Abstract
The effects of different spectral light distribution on in vitro induction and proliferation of Oncidium protocorm-like bodies (PLBs) and subsequent growth of plantlets were investigated. Shoot tips (5 mm in length) of proliferating shoots of Oncidium “Gower Ramsey” were vertically incubated on 1/2 Murashige and Skoog (MS) medium supplemented with 1.0 mg l−1 6-benzyladenine (BA), and grown under either monochromatic red light-emitting diodes (LEDs) (RR), blue LEDs (BB), yellow LEDs (YY) or green LEDs (GG). Cultures grown under fluorescent lamps (FL) were used as control. Selected FL-induced PLBs were cut into 3- to 4-mm sections and incubated on MS medium supplemented with 1.0 mg l−1 BA and 0.5 mg l−1 α-naphthaleneacetic acid (NAA), and grown under RR, BB, YY, GG, or FL. Moreover, FL-differented shoots (15 mm in length with two leaves) were incubated on 1/2 MS medium with 0.5 mg l−1 NAA, and grown under either FL, RR, 10% blue + 90% red LEDs (1BR), 20% blue + 80% red LEDs (2BR), 30% blue + 70% red LEDs (3BR), BB, 80% red + 10% blue + 10% far-red LEDs (RBFr), or 80% red + 10% blue + 10% green LEDs (RBG). Overall, the red light spectrum enhanced induction, proliferation, and the carbohydrate contents of PLBs, as well as subsequent plantlet lengths, while the blue spectrum promoted differentiation, protein accumulation, and enzyme activities in PLBs, as well as pigment content accumulation in PLBs and developing plantlets. The combination of red and blue LEDs resulted in higher energy efficiency as well as dry weight and enzyme activities in these plantlets.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oncidium orchids are economically important and are cultivated for cut flowers and potted plants. Oncidium plantlets are now commercially grown in many countries of the world (Jheng et al. 2006; Su et al. 2006; Chen and Chang 2002). The need for mass propagation of selected elite genotypes has led to the development of several in vitro methods (Arditti and Ernst 1993). Plant regeneration of Oncidium from somatic embryogenesis has been established by using flower stalk internodes (Chen and Chang 2000a), leaves, roots and stems (Chen and Chang 2000b) as explants. The effects of the carbohydrate sources (Jheng et al. 2006; Li et al. 2005), plant growth regulators (Wu et al. 2004; Chen and Chang 2003), on somatic embryogenesis have been investigated in details in Oncidium. In these reported experiments on Oncidium, the influences of spectra were not documented due to the difficulty in modulation of the spectra emanating from fluorescent lamps (FL).
Among the various environmental factors, light is one of the most important variables affecting growth and development of tissue cultures of many plant species (Kozai et al. 1997; Nguyen et al. 1999; Sivakumar et al. 2006). Light spectra effects have been previously investigated on callus and somatic embryogenesis, as well as on plantlets at the rooting stage in various species, such as Cymbidium (Hamada et al. 2009), Lilium (Lian et al. 2002), Hyacinth orientalis (Anna and Alicja 2001) and Zantedeschia (Jao et al. 2005) in vitro. In recent years, the effects of the light spectra on the growth and development of orchids have attracted considerable interest. Hamada et al. (2009) determined that the red spectrum emanating from red fluorescent films increased the proliferation and number of PLBs of Cymbidium finlaysonianum. Cybularz-Urban et al. (2007) demonstrated that different spectra can alter organogenesis in such slow-growing hybrids as Cattleya intermedia × C. aurantiaca, and it can alter the morphology and anatomy of orchid plantlets obtained adventitiously in vitro. Huan and Tanaka (2004) reported that the red spectrum emitted from red LEDs was the most effective for Cymbidium callus induction from PLB segments and that the spectra composed of red and blue LEDs were optimal for Cymbidium callus proliferation and PLB formation. Tanaka et al. (1998) found that the red spectrum emitted from red LEDs promoted leaf growth of Cymbidium plantlets in vitro, but that it decreased chlorophyll (Chl) content, and that this effect was reversed by the blue spectrum emitted from blue LEDs. However, fewer studies have been reported about the spectrum effects on some physiological and biochemical parameters, such as the carbon–nitrogen (C–N) metabolism, pigment content and enzyme activities of tissue cultures of Oncidium.
