (with a minimum of 10 explants per treatment). Pearson linear correlation coefficients were calculated to evaluate associations among variables. The statistical significance of the differences (P < 0.05) between groups was tested using one-way analysis of variance (ANOVA) combined with Tukey’s honestly significant difference (HSD) post hoc test. All the statistical analyses were performed using the SPSS software package (version 19.0; SPSS Inc., Chicago, IL, USA).
Results
Effects of SA treatment on chl a fluorescence, photosynthetic pigment contents, and shoot growth
Chlorophyll a fluorescence is widely used for non-invasively monitoring the photosynthetic performance of plants (Maxwell and Johnson 2000; Baker 2008). Two related ratios F v/F m (where F v = F m−F 0) and F v/F 0 are often used for determining the potential quantum efficiency of PSII (Serret et al. 2001; Lichtenthaler et al. 2005). The first ratio is routinely used as an indicator of damage to the PSII reaction centre and, typical F v/F m values for non-stressed leaves ex vitro are 0.74 − 0.85 (Lichtenthaler et al. 2005). As shown in Table 1, there was a small, but not statistically significant, decrease in the F v/F m and F v/F 0 ratios in SA-treated shoots. A dose of 10 μM SA significantly increased the levels of chlorophyll a, b and carotenoids (by 30, 23 and 52 %, respectively), although their levels fell as the concentration of SA was increased, reaching levels below the control levels at the maximum SA concentration used (1,000 μM).
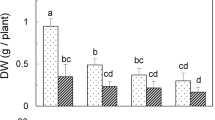
No major changes (Tukey’s HSD test, P < 0.05) in growth, estimated by both fresh shoot mass and shoot length, between the control, l0 μM SA, and 100 μM SA-treated explants were found. Exposure to a high SA concentration (1,000 μM) resulted in drastic growth inhibition of 30–50 % (see Supplemental Fig. 1).
Effect of SA on H2O2 level, lipid peroxidation, proline content, and accumulation of free and bound SA
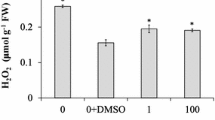
To determine whether SA doses have a toxic effect, some biochemical stress markers were evaluated. No significant changes were noted in the hydrogen peroxide level in SA-treated shoots compared with the control, whereas SA at 100 μM significantly increased proline accumulation and lipid peroxidation, as measured by the TBARS assay, and the endogenous levels of both free (>2.5-fold) and bound (>eightfold) SA in the shoots (Fig. 1), although no visible phytotoxic effects on the shoots were observed. The total content of free and bound SA in 10 μM SA-treated shoots were close to the basal levels observed in the control treatments.
Effect of salicylic acid (SA) concentrations on H2O2 level, and lipid peroxidation (A), proline content (B), and accumulation of free and bound SA (C) in shoots of 40-day-old plantlets of Cistus heterophyllus grown for 2 months with or without the elicitor on MS/2 basal medium. Vertical bars represent mean ± standard error
Effect of SA on PAL and Prx activities
SA treatments caused a significant increase (>tenfold) in PAL activity (Fig. 2). Soluble peroxidase activities also increased in a dose-dependent manner, whereas cell-wall-bound peroxidase activity increased by about 35 % compared with the control levels in 10 μM-SA-treated shoots, although at a dose of 100 μM SA the activity was in the range of the untreated controls (Fig. 2).
Effect of salicylic acid (SA) concentrations on the activities of PAL and Prx in shoots of 40-day-old plantlets of Cistus heterophyllus grown for 2 months with or without the elicitor on MS/2 basal medium. Vertical bars represent mean ± standard error. S-Prx soluble peroxidase activity, CW-Prx cell wall-bound peroxidase activity
Effects of SA treatment on the phenolic content of Cistus extracts
Because SA treatments resulted in a substantial increase in PAL and Prx activities, its effect on the accumulation of phenolic compounds and flavanols, one of the major groups of phenolics present in Cistus species was analyzed. No major differences in the soluble total phenol content (TPC) between control and SA-treated explants were observed (Table 2). However, higher doses of SA tended to decrease the levels of soluble flavanols and increased the levels of proanthocyanidins present in Cistus shoots. HPLC analyses were carried out to verify whether SA caused a variation in the phenolic patterns of Cistus shoots. HPLC chromatograms at 280 and 310 nm showed that SA-treated shoots contained roughly the same type of phenolic compounds, even though there were differences in the concentration of individual compounds (see Supplemental Fig. 2). The HPLC phenolic profile was dominated by phenolic acids and flavonoids, whereas hydroxycinnamic acid derivatives were detected in lower amounts. As shown in Table 2, the lignin content increased twofold in 10 μM-SA-treated shoots, whereas with 100 μM-SA its content was in the range of the controls.
