Abstract
Callus cultures from nodal and leaf explants of Phyllanthus amarus were established on Murashige and Skoog (MS) medium with various combinations of auxins and cytokinins. The leaf-derived callus induced on 2.26 μM 2,4-dichlorophenoxyacetic acid (2, 4-D) + 2.32 μM Kinetin (Kin) upon transfer to medium containing thidiazuron (TDZ) exhibited higher shoot regeneration (32.4 ± 1.3 shoots per culture). Four-week-old shoots rooted readily on 1.5 μM Indol acetic acid (IAA)-containing medium and were successfully acclimatized with 98% survival. The lignans, Phyllanthin (PH) and Hypohyllanthin (HPH), of leaf extracts from naturally grown plants were identified by using TLC, HPLC and H1-NMR. The PH and HPH production in the regenerated shoots was compared to their production in callus cultures, plants under field conditions and in naturally grown plants. The regenerated shoots on MS + 2.27 μM TDZ produced about two times higher PH and HPH than the leaves of naturally grown plant. The present study provides a useful system for further studies on in vitro morphogenesis, elicitor-assisted production of PH and HPH and A. rhizogenes-mediated genetic transformation in Phyllanthus amarus.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phyllanthus amarus Shcum and Thonn (Euphorbiaceae) is an annual herb widely distributed in the tropical and subtropical regions of India and Central and South American countries (Ghanti et al. 2004). Due to its numerous pharmaceutical applications, this herb is used in traditional folk medicines in India (Unander et al. 1995; Sharma et al. 2001). It is often used for the treatment of fever, jaundice, ascites, hemorrhoid, frequent menstruation, skin ulcer and diabetes (Rai and Mehrotra 2007). Apart from these medicinal uses, several reports showed that the whole plant possesses hypoglycemic, antibacterial, antifungal and antiviral (Sharma et al. 2001), antinociceptive (Santos et al. 2000), antitumor (Islam et al. 2008), antimutgenic (Kumar and Kuttan 2005), and anti-inflammatory (Kiemer et al. 2003) properties. It is employed in an Ayurvedic preparation used as a health promotive and disease preventive tonic (Govindarajan et al. 2007).
This herb has strong activity against hepatitis B virus (HBV) (Shim et al. 2000) and C virus (HCV) (Bhattacharyya et al. 2003). The hepatoprotective active ingredients were shown to be phyllanthin (PH) and (HPH) hypophyllanthin (Shim et al. 2000). The chemical synthesis of PH and HPH is difficult due to their complex structure.
In nature, P. amarus appears along with other herbs after the first showers of rain and disappears on completion of the monsoon. The rate of propagation through seed is unreliable due to poor seed germination and seed heterothermy (Singh et al. 2006). As the plant parts are smaller in size, the resultant biomass is insufficient. Overexploitation of this species by pharmaceutical companies, firms preparing Ayurvedic medicines and grazing animals has resulted in rapid depletion of its natural stock. Considering the ever-increasing demand for this plant, it is necessary to develop alternate propagation strategies. There are no well-defined methods for organized cultivation of this plant. Therefore, the plants growing in the uncultivated areas have to be used which also limits the supply of the quality material. During the last few decades, organ, tissue and callus culture have been reported as an alternative method for production of biomass used for extraction of active metabolites (Rao and Ravishankar 2002).
Despite having immense medicinal importance, P. amarus has so far received inadequate attention. In vitro propagation techniques have been reported for P. caroliniensis (Catapan et al. 2000), P. urinaria (Catapan et al. 2002), and P. stipulatus (Catapan et al. 2001). Synthetic seeds of alginate encapsulated shoot tips were reported in P. amarus (Singh et al. 2006). Shoot tip, nodal and internodal segments were used for in vitro plantlet regeneration in P. amarus (Bhattacharyya and Bhattacharyya 2001; Ghanti et al. 2004). Genetic transformation protocol has been recently described for P. amarus (Banerjee and Chattopadyay 2009; Abhyankar et al. 2010). However, there are no reports on shoot organogenesis in relation to accumulation of phyllanthin and hypophyllanthin. Therefore, the aim of the present investigation was to establish a protocol for efficient propagation through callus culture, to study the organogenic response mediated through different plant growth regulators and biosynthesis of PH and HPH.
Materials and methods
Plant material and surface sterilization
The plants of P. amarus were collected from naturally grown collections found on the campus of University of Pune during the months of June and August in the years 2005, 2006 and 2007. A voucher specimen (BSI/WRC/Tech/2010/NITKPA-1) was deposited in the herbarium of the regional office of the Botanical Survey of India, Pune 411 001. The nodal and leaflet explants (10 mm) were randomly selected from healthy mother plants (height about 20–30 cm). The explants were thoroughly washed with tap water and surface sterilized with 0.1% (W/V) mercuric chloride for 5 min, followed by five washes with sterile distilled water.