Compared to conventional light sources such as fluorescent lamps, metal halide and high-pressure sodium, LEDs are particularly well suited for plant growth chambers (Kozai et al. 1997). Moreover, LEDs can be used as the light source for photo-biological research because of its convenience in modulating fine light spectra and intensity. LEDs are also useful in plant tissue culture because of their lower heat radiation, higher energy efficiency and longer lifespan (Nhut et al. 2003; Kim et al. 2004a). The light spectra in many reported experiments, which were obtained by using LEDs, colored membranes or fluorescent lamps with fluorescent films, were inconsistent, and the light intensity was non-uniform because the investigators were unable to precisely modulate and quantify the spectrum energy parameters. Furthermore, the experimental results may have been influenced in part by differences in the light intensity (Cybularz-Urban et al. 2007), and this often presents a problem when comparing results from experiments conducted under inconsistent light parameters.
The objective of this study was to investigate the effects of different LED-generated spectra of uniform intensity on Oncidium PLB induction, proliferation and in vitro plantlets at the rooting stage, as well as on the C–N metabolism, pigment content and enzyme activities of tissue cultures of Oncidium orchids. A secondary objective was to identify the most effective spectrum for the rapid propagation of Oncidium plantlets in vitro.
Materials and methods
LED devices with different light spectra
FL (Huadian Fluorescent Lamp, FL40D-EX/38) was used as the control in our study. All the LED lights were designed by Agronomy College, Nanjing Agricultural University. The spectral distribution in relative energy of the blue (peak at 460 nm), red (peak at 660 nm), yellow (peak at 590 nm), green (peak at 530 nm) and far-red (peak at 715 nm) regions were measured by a spectroradiometer (LI-250A; LI-COR, USA) in the range of 300–800 nm; the half bandwidth of peak wavelength is ±10 nm. Light treatments for Oncidium PLB induction, proliferation and differentiation were red light-emitting diodes (LEDs) (RR), blue LEDs (BB), yellow LEDs (YY) and green LEDs (GG), and the photon flux density (PFD) was at 11 μmol m−2 s−1. The light treatments for Oncidium plantlet rooting were RR, 10% blue + 90% red LEDs (1BR), 20% blue + 80% red LEDs (2BR), 30% blue + 70% red LEDs (3BR), BB, 80% red + 10% blue +10% far-red LEDs (RBFr), or 80% red + 10% blue + 10% green LEDs (RBG), and the PFD was at 50 μmol m−2 s−1, while the power of each treatment was 10.3, 11.0, 11.8, 12.4, 17.3, 11.8, and 11.8 W, respectively.
The LED device used in the experiments was an aluminum, rectangular box. The ceiling of the box was the LED board with an adjustable vertical height. As a light source, many pieces of LEDs, comprising various wavelengths, were arranged on the LED board. According to the various requirements in the experiments, the light spectra in the boxes were adjusted and were different from each other, while the light intensity in all boxes was uniformly controlled by adjusting both the electric current for the LEDs and the height of the LED board.
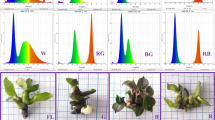
All boxes were placed in a culture room and were arranged as many separate plots with different light spectra (Fig. 1). In the culture room, the relative humidity of 80 ± 5% was maintained with a 16-h photoperiod and a temperature of 25 ± 2°C.
Experimental materials and processing
PLB induction stage
Shoot tips (5 mm) were dissected aseptically from Oncidium ‘Gower Ramsey’ plantlets grown in vitro and provided by the Floriculture Institute of Nanjing Agricultural University. The shoot tips were inoculated vertically in 1/2 MS medium (Murashige and Skoog 1962) with 1.0 mg l−1 6-benzyladenine (BA), 30 g l−1 sucrose and 6 g l−1 agar. Sixty bottles containing this medium and the shoot tips were randomized into five groups and were placed under FL or in the boxes with RR, BB, YY or GG. FL was used as the control.
PLB proliferation and the differentiation stage
White FL-induced PLBs were selected as material and were cut into small sections of 3–4 mm diameter. After weighing in sterile conditions, the sections were cultured in a medium containing MS with 1.0 mg l−1 BA, 0.5 mg l−1α-naphthaleneacetic acid (NAA), 30 g l−1 sucrose and 6 g l−1 agar. Sixty bottles containing the medium and the PLB sections were randomized into five groups and were placed under FL and in the boxes with RR, BB, YY or GG. FL was used as the control.