Effects of SA treatment on the antioxidant activities of Cistus extracts
In the assessment of the effect of SA on the antioxidant activity of Cistus extracts, four different assays were carried out: DPPH radical scavenging activity, the electron donation capacity by Fe(III) reduction in the presence of TPTZ, the inhibition of lipid autoxidation by the conjugated diene method, and the hypochlorous acid scavenging potential.
As shown in Fig. 3, increasing SA concentration tends to decrease the free-radical scavenging activity, as measured by the DPPH method, and the ferric reducing capacity of the Cistus extracts, although the differences with the control were not statistically significant at lower SA concentrations. A strong correlation was obtained when these values were compared with the total flavanol content (r = 0.962 and 0.916, P < 0.01, respectively). SA also provoked a dose-dependent increase in the protection against both lipid peroxidation and HOCl-induced BSA oxidation. In all the SA treatments, the inhibition of BSA degradation was significantly greater than that observed in the 500 μM-ascorbic acid treatment (71 ± 3 % above control level) and similar to that observed with (+)-catechin at 500 μM (121 ± 16 % above control level). Good correlations were found between protection against HOCl and free SA (r = 0.810; P < 0.01) and bound SA (r = 0.783; P < 0.01), PAs (r = 0.888; P < 0.01), and total flavanol content (r = −0.606; P < 0.01).
Antioxidant activity of SA-treated Cistus shoots. Antioxidant activity of methanol extracts of Cistus heterophyllus shoots grown in vitro in the presence (10, 100 and 1,000 μM) of SA for 2 months. Each value is expressed as percentage of respective control value in the absence of SA. Values followed by the same letter on every axes are not significantly different at P < 0.05 level (one-way ANOVA followed by Tukey’s HSD test). Letter “a” denotes that the value is not significantly different (P < 0.05) from control (no SA-treated material). CD, conjugated diene assay; HClO hypochlorous acid scavenging assay, FRAP ferric reducing antioxidant power assay, DPPH DPPH scavenging assay
Discussion
In the present study, 40-day-old C. heterophyllus shoots were cultured for 8 weeks on MS/2 solid medium in the presence of increasing doses of SA (10, 100, and 1,000 μM) in order to evaluate its possible beneficial effect on plantlet vigour, which was assessed by measuring shoot growth, photosynthetic activity and antioxidant capacity. Oxidative stress and poor development of the photosynthetic system in in vitro cultures have been cited as major factors in transplant vulnerability (Lee et al. 1985; Cassells and Curry 2001). In the present work, low doses of SA (10 and 100 μM) did not affect the maximum efficiency of PSII or shoot growth, but enhanced chlorophyll and carotenoid levels, while a dose of 1,000 μM SA caused a visible growth inhibition, and a decline in both Chl and carotenoid levels. The growth retardation effect of high SA doses is well documented both in SA-accumulating plants (Jirage et al. 2001; Mateo et al. 2006) and when the regulator is applied exogenously (Kováčik et al. 2009a; Hayat et al. 2010). Although there are few references in the literature to the effect of long-term exposure to SA on photosynthetic performance, an increase in chlorophyll concentration after the application of low SA concentrations has been reported in short-term experiments in other plant species, including Arabidopsis thaliana (Rao et al. 1997), several genotypes of cowpea (Chandra and Bhatt 1998), mustard (Fariduddin et al. 2003) and sunflower (El-Tayeb et al. 2006). Nevertheless, using low concentrations of SA, no change (Drazic and Mihailovic 2005; Abreu and Munné-Bosch 2008) and even a decrease (Moharekar et al. 2003) in photosynthetic pigments have also been mentioned. These seemingly contradictory results confirm that SA influences growth and productivity-related physiological processes in plants, such as the photosynthetic rate, depending on its endogenous levels in particular plant species under specific developmental and/or environmental circumstances (Raskin 1992; Horváth et al. 2007; Hayat et al. 2010).