Culture conditions and establishment of callus culture
Surface sterilized explants were transferred to MS (Murashige and Skoog 1962) medium supplemented with cytokinins (BA, Kin and TDZ) (0–22 μM) and auxins (IAA, NAA and 2, 4-D) (0–22 μM), singly and in combinations. The media were fortified with 3% (W/V) sucrose, 0.8% agar (W/V), and pH was adjusted to 5.8. The developed calluses were separated from the initial explants and healthy masses subsequently sub-cultured at about 4 weeks interval. Fresh callus (1 g) was used as inoculum. The calluses were harvested after 4 weeks and dried in an oven at 60°C until constant weight was obtained. The calluses obtained on different media were analyzed for PH and HPH. Cultures were incubated at 25 ± 2°C under 8 h photoperiod, with light of 30 μmol m−2 s−1 intensity from cool fluorescent tubes (40 W; Philips India) for a period of 4 weeks.
Shoot and root formation in the callus
To study the influence of different concentrations and types of cytokinins and auxins on shoot and root regeneration, MS basal medium supplemented with BA (00–22.19 μM), Kin (2.32–23.23 μM), TDZ (00–40 μM), IAA (0.57–28.54 μM), NAA (0.54–26.85 μM) and 2, 4-D (0.45–22.62 μM) singly and in combinations was used. Pieces of about 1 g calluses were transferred onto medium for shoot and root regeneration. Cultures were incubated at 25 ± 2°C, under 8 h photoperiod, with light intensity of 30 μmol m−2 s−1 provided by cool fluorescent tubes (Philips India) for a period of 4 weeks.
Rooting and transplantation
After 4 weeks of incubation, the regenerated shoots (2–3 cm) were separated from clumps and harvested for rooting in MS medium supplemented with IAA or NAA (00–22 μM). The frequency of rooting and average number of roots per shoot were determined and recorded after 28 days of culture. The plantlets were removed and washed with water to remove traces of agar. The young plants were transplanted into earthen pots containing soil and sand mixture (2:1) and kept in glass house for 2 weeks (light 60 μmol m2 s−1, humidity 80–90% and temperature 25 ± 4°C) and then gradually exposed to ambient humidity.
Isolation of PH and HPH
PH and HPH were isolated as per the method of Row and Srinivasulu (1966). The oven-dried leaves (300 g) were mixed with lime (90 g) and water (90 ml) and allowed to dry overnight before extraction with petroleum ether (4 syphonings). The bright yellow semi-solid residue (7 g), obtained after removal of the solvent by distillation, was boiled with ethanol (100 ml) for 10 min, cooled to room temperature, and then filtered. The filtrate after evaporation gave pale yellowish residue (4–5 g). The residue was boiled with petroleum ether and left until separation of yellow oil was complete. On cooling, it gave a mixture of yellow and white powder (2–3 g). Part of the residue (1 g) was layered over Si gel (60–120 mesh LR 40 g) column and was eluted with hexane and mixture of hexane and ethyl acetate. The polarity of the eluting solvent was increased gradually and fractions of 50 ml volume were collected. At 20% polarity, fraction number 75–84 gave mixture of PH and HPH after removal of the solvent, which was confirmed on TLC. These fractions were pooled and evaporated to yellow residue (50 mg). Part of this residue (10 mg) was further purified on preparative HPLC by using MeOH: water (65:35 v/v) as mobile phase.
NMR analysis
H1 (proton) spectra were recorded in CDCl3 using a 300 MHz Varian Mercury (Bruker, Dubendorf, Switzerland) equipped with a QNP probehead. Chemical shifts (δ) were reported in parts per million (ppm) related to tetramethylsilane as internal standard and coupling constant (j) was given in Hertz. The spectra were in complete agreement with those reported in literature.
Preparation of sample extract
About 0.5 g of powdered callus, natural material (fruit, leaves, and stem) and part of in vitro raised shoots (fruit, leaves, and stem) of P. amarus were extracted in 5 ml petroleum ether in an ultrasonic bath for 30 min. After centrifugation, the supernatant was evaporated to dryness. The residue was dissolved in 100 μl HPLC grade methanol, 20 μl of which was submitted to HPLC analysis.
Estimation of PH and HPH
The isocratic HPLC system (DIONEX) comprised P-680 solvent delivery pump, C18 (250 × 4.6 mm, 5 μm) column and UV detector (170 U). Mobile phase used was methanol–water (65:35 v/v) with a flow rate 1.0 ml/min at 25°C. Twenty μl sample was injected and scanned at 254 nm. One mg powder of PH and HPH was dissolved in 1 ml HPLC grade methanol. The peaks corresponding to these lignans were confirmed by spiking the sample extract along with standard. The PH and HPH in the samples were quantified by comparing the peak areas with those of the standard PH and HPH.