The rooting culture stage
White FL-differentiated shoots (15 mm tall, with two leaves) were selected as the plantlets. The plantlets were inoculated in a culture medium containing 1/2 MS with 0.5 mg l−1 NAA, 30 g l−1 sucrose and 6 g l−1 agar. Ninety-six bottles containing this medium and the plantlets were randomized into eight groups and were placed under FL or in the boxes with RR, 1BR, 2BR, 3BR, BB, RBFr or RBG. FL was used as the control.
Data collection and statistical analysis
After 30 days in the PLB induction stage, the induction rate of PLBs from the shoot tips was observed. After 30 days in PLB proliferation and differentiation stage, the PLBs were collected and weighed, and the proliferation and differentiation rate were determined. After 30 days in the rooting stage, the shoot and root length, rooting percentage, the fresh weight (FW) and dry weight (DW) of plantlets were recorded.
The Chl content was measured by spectrophotometry using the method of Zhang et al. (2009). Chl was extracted from the leaves of five plantlets at a similar position in each treatment. Leaves were weighed, and 0.1-g (FW) samples were put into a mortar with quartz sand and ground in 10 ml 80% acetone until the leaf material turned white. The optical density was measured with a UV-1200 spectrophotometer (Jin Peng, China) at 663 nm for Chl a and at 645 nm for Chl b. The concentrations of Chl a, Chl b and carotenoids (Car) were determined from the following equations (Arnon 1949):
The contents of soluble sugar and starch were measured by the modified anthrone method of Fairbairn (1953). Leaves (0.05 g DW) were put into a 20-ml test tube, to which 5 ml distilled water was added and mixed. After 30 min (min) in a water bath at 85°C, the supernatant was collected; this step was repeated twice, and then distilled water was added to obtain a volume of 10 ml. The soluble sugar content was determined with the sulfuric acid anthrone method, at a wavelength of 620 nm. The content of sucrose was determined by the phloroglucinol method, at a wavelength of 480 nm. The content of free amino acids was determined by the ninhydrin method, using 1 ml of the extract and a wavelength of 570 nm. The method of Takahashi et al. (1995) was used for the extraction of starch. The residue that was obtained after the distilled-water extraction was dried, and then 1 ml distilled water was added, and the mixture was put in a water bath for 30 min at 100°C. After cooling, the gelatinized starch was digested with 1 ml 9.2 N perchloric acid for 10 min, 2 ml distilled water was added, and then the mixture was centrifuged at 8,000g for 6 min. The extract was then transferred to a 20-ml test tube, 1 ml 4.6 N perchloric acid was added and stirred for 10 min; after centrifuging, 3 ml distilled water was added to a final volume of 20 ml. The content of starch was determined by the same method as the soluble sugar (Fairbairn 1953).
Leaves (0.05 g, FW) were ground in a mortar with liquid nitrogen, to which 3 ml phosphate buffer solution (pH = 7.0) was added. The extract was centrifuged at 13 000g for 15 min at 4°C, and 0.1 ml of the supernatant was combined with 4.9 ml Coomassie brilliant blue G-250 solution (0.1 g l−1). After 2 min, the soluble protein content was determined at a wavelength of 595 nm (Bradford 1976).
The content of free amino acids was determined by the ninhydrin method (Van Slyke et al. 1941). Superoxide dismutase (SOD) activities were measured by the nitroblue tetrazolium (NBT) method (Xiao et al. 1999). Peroxidase (POD) activities were determined by the guaiacol method (Pütter 1974). Catalase (CAT) activities were measured with potassium permanganate titration (Charles and Frederick 1948). The content of malondialdehyde (MDA) was determined by thiobarbituric acid colorimetry (Zhao et al. 1994). C/N = Soluble sugar/Free amino acid and energy efficiency = DW of whole plantlet/ower consumed by LEDs.
Root activity
The lateral roots (0.5 g FW) of five random plantlets were sampled at a similar position for each treatment, and the concentrations of root activity were determined by the method of Li et al. (2010).
Results
The effects of different spectra on the induction and differentiation of Oncidium PLBs
The induction rate of PLBs was determined to be 96.7% under RR, 83.3% under BB, and 76.7% under FL, while only ~66.7% under GG and ~55.7% under YY treatments. The highest fresh weight for PLBs was under RR, and the highest propagation coefficient under RR was comparable to other treatments (Fig. 2).