In addition, it is considered that low doses of SA (5–500 μM) cause a moderate stress that affects cell redox homeostasis in a similar way to stress acclimation processes (Rao et al. 1997; Horváth et al. 2007). Indeed, it had been proposed that SA can play a pro-oxidant or antioxidant role according to its free endogenous level (Yang et al. 2004). In the present study, there were no great changes in the levels of H2O2 in the different treatments, but the extent of lipid peroxidation and the accumulation of proline, which are considered to be indicators of disturbed physiological conditions, increased in 100 μM-SA-treated shoots. Nevertheless, the absence of visible injuries in shoots treated with low doses of SA (10 and 100 μM) suggests that no severe oxidative damage was caused to plantlets under these conditions, probably due to the induction of antioxidant mechanisms. In this respect, several studies have suggested an antioxidant role for proline during stress (Hong et al. 2000; Matysik et al. 2002). In fact, proline has been found to counteract lipid peroxidation (Szabados and Savouré 2010; and references herein) by forming long-lived adducts with ROS (Alia et al. 1997). So, it is likely that SA-induced proline accumulation contributes to lowering ROS levels and, consequently, to protecting Cistus tissues against oxidative stress, as has been reported in several plant species under both normal and stress conditions, including lentil (Misra and Saxena 2009), sunflower (El-Tayeb et al. 2006) and wheat (Shakirova et al. 2003; Hussein et al. 2007). Moreover, a model that relates the proline-linked pentose phosphate pathway to the shikimate and phenylpropanoid pathways has been proposed by Shetty (2004). According to this model, proline biosynthesis coupled to the pentose phosphate pathway could stimulate the production of phenolic phytochemicals many of which are considered to be powerful antioxidants which would enhance the ROS-deactivating capacity of SA-treated plantlets.
When the endogenous SA content was evaluated, a marked increase in both free and bound SA was observed in the 100 μM-treated shoots. A recent work (Szalai et al. 2011) suggested that SA treatment induces its de novo synthesis by activating the expression of SA biosynthetic genes. This indicates that the endogenous SA content is subject to strict control and maintained at a level that would allow the induction of appropriate defence responses. Thus, it has been proposed that excessive SA accumulation can induce the programmed cell death pathway (Borsani et al. 2001), whereas, at moderately high levels, SA may be directly involved in the scavenging of free radicals acting as an antioxidant (Dinis et al. 1994; Szalai et al. 2011) and/or indirectly alter the redox balance through the activation of antioxidant responses (Horváth et al. 2007; Szalai et al. 2011).
Phenylpropanoid metabolism is one of the major pathways stimulated during stress and acclimation responses (Dixon and Pavia 1995; Winkel-Shirley 2002). Increasing information suggests that endogenous and exogenous SA induce both gene expression and the enzymatic activity of PAL (Chen et al. 2006; Kováčik et al. 2009a), which, in turn, provokes the accumulation of phenylpropanoids (Bate et al. 1994). In the present study, long-term exposure of Cistus explants to SA markedly enhanced PAL activity, although the levels of TPC remained unchanged. Nevertheless, SA induced marked changes in the content of certain phenolic compounds. As regards the family of flavanols, increasing SA concentrations decreased the levels of soluble flavanols and increased the content of soluble proanthocyanidins (PAs) present in shoots. There is general agreement that flavanols can polymerize, possibly following an oxidative process, yielding PAs that are stored in the vacuole (Pourcel et al. 2007; Hernández et al. 2009). The oxidation of flavanols could be catalyzed by both polyphenol oxidases (in the presence of oxygen) and Prx (in the presence of H2O2). In this way, the Prx-flavonoid pair constitutes a well-known important sink/buffer of excess H2O2 (Yamasaki et al. 1997; Pérez et al. 2002; Takahama 2004). However, in a recent work (Ferreres et al. 2011), this system was also seen to be involved in the fine homeostasis of H2O2 levels. Therefore, it is plausible that SA is involved in the regulation of this Prx/phenol system, which in turn, would permit the fine-tuned regulation of ROS levels, depending on its endogenous level.