The PLBs under RR-treated were loosely formed, moreover, their FW and multiplication coefficients were significantly greater than for other spectral treatments, which may be due to hyperhydricity to a certain extent. More compact PLBs and the lowest FW were observed under BB treatment (Fig. 3). The PLBs differentiated into green buds during the proliferation phase, and after 30 days, a large number of adventitious buds differentiated under all treatments. Compared to the FL, the differentiation of PLBs was inhibited by GG and RR and enhanced under YY and BB, particularly under BB. The differentiation rates of BB and YY were 90 and 80%, respectively, and 70% under FL, while lower values were observed under GG (60%) and RR (50%).
The effects of different spectra on the pigment content of Oncidium PLBs
The Car content in the PLBs was the highest under BB (Fig. 4). The Car content data did not show significant differences between BB and FL treatments. Similar responses to different light spectra were observed on the Chl a, Chl b and Chl (a + b) contents in PLBs, which were consistent with the results shown in Fig. 3, where the BB-treated cultures were dark green, the GG-treated and FL-treated PLBs were light green, RR-treated cultures were yellow and YY-treated cultures were light yellow. Our study showed that Oncidium PLBs might have the greatest Chl and Car contents under blue LED and that this spectrum might be beneficial for the accumulation of pigments.
The effects of different spectra on the carbon–nitrogen metabolism of Oncidium PLBs
The data on the soluble sugar, starch and carbohydrate contents, the free amino acids and the soluble protein content of PLBs are shown in Table 1. The soluble sugar and carbohydrate contents did not show significant differences among RR, FL, YY and GG treatments, the lowest values were observed under BB: 9.125 mg g−1(soluble sugar), 11.566 mg g−1(carbohydrate contents). Moreover, they were statistically different from those induced by RR. A similar result was observed for the starch content, and it was higher under RR (2.609 mg g−1), YY (2.441 mg g−1), BB (2.294 mg g−1) and FL (2.438 mg g−1) than under GG (1.870 mg g−1). Under RR, BB and YY, the free amino acid content was higher than that measured under FL and GG. The highest value of the soluble protein content was observed in the BB treatments, 1.705 mg g−1, and it was significantly higher than those under RR and GG. Our results showed that the red LED seemed to promote the accumulation of soluble sugars, starch and carbohydrates.
The effects of different spectra on the enzyme activities of Oncidium PLBs
The POD, SOD and CAT activities of PLBs were higher under BB and YY (Fig. 5). Both the values of SOD and POD activities were the highest in PLBs illuminated with BB, ~198.21 U g−1 and ~14.054 U min−1 g−1, respectively, and they showed statistical differences with those under RR: ~83.960 U g−1 and ~5.853 U min−1 g−1, respectively. The enzyme activities of PLBs under BB were higher than those under RR, and it was observed (Fig. 2) that the differentiation rate was greater under BB and lower under RR.
The effects of different spectra on the rooting and morphogenesis of Oncidium plantlets
The stem elongation of Oncidium plantlets in vitro was marked under RR (3.925 cm), whereas the inhibition of elongation was marked under BB (2.675 cm) (Table 2). Longer roots were observed on the Oncidium plantlets treated by the composite spectra, 2BR (1.950 cm) and 3BR (1.850 cm), and were significantly longer than the FL control (1.175 cm). The rooting rate of plantlets treated with composite spectra of LEDs was noticeably higher than those observed for BB, RR and FL. The lowest value of root activity was found under RR treatment (192.29 μg g−1 h−1), but it was not statistically different compared to the value under FL (277.51 μg g−1 h−1). For the 2BR and 3BR treatments, the dry weight per plantlet and the energy efficiency were the highest. Our results suggest that a composite spectrum of 2BR and 3BR afforded the greatest rooting and morphogenesis response in Oncidium plantlets.
The effects of different spectra on the pigment content of rooted Oncidium plantlets
The concentration of pigments in rooted Oncidium plantlets also depended upon the wavelength of the applied light. Data on the pigment content per fresh weight of plants are shown in Fig. 6. The Chl a, Chl b and Chl (a + b) contents of leaves grown under RR was the lowest, ~0.226 mg g−1, ~0.067 mg g−1, and ~0.293 mg g−1, respectively, and there were no statistical differences among other treatments. The value of the Car content was the highest under RBG (0.322 mg g−1), the lowest under RR (0.084 mg g−1), and there were no significant differences among the values of the leaves under BB, 1BR, 2BR, 3BR, RBFr and FL. Photosynthetic pigment levels were lower under illumination with monochromatic red light than under other treatments, and the light treatments that included blue light seem to be more favorable for the accumulation of pigments in Oncidium plantlets.
The effects of different spectra on enzyme activities and the protein in the leaves of Oncidium plantlets
The SOD, POD and CAT activities in the leaves under the treatments of 1BR, 2BR, 3BR, BB, RBFr and RBG were markedly higher than those under FL; in addition, there were no statistical differences between the RR treatment and FL (Fig. 7). The SOD and POD activities of leaves under RBG were the highest, ~197.08 U g−1 and ~12.669 U min−1 g−1, respectively. The CAT activity of leaves under BB was the highest, 1.367 mol g−1 min−1. There were no significant differences in the MDA content of the leaves between all of the LED treatments and the control. The soluble protein content in leaves was the highest in the BB treatment, 3.562 mg g−1, while the lowest value was observed for the RR treatment, 2.145 mg g−1. In our study, the light treatments that included blue light proved to be more advantageous to enhancing the enzyme activities and to the accumulation of protein in the leaves of Oncidium plantlets.
Discussion
The effects of the red spectrum on plant cell, tissue and organ culture
The highest PLB induction rate in Oncidium appeared in the RR treatment (Fig. 2). This result was in accordance with previous reports that the red spectrum promoted the induction of callus in monocots such as Phalaenopsis (Tanaka et al. 2001), Cymbidium (Huan and Tanaka 2004), and Saccharum sinense (Liang and Chen 2006).
The highest propagation coefficient and FW of Oncidium PLBs were observed under RR treatment (Fig. 2), which is in agreement with observations from Cattleya (Cybularz-Urban et al. 2007). Hamada et al. (2009) found that red fluorescent films increased the PLB proliferation of C. finlaysonianum, even if the film was used only during the early stage of the culture. The red spectrum produced similar positive effects on Cymbidium callus proliferation (Huan and Tanaka 2004) and the somatic embryo output of Cydonia oblonga (Onofrio et al. 1998). However, Zhang et al. (2008) reported that the propagation coefficient of Vitis vinifera callus under the yellow spectrum was appreciably higher than that under the red spectrum. We suspect that such inconsistencies might be due to an “impurity of light quality”, for there was spectral overlap of 50 nm wave-widths between the yellow (520–650 nm) and red (600–900 nm) light source used in the experiment reported by Zhang et al. (2008).
Our results showed that the red LED seemed to promote the accumulation of soluble sugar, starch and carbohydrate (Table 1), which are in agreement with those of Kowallik (1982), who showed that light quality regulated carbohydrate metabolism in higher plants and that the carbohydrate content was high under red light. Red light is important for the development of the photosynthetic apparatus of plants and increases starch accumulation (Schuerger et al. 1997). Li et al. (2010) suggested that Upland Cotton plantlets might generate abundant photosynthetic products but that the plantlets might not transport them efficiently, resulting in the accumulation of starch in the leaves. In our study, the soluble sugars, starch and carbohydrates provided the substrate and energy for proliferation, and this finding may explain why the propagation coefficient under red LEDs was higher than that under other treatments.
In the present experiment, the tallest Oncidium plantlets were observed under the RR treatment (Table 2), but the difference was not statistically significant, as compared with the other light treatments, with the exception of the BB treatment. Sivakumar et al. (2006) showed that the continuous red radiation significantly stimulated shoot elongation of sweet potato plantlets in vitro. Kim et al. (2004b) reported that red LEDs induced the greatest stem length of Chrysanthemum plantlets but that it also resulted in stem fragility due to excessive elongation of the third internode. Moreover, Nhut et al. (2003) determined that in vitro strawberry plantlets that clearly elongated were weak and abnormal under red LEDs, as compared with plantlets under other light conditions. Cybularz-Urban et al. (2007) found that red light caused collapse of part of the mesophyll cells of Cattleya plantlets in vitro, and reduction of leaf blades. Red light promoted leaf growth but decreased the Chl and Car content of plantlets in vitro (Tanaka et al. 1998; Nhut et al. 2003; Cybularz-Urban et al. 2007), and this finding is consistent with our results (Fig. 6). These results imply that a monochromatic red spectrum would elongate the stem and produce weak and morphologically abnormal plantlets in vitro. Moreover, it has been indicated that using a monochromatic red spectrum for Cattleya plantlets in vitro is not acceptable (Cybularz-Urban et al. 2007); our data for SOD activities (Fig. 7) have supported this assertion. The SOD activity in Oncidium leaves under the RR treatment was markedly lower than that in the other LED treatments (Fig. 7), and this result implies that a monochromatic red spectrum would induce less SOD activity in Oncidium plantlets. Dewir et al. (2006) found that SOD activity was significantly higher in Euphorbia millii hyperhydric tissue in vitro as compared to non-hyperhydric normal leaf tissue. Increased SOD activity scavenges O2∙− radicals to protect cellular components from oxidative damage, and it is important in determining the ability of plants to survive. It may be conjectured that a monochromatic red spectrum is not advantageous to the vigorous culture of plantlets in vitro.