Furthermore, soluble and cell wall-bound Prxs exhibited a distinct behaviour in 10 and 100 μM SA-treated shoots, suggesting that peroxidase isoforms were differentially affected by SA. These results agreed with previous investigations reporting that SA may interfere positively or negatively with different metabolic pathways mediated by Prx (Kawano 2003), and also with the induction of specific Prx genes (Almagro et al. 2009; and references herein). Thus, the differential influence of SA on Prx-mediated metabolic processes could explain the rise in the lignin content of 10 μM SA-treated shoots, which correlated with the increase in cell wall-bound Prx activity, while the accumulation of PAs in 100 μM SA-treated shoots correlated with the rise of soluble Prx activities. Accordingly, the different levels of flavanols and hydroxycinnamic acid derivatives observed in SA-treated shoots could depend, at least in part, on the SA dose-dependent stimulation and down-regulation of these Prx isoenzyme groups.
Similar relationships between TPC, polymeric phenolics and phenolic-metabolizing enzymes have been previously described and related to SA-mediated stress tolerance. For example, changes in the phenylpropanoid profile have been reported in chamomile plants as a consequence of exogenous SA application, which leads to only slight changes in TPC but stimulates the conversion of SA to gentisic acid, which, in turn, activates the expression of the mechanisms involved in the tolerance to some heavy metals (Kováčik et al. 2009b). In contrast to the above-mentioned small changes in TPC and reflecting our findings in the present study, SA treatment may also stimulate the increase in polymeric phenolics, including cell wall-immobilized, phenolics. This could affect the tolerance to stress provoked by different agents, mediating, for instance, the endogenous accumulation of some heavy metals (Kováčik et al. 2011).
Bearing in mind that many plant phenols display antioxidant properties and taking into account that the location and timing of ROS and antioxidant formation are key factors for cell signalling and defence responses (Foyer and Noctor 2009), the effect of SA on total antioxidant capacity was examined. No effect of low SA doses on the total antioxidant capacity was found when measured by the DPPH and FRAP methods, indicating that neither the free radical scavenging activity nor the reducing capacity of Cistus was affected by such SA doses. This contrasts with the results obtained for Thymus membranaceus shoots grown in similar conditions, in which SA treatments, especially at 10 μM, greatly enhanced the antioxidant capacity (Pérez-Tortosa et al. 2012). A high correlation between soluble flavanols and antioxidant activity, as assessed by the DPPH (r = 0.962; P < 0.01), and FRAP (r = 0.916; P < 0.01) methods, was found. Using the same methods, several authors have also reported significant correlation between catechin levels and antioxidant capacity in wines (Katalinić et al. 2004), green tea (Xu et al. 2004) and in different fruits, vegetables, and beverages (Floegel et al. 2011). The antioxidant activity of flavanols has been attributed to the presence of a catechol group on the B ring, which chelates redox-active metals and traps free radicals, namely superoxide radical, singlet oxygen and lipophilic alkyl peroxyl radical (Nakao et al. 1998; Pannala et al. 2001). Furthermore, PAs (the polymeric condensation products of flavanols) have also been seen to possess strong superoxide anion (O2·−), hydroxyl radical (OH·) and hypochlorous acid (HOCl) scavenging activities in both in vitro and in vivo models, as well as in human clinical studies (Cos et al. 2004; Maldonado et al. 2005; Aron and Kennedy 2008). Thus, it was not surprising to find good correlations between PA levels and the anti-lipid oxidation activity and the HOCl scavenging potential (r = 0.575, P < 0.05; r = 0.835, P < 0.01, respectively) in Cistus shoots. It is important to point out that the overall antioxidant capacity displayed by complex extracts in these assays was probably influenced by the occurrence of additive, synergistic and/or antagonistic effects of the different individual compounds. Thus, although it is not easy to attribute the antioxidant capacity displayed by plant extracts to the contribution of a specific phenolic class, it is likely that the oxidation of flavanols to PAs observed in SA-treated shoots could serve as a backup defense system against oxidative stress that could contribute to the attenuation of lipid peroxidation.