The effects of the blue spectrum on plant cell, tissue and organ culture
In our study, the SOD, POD, CAT activities in leaves under the treatments that included the blue spectrum were markedly higher than those under RR and FL; in addition, there was no statistical difference between the RR treatment and the control FL (Fig. 7). However, there is little information available on the mechanism of blue light on antioxidant metabolism in higher plants. Oxidative stress is a key component of environmental stresses (Asada 1999). Dewir et al. (2006) suggested that SOD is usually considered the first line of defense against oxidative stress and that the increased peroxidation of lipids and POD activity could reflect a similar process of oxidative stress, with the implication of POD activity as a part of the antioxidant response against H2O2. Moreover, the increase in CAT activity could be necessary to scavenge H2O2 in peroxisomes and the cytosol. Consequently, our study showed that the blue LED may be more satisfactory for activating different defensive systems to reduce excessive amounts of reactive oxygen species.
In the present experiment, the shortest Oncidium plantlets were observed in the BB treatment (Table 2), and it may be concluded that stem elongation was inhibited by a blue spectrum. A similar result was observed in vitro for pepper plantlets (Schuerger et al. 1997), Chrysanthemum (Kim et al. 2004b) and Zantedeschia (Jao et al. 2005). In the experiment of Jao et al. (2005), a shorter plant height and higher Chl content was found in the red plus blue light treatment, and it was shown that blue light may be involved in both plant height and Chl development control. Nhut et al. (2003) found that the growth of strawberry plantlets under blue LEDs was inhibited but that growth without blue LEDs led to an imbalance during the growth and development of the plantlets. Therefore, it seems that a light treatment that includes blue light is favorable for the vigorous growth of plantlets.
It has been reported previously that blue light increased both Chl and Car contents (Tanaka et al. 1998; Nhut et al. 2003); this finding is consistent with our results (Fig. 6). Cybularz-Urban et al. (2007) found that photosynthetic pigment levels were lower under illumination with monochromatic light than under white light, but in terms of the analyzed plant material’s morphological and anatomical features, irradiation with blue monochromatic light was the most favorable for Cattleya intermedia × C. aurantiaca cultures. In our study, the Chl a, Chl b, Chl (a + b) and Car contents in the leaves under RR were the lowest, and there were no statistical differences among other treatments, with the exception of RBG (Fig. 6). Our study shows that a blue spectrum might be beneficial to the accumulation of pigments in Oncidium PLBs and plantlets.
Metabolically, protein synthesis and enzyme activation in unicellular green algae and higher plants are triggered by blue light (Senge and Senger 1991). Maevskaya and Bukhov (2005) concluded that the photoregulatory action of blue LEDs on nitrate reductase activity determined the different rates of nitrogen assimilation between blue- and red-grown radish plants. Moreover, the protein content in blue-grown leaves was found to be always higher than in red-grown leaves of the same age. In our study, the amounts of soluble protein in the PLBs and leaves were the highest in the BB treatment (Table 1; Fig. 7), which suggests that the blue spectrum was advantageous for protein synthesis.
The feasibility of LED use in plant cell, tissue and organ culture
The spectral energy distribution radiating from fluorescent lamps could not perfectly satisfy the demands of plant tissue culture and micropropagation, although they are commonly used at present. The amount of heat radiation from fluorescent lamps is higher, and the bio-energy efficiency of fluorescent lamps is lower, than LEDs. Fluorescent lamps in plant tissue culture and micropropagation consume more electricity, and lighting costs account for 65% of the total expenditure on electricity (Huan and Tanaka 2004; Kim et al. 2004a, b; Andrea and Francisco 1999). In our study, the largest dry weight per plantlet and the highest energy efficiency were obtained under the 2BR and 3BR treatments, whereas the lowest energy efficiency was obtained under FL (Table 2). This result implies that the composite spectra of red and blue LEDs has excellent energy efficiency when used for the growth of plantlets in vitro. To control plant photomorphogenesis, LEDs can be successfully applied for growing plantlets in vitro at a lower cost (Kozai et al. 1997).