In conclusion, the changes in the phenolic contents observed in SA-treated shoots appear to result, at least partially, from the differential influence of SA on PAL and soluble and cell wall-bound Prxs. Thus, the SA dose-dependent oxidation of flavanols to PAs by soluble Prxs could contribute to attenuating oxidative stress in plants by preventing lipid peroxidation, as suggested by the high anti-lipid oxidation activity of extracts obtained from shoots. Besides growth-stimulating effects, the protection conferred against oxidative stress induced by low concentrations of exogenously applied SA could improve the vigour of Cistus plantlets, providing potentially superior material for conservation programmes. Moreover, the findings open up the possibility of using SA-treated Cistus shoot cultures as a production system for obtaining phytochemicals with pharmacological applications.
Abbreviations
- Chl:
-
Chlorophyll
- DPPH:
-
1,1-Diphenyl-2-picrylhydrazyl
- FRAP:
-
Ferric Reducing Antioxidant Power
- MS/2:
-
Murashige and Skoog’s medium with macronutrients at half-strength
- PAL:
-
Phenylalanine ammonia-lyase
- PAs:
-
Proanthocyanidins
- Prx:
-
Class III plant peroxidase
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- SA:
-
Salicylic acid
- TBARS:
-
Thiobarbituric acid-reacting substances
- TPC:
-
Total phenol content
References
Abreu ME, Munné-Bosch S (2008) Salicylic acid may be involved in the regulation of drought-induced leaf senescence in perennials: a case study in field-grown Salvia officinalis L. plants. Environ Exp Bot 64:105–112
Alia, Saradhi PP, Mohanty P (1997) Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J Photochem Photobiol B Biol 38:253–257
Almagro L, Gómez Ros LV, Belchí-Navarro S, Bru R, Ros Barceló A, Pedreño MA (2009) Class III peroxidases in plant defence reactions. J Exp Bot 60:377–390
Amoo SO, Aremu AO, Staden JV (2012) In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tissue Organ Cult 111:345–358
Aron PM, Kennedy JA (2008) Flavan-3-ols: nature, occurrence and biological activity. Mol Nutr Food Res 52:79–104
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Ann Rev Plant Biol 59:89–113
Barrajón-Catalán E, Fernández-Arroyo S, Saura D, Guillén E, Fernández-Gutiérrez A, Segura-Carretero A, Micol V (2010) Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem Toxicol 48:2273–2282
Bate NJ, Orr J, Ni W, Meroni A, Nadler-Hassan T, Doerner PW, Dixon RA, Lamb CJ, Elkind Y (1994) Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc Natl Acad Sci USA 91:7608–7612
Bates LS, Waldren RP, Teare IB (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Borsani O, Valpuesta V, Botella MA (2001) Evidence for a role of salicylic acid in the oxidative damage generated by NaCl and osmotic stress in Arabidopsis seedlings. Plant Physiol 126:1024–1030
Cassells AC, Curry RF (2001) Oxidative stress and physiological, epigenetic and genetic variability in plant tissue culture: implications for micropropagators and genetic engineers. Plant Cell Tissue Organ Cult 64:145–157
Chandra C, Bhatt RK (1998) Biochemical and physiological response to salicylic acid in relation to the systemic acquired resistance. Photosynthetica 35:255–258
Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57:2435–2444
Chen JY, Wen PF, Kong WF, Pan QH, Zhan JC, Li JM, Wan SB, Huang WD (2006) Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Postharvest Biol Technol 40:64–72
Cos P, De Bruyne T, Hermans N, Apers S, Berghe DV, Vlietinck AJ (2004) Proanthocyanidins in health care: current and new trends. Curr Med Chem 11:1345–1359
Dinis TC, Maderia VM, Almeida LM (1994) Action of phenolic derivates (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys 315:161–169