The various growth indexes in the present experiment showed that red LEDs promoted PLB induction in Oncidium, and that blue LEDs promoted differentiation. It has been reported that LEDs are an effective light source for callus induction, callus proliferation and PLB formation from callus (Huan and Tanaka 2004). The composite spectra of red and blue LEDs resulted in many positive effects on growth, dry matter accumulation and root activity (Tanaka et al. 1998; Nhut et al. 2003; Sivakumar et al. 2006; Cybularz-Urban et al. 2007), which are supported by our results. We suggest that LEDs are an appealing alternative light source for plant tissue culture and large-scale micropropagation.
References
Andrea K, Francisco JZA (1999) Natural light as an alternative light source for the in vitro culture of banana. Plant Cell Tissue Organ Cult 55:141–145
Anna B, Alicja K (2001) Effect of light quality on somatic embryogenesis in Hyacinthus orientalis L. ‘Delft’s Blue’. Biol Bull Poznan 38:103–107
Arditti J, Ernst R (1993) Micropropagation of orchids. Wiley, New York
Arnon DL (1949) Copper enzymer in isolated chloroplast polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Mol Biol 50:601–639
Bradford E (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Charles EH, Frederick GK (1948) The accuracy of estimation of hydrogen peroxide by potassium permanganate titration. J Am Chem Soc 70(4):1640–1644
Chen JT, Chang WC (2000a) Plant regeneration via embryo and shoot bud formation from flower-stalk explants of Oncidium ‘Sweet Sugar’. Plant Cell Tissue Organ Cult 62:95–100
Chen JT, Chang WC (2000b) Efficient plant regeneration through somatic embryogenesis from callus cultures of Oncidium (orchidaceae). Plant Sci 160:87–93
Chen JT, Chang WC (2002) Effects of tissue culture conditions and explant characteristics on direct somatic embryogenesis in Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult 69:41–44
Chen JT, Chang WC (2003) Effects of GA3, ancymidol, cycocel and paclobutrazol on direct somatic embryogenesis of Oncidium in vitro. Plant Cell Tissue Organ Cult 72:105–108
Cybularz-Urban T, Hanus-Fajerska E, Swiderski A (2007) Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol Crac Ser Bot 49(1):113–118
Dewir YH, Chakrabarty D, Ali MB, Hahn EJ, Paek KY (2006) Lipid peroxidation and antioxidant enzyme activities of Euphorbia millii hyperhydric shoots. Environ Exp Bot 58:93–99
Fairbairn NJ (1953) A modified anthrone reagent. Chem Ind (4):86
Hamada K, Shimassaki K, Nishimura Y, Egawa H, Yoshida K (2009) Effect of red fluorescent films on the proliferation of Cymbidium finlaysonianum Lindl. PLB cultured in vitro. Hort Environ Biotechnol 50:319–323
Huan LVT, Tanaka M (2004) Effects of red and blue light-emitting diodes on callus induction, callus proliferation, and protocorm-like body formation from callus in Cymbidium orchid. Environ Control Biol 42:57–64
Jao RC, Lai CC, Fang W, Chang SF (2005) Effects of red light on the growth of Zantedeschia plantlets in vitro and tuber formation using light-emitting diodes. HortScience 40(2):436–438
Jheng FY, Do YY, Liauh YW, Chung JP, Huang PL (2006) Enhancement of growth and regeneration efficiency from embryogenic callus cultures of Oncidium ‘Gower Ramsey’ by adjusting carbohydrate sources. Plant Sci 170:1133–1140
Kim HH, Goins GD, Wheeler RM (2004a) Green-light supplementation for enhanced lettuce growth under red- and blue-light-emitting diodes. HortScience 39:1617–1622
Kim SJ, Hahn EJ, Heo JW (2004b) Effects of LEDs on net photosynthetic rate, growth and leaf stomata of Chrysanthemum plantlets in vitro. Sci Hortic 101:143–151
Kowallik W (1982) Blue light effects on respiration. Plant Physiol 33:51–72
Kozai T, Kubota C, Jeong BR (1997) Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tissue Organ Cult 51:49–56
Li SH, Kuoh CS, Chen YH, Chen HH, Chen WH (2005) Osmotic sucrose enhancement of single-cell embryogenesis and transformation efficiency in Oncidium. Plant Cell Tissue Organ Cult 81:183–192