Dixon RA, Pavia NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Drazic G, Mihailovic N (2005) Modification of cadmium toxicity in soybean seedlings by salicylic acid. Plant Sci 168:511–517
El-Tayeb MA, El-Enany AE, Ahmed NL (2006) Salicylic acid-induced adaptive response to copper stress in sunflower (Helianthus annuus L.). Plant Growth Regul 50:191–199
Everette JD, Bryant QM, Green AM, Abbey YA, Wangila GW, Walker RB (2010) Thorough study of reactivity of various compound classes toward the Folin–Ciocalteu reagent. J Agric Food Chem 58:8139–8144
Fariduddin Q, Hayat S, Ahmad A (2003) Salicylic acid influences net photosynthetic rate, carboxylation efficiency, nitrate reductase activity and seed yield in Brassica juncea. Photosynthetica 41:281–284
Ferrer MA, Calderón AA, Muñoz R, Barceló A (1990) 4-Methoxy-alpha-naphthol as a specific subtrate for kinetic, zymographic and cytochemical studies on plant peroxidase activities. Phytochem Anal 1:63–69
Ferreres F, Figueiredo R, Bettencourt S, Carqueijeiro I, Oliveira J, Gil-Izquierdo A, Pereira D, Valentao P, Andrade P, Duarte P, Ros Barceló A, Sottomayor M (2011) Identification of phenolic compounds in isolated vacuoles of the medicinal plant Catharanthus roseus and their interaction with vacuolar class III peroxidase: an H2O2 affair? J Exp Bot 62:2841–2854
Floegel A, Kim D-O, Chung S-J, Koo SI, Chun OK (2011) Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compost Anal 24:1043–1048
Foyer C, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation and practical implications. Antioxid Redox Signal 11:1–45
Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagege D, Didier Courtois D, Joseph C (2012) The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-012-0248-0
Gratão PL, Pompeu GB, Capaldi FR, Vitorello VA, Lea PJ, Azevedo RA (2008) Antioxidant response of Nicotiana tabacum cv. Bright Yellow 2 cells to cadmium and nickel stress. Plant Cell Tissue Organ Cult 94:73–83
Grippa E, Pavone F, Gatto MT, Petrucci R, Marrosu G, Silvestrini B, Saso L (2000) In vitro evaluation of antioxidant activity by electrophoresis and high performance liquid chromatography. Biochem Biophys Acta 1524:171–177
Hatzilazarou SP, Syros TD, Yupsanis TA, Bosabalidis AM, Economou AS (2006) Peroxidases, lignin and anatomy during in vitro and ex vitro rooting of gardenia (Gardenia jasminoides Ellis) microshoots. J Plant Physiol 163:827–836
Hayat Q, Hayat S, Irfan M, Ahmed A (2010) Effect of exogenous salicylic acid under changing environment: a review. Environ Exp Bot 68:14–25
Hernández I, Alegre L, van Breusegem F, Munné-Bosch S (2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14:125–132
Hodges DM, DeLong JM, Forney CF, Prange RK (1999) Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 207:604–611
Hong Z, Lakkineni K, Zhang Z, Verma DPS (2000) Removal of feedback inhibition of 1-pyrroline-5-carboxylate synthetase results in increased proline accumulation and protection of plants from osmotic stress. Plant Physiol 122:1129–1136
Horváth E, Szalai G, Janda T (2007) Induction of abiotic stress tolerance by salicylic acid signaling. J Plant Growth Regul 26:290–300
Hussein MM, Balbaa LK, Gaballah MS (2007) Salicylic acid and salinity effects on growth of maize plants. Res J Agric Biol Sci 3:321–328
Jirage D, Zhou N, Cooper B, Clarke JD, Dong X, Glazebrook J (2001) Constitutive salicylic acid-dependent signaling in cpr1 and cpr6 mutants requires PAD4. Plant J 26:395–407
Katalinić V, Milos M, Modun D, Musić I, Boban M (2004) Antioxidant effectiveness of selected wines in comparison with (+)-catechin. Food Chem 86:593–600
Kawano T (2003) Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep 21:829–837
Kováčik J, Grúz J, Bačkor M, Strnad M, Repčák M (2009a) Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep 28:135–143
Kováčik J, Grúz J, Hedbavny J, Klejdus B, Strnad M (2009b) Cadmium and nickel uptake are differentially modulated by salicylic acid in Matricaria chamomilla plants. J Agric Food Chem 57:9848–9855
Kováčik J, Klejdus B, Hedbavny J, Zoń J (2011) Significance of phenols in cadmium and nickel uptake. J Plant Physiol 168:576–584
Lee N, Wetzstein HY, Sommer HE (1985) Effects of quantum flux density on photosynthesis and chloroplast ultrastructure in tissue-cultured plantlets and seedlings of Liquidambar styraciflua L. towards improved acclimatization and field survival. Plant Physiol 78:637–641
Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 11:591–592
Lichtenthaler HK, Buschmann C, Knapp M (2005) How to correctly determine the different chlorophyll fluorescence parameters and the chlorophyll fluorescence decrease ratio RFd of leaves with the PAM fluorometer. Photosynthetica 43:379–393
López-Arnaldos T, Muñoz R, Ferrer MA, Calderón AA (2001) Changes in phenol content during strawberry (Fragaria x ananasa, cv. Chandler) callus culture. Physiol Plant 113:315–322
Maldonado PD, Rivero-Cruz I, Mata R, Pedraza-Chaverri J (2005) Antioxidant activity of A-type proanthocyanidins from Geranium niveum (Geraniaceae). J Agric Food Chem 53:1996–2001
Mateo A, Funck D, Mühlenbock P, Kular B, Mullineaux PM, Karpiński S (2006) Controlled levels of salicylic acid are required for optimal photosynthesis and redox homeostasis. J Exp Bot 57:1795–1807
Matkowski A (2008) Plant in vitro culture for the production of antioxidants—a review. Biotechnol Adv 26:548–560
Matysik J, Alia Bhalu B, Mohanty P (2002) Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr Sci 82:525–532
Maxwell K, Johnson GN (2000) Chlorophyll fluorescence—a practical guide. J Exp Bot 51:659–668
Misra N, Saxena P (2009) Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Sci 177:181–189
Moharekar ST, Lokhand SD, Hara T, Tanaka R, Tanaka A, Chavan PD (2003) Effect of salicylic acid on chlorophyll and caroteniods contents of wheat and moong seedlings. Photosynthetica 41:315–317
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nakao M, Takio S, Ono K (1998) Alkyl peroxyl radical-scavenging activity of catechins. Phytochemistry 49:2379–2382
Olsen KM, Lea US, Slimestad R, Verheul M, Lillo C (2008) Differential expression of the four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental triggered flavonoid synthesis. J Plant Physiol 165:1491–1499
Pannala AS, Chan TS, O’Brien PJ, Rice-Evans C (2001) Flavonoid B-ring chemistry and antioxidant activity: fast reaction kinetics. Biochem Biophys Res Commun 282:1161–1168
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Pérez FJ, Villegas D, Mejia N (2002) Ascorbic acid and flavonoid-peroxidase reaction as a detoxifying system of H2O2 in grapevine leaves. Phytochemistry 60:573–580
Pérez-Tortosa V, López-Orenes A, Pérez-Martínez A, Ferrer MA, Calderón AA (2012) Antioxidant activity and rosmarinic acid changes in salicylic acid-treated Thymus membranaceus shoots. Food Chem 130:362–369
Pourcel L, Routaboul JM, Cheynier V, Lepiniec L, Debeaujon I (2007) Flavonoid oxidation in plants: from biochemical properties to physiological functions. Trends Plant Sci 12:29–36
Rao MV, Paliyath VG, Ormorod P, Mur P, Watkins CB (1997) Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Plant Physiol 115:137–149
Raskin I (1992) Role of salicylic acid in plants. Annu Rev Plant Physiol Plant Mol Biol 43:439–463
Rivas-San Vicente M, Plasencia J (2011) Salicylic acid beyond defence: its role in plant growth and development. J Exp Bot 62:3321–3338
Sánchez-Gómez P, Guerra J, Carrión MA (2002) Libro rojo de la flora silvestre protegida de la Región de Murcia I & II. Consejería de Agricultura Agua y Medio Ambiente, Dirección General del Medio Natural, Murcia