Li HM, Xu ZG, Tang CM (2010) Effect of light-emitting diodes on growth and morphogenesis of upland cotton (Gossypium hirsutum L.) plantlets in vitro. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-010-9763-z
Lian ML, Murthy HN, Paek KY (2002) Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’. Sci Hortic 94:365–370
Liang JX, Chen B (2006) Influence of light quality on seedling differentiation from Sugarcane callus. Sugar crops of China 3:9–11 (in Chinese)
Maevskaya SN, Bukhov NG (2005) Effect of light quality on nitrogen metabolism of radish plants. Russ J Plant Physiol 52(3):304–310
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Nguyen QT, Kozai T, Niu G, Nguyen UV (1999) Photosynthetic characteristics of coffee (Coffeaarabusta) plantlets in vitro in response to different CO2 concentrations and light intensities. Plant Cell Tissue Organ Cult 55:133–139
Nhut DT, Takamura T, Watanabe H (2003) Responses of strawberry plantlets cultured in vitro under superbright red and blue light-emitting diodes (LEDs). Plant Cell Tissue Organ Cult 73:43–52
Onofrio CD, Morini S, Bellocchil G (1998) Effect of light quality on somatic embryogenesis of quince leaves. Plant Cell Tissue Organ Cult 53:91–98
Pütter J (1974) Peroxidases. In: Bergmeyer HU (ed) Methods of enzymatic analysis, vol 2. Academic Press, NY, pp 685–690
Schuerger AC, Brown CS, Stryjewski EC (1997) Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann Bot 79:273–282
Senge M, Senger H (1991) Adaptation of photosynthetic apparatus of Chlorella and Ankistrodesmus to blue and red light. Bot Acta 104:139–143
Sivakumar G, Heo JW, Kozai T, Peak KY (2006) Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J Hortic Sci Biotechnol 81:546–548
Su YJ, Chen JT, Chang WC (2006) Efficient and repetitive production of leaf-derived somatic embryos of Oncidium. Biologia Plantarum 50:107–110
Takahashi K, Fujino K, Kikuta Y, Koda Y (1995) Involvement of the accumulation of sucrose and the synthesis of cell wall polysaccharides in the expansion of potato cells in response to jasmonic acid. Plant Sci 111:11–18
Tanaka M, Takamura T, Watanabe H, Endo M, Yanagi T, Okamoto K (1998) In vitro growth of Cymbidium plantlets cultured under superbright red and blue light-emitting diodes (LEDs). J Hortic Sci Biotechnol 73:39–44
Tanaka M, Watanabe T, Giang DT, Tanaka M, Takamura T, Watanabe H (2001) Morphogenesis in the PLB segments of Phalaenopsis cultured under LED irradiation system (Abstract). J Jpn Soc Hortic Sci 70(Suppl. 1):306 (in Japanese)
Van Slyke DD, Dillon RT, MacFadyen DA, Hamilton P (1941) Gasometric determination of carboxyl groups in free amino acids. J Biol Chem 141:627–669
Wu IF, Chen JT, Chang WC (2004) Effects of auxins and cytokinins on embryo formation from root-derived callus of Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult 77:107–109
Xiao P, Zhong WJ, Zhang RQ (1999) The comparison of chemical luminescence and chemical colorimetric determination method of SOD. Shanghai J Prev Med 11(2):54–56 (in Chinese)
Zhang Z, Li S, Li W (2008) Effects of different light qualities on the multiplication of the callus from Vitis vinifera L. and resveratrol content. Plant Physiol Commun 44:106–108 (in Chinese)
Zhang YS, Huang X, Chen YF (2009) Experimental course of plant physiology. Higher Education Press, Beijing (in Chinese)
Zhao SJ, Xu CC, Zhou Q (1994) The plant tissue malondialdehyde determination method of improvement. Plant Physiol Commun 30(3):207–210 (in Chinese)
Acknowledgments
This project was partially funded by National Science and Technology Support Project of China (No: 2010AA03A165) and Natural Science Fundation of China (No: 30972035).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mengxi, L., Zhigang, X., Yang, Y. et al. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tiss Organ Cult 106, 1–10 (2011). https://doi.org/10.1007/s11240-010-9887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9887-1