Serret MD, Trillas MI, Matas J, Araus JL (2001) The effect of photoautotrophy on photosynthesis and photoinhibition of gardenia plantlets during micropropagation. Photosynthetica 39:245–255
Shakirova FM, Sakhabutdinova AR, Bezrukova MV, Fatkhutdinova RA, Fatkhutdinova DR (2003) Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci 164:317–322
Sharma V, Gulati A, Ravindranath SD, Kumar V (2005) A simple and convenient method for analysis of tea biochemicals by reverse phase HPLC. J Food Compos Anal 18:583–594
Shetty K (2004) Role of proline-linked pentose phosphate pathway in biosynthesis of plant phenolics for functional food and environmental applications: a review. Process Biochem 39:789–803
Soobrattee MA, Neergheen VS, Luximon-Ramm A, Aruoma OI, Bahorun T (2005) Phenolics as potential antioxidant therapeutic agents: mechanism and actions. Mutat Res 579:200–213
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97
Szalai G, Horgosi S, Soós V, Majláth I, Balázs E, Janda T (2011) Salicylic acid treatment of pea seeds induces its de novo synthesis. J Plant Physiol 168:213–219
Takahama U (2004) Oxidation of vacuolar and apoplastic phenolic substrates by peroxidase: physiological significance of the oxidation reactions. Phytochem Rev 3:207–219
Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J (2007) Free radicals and antioxidant in normal physiological function and human disease. Int J Biochem Cell Biol 39:44–84
Verberne MC, Brouwer N, Delbianco F, Linthorst HJM, Bol JF, Verpoorte R (2002) Method for the extraction of the volatile compound salicylic acid from tobacco leaf material. Phytochem Anal 13:45–50
Vermerris W, Nicholson R (2006) Phenolic compound biochemistry. Springer, Dordrecht, The Netherlands, pp 151–196
Vlot AC, Dempsey DA, Klessig DF (2009) Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47:177–206
Vogt T (2010) Phenylpropanoid biosynthesis. Mol Plant 3:2–20
Winkel-Shirley B (2002) Biosynthesis of flavonoids and effects of stress. Curr Opin Plant Biol 5:218–223
Xu JZ, Yeung SY, Chang Q, Huang Y, Chen ZY (2004) Comparison of antioxidant activity and bioavailability of tea epicatechins with their epimers. Br J Nutr 91:873–881
Yamasaki H, Sakihama Y, Ikehara N (1997) Flavonoid-peroxidase reaction as a detoxification mechanism of plant cells against H2O2. Plant Physiol 115:1405–1412
Yang Y, Qi M, Mei C (2004) Endogenous salicylic acid protects rice plants from oxidative damage caused by aging as well as biotic and abiotic stress. Plant J 40:909–919
Yuan S-x, Su Y-b, Liu Y-m, Fang Z-y, Yang L-m, Zhuang M, Zhang Y-y, Sun P-t (2012) Effects of pH, MES, arabinogalactan-proteins on microspore cultures in white cabbage. Plant Cell Tissue Organ Cult 110:69–76
Zawoznik MS, Groppa MD, Tomaro ML, Benavides MP (2007) Endogenous salicylic acid potentiates cadmium-induced oxidative stress in Arabidopsis thaliana. Plant Sci 173:190–197
Acknowledgments
The study was supported by the Ministerio de Ciencia e Innovación (project number CGL2006-11569), the Fundación Séneca (project number 08799/PI/08), and the Consejería de Educación, Ciencia e Investigación (CARM, CLUSTER 465.03.08). A. López-Orenes hold a grant from the Universidad Politécnica de Cartagena. Part of this work was carried out at the Instituto de Biotecnología Vegetal, Universidad Politécnica de Cartagena.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to Professor Alfonso Ros Barceló “in memoriam”.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
López-Orenes, A., Martínez-Moreno, J.M., Calderón, A.A. et al. Changes in phenolic metabolism in salicylic acid-treated shoots of Cistus heterophyllus . Plant Cell Tiss Organ Cult 113, 417–427 (2013). https://doi.org/10.1007/s11240-012-0281-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-012-0281-z