Statistical analysis
All the experiments were set up in completely randomized design. Each treatment consisted of at least 21 replicates. All the treatments were repeated thrice. Mean values were compared using Duncan’s Multiple Range Test (DMRT) at 5% probability (Duncan 1955).
Result and discussion
Callus induction and growth of callus
Leaf and nodal explants of P. amarus grown on MS medium supplemented with BA (2.22 μM–22.19 μM) or Kin (2.32 μM–23.23 μM) or TDZ (2.27 μM–40 μM) formed calluses from the cut ends within 2 weeks of culture, and at the end of the fourth week, the entire surface of explant was covered with callus. The amount, texture, color and morphogenic response and PH and HPH contents of these calluses varied with the type and concentration of cytokinin (Tables 1, 2).
Extensive proliferation and growth of callus (dry weight basis) in both the explants were observed with significant differences on medium supplemented with lower concentrations of BA (2.22 μM) or TDZ (2.27 μM) or Kin (2.32 μM) (Tables 1, 2). However, the leaf-derived callus showed significantly higher growth (295 mg DW per culture) compared to the node-derived callus (136 mg DW per culture) on 2.22 μM BA-containing medium (Table 1). The callus was dark green, nodular and hard on medium with BA and TDZ, while loose and pale green on medium with Kin. BA appears to be superior compared to Kin and TDZ for callus induction in P. amarus. The use of MS medium containing BA was also shown to be effective for callus induction from nodal explant in P. stipulatus (Catapan et al. 2001).
It is well known that 2, 4-D promotes cell division and is mostly employed for callus induction in tissue culture studies (Constabel 1987). In the present investigation, among the auxins used, maximum growth of callus (199 mg DW per culture) in leaf explants was recorded on medium containing 4.52 μM 2, 4-D. Substantial callus formation was also observed with 10.74 μM NAA and 11.42 μM IAA. Similar trend of callus formation was also recorded in nodal explants on equimolar concentrations of 2, 4-D, NAA and IAA (Table 3) with significant differences. Concurrent results were reported for establishment of callus in P. urinaria, P. abnormis and P. caroliniensis (Catapan et al. 2000, 2002). Therefore, 2, 4-D seems to be superior over other auxins when used singly for callus formation in Phyllanthus sp.
In the present investigation, the callus produced on media supplemented with IAA and NAA was off-white and friable and produced numerous roots (1–12 roots per culture) in the third and fourth weeks of culture. However, callus produced on 2,4-D-fortified media was yellowish and loose without any root formation (Table 3). In an earlier report in P. stipulatus, root formation in the callus was recorded on media fortified with 1.25–5 μM NAA (Catapan 1999).Use of cytokinins in conjunction with auxins facilitated only callus formation (Table 4). From a total of more than 240 combinations of auxins and cytokinins tested at various concentrations (0.45–40 μM), significant callus proliferation from leaf explant was observed with 4.65 μM Kin + 2.15 μM NAA, while from nodal explant, it was observed with 4.44 μM BA + 0.54 μM NAA (Table 4). Therefore, growth regulator requirement for callus induction appeared to vary with the source of explant.
Though substantial callus growth was also obtained with other combinations of growth regulators (Tables 1, 2, 3 and 4), texture and color of the callus and rhizogenesis response depended on auxin and cytokinin. The higher proportion of IAA or NAA turned the callus friable and whitish in color and showed rhizogenesis at the end of the fourth week (Table 3). On the other hand, a lower proportion of auxin made the callus dark or pale green and nodular. Both leaf and nodal explant-derived calluses with regular sub-culturing to fresh medium after every 28 days were kept in an actively growing condition over 2 years. These results reveal tha,t in P. amarus, the best callogenic response can be obtained in the presence of 2.22 μM BA in the MS medium (Table 1; Fig. 1a).
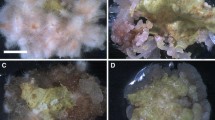
Callus culture and in vitro propagation of P. amarus. a Extensive proliferation of callus on MS + 2.22 μM BA. b Regeneration of shoot from leaf derived callus on MS + 2.27 μM TDZ. c Proliferation of multiple shoot from the leaf derived callus on MS + 2.27 μM TDZ. d Rooting of shoot on MS + 1.5 μM IAA. e Regenerated plants in field conditions
Accumulation of PH and HPH in callus
The structures of PH and HPH isolated from the leaves of P. amarus were confirmed by comparing their NMR spectra with those reported previously (Somanabandhu et al. 1993). These PH and HPH extracted and separated were used as standard compounds. Considering the retention time with the standards, the peak with retention time of 14.6 ± 0.8 min and 12.9 ± 0.5 min was assigned to PH and HPH, respectively.
The accumulation of PH and HPH was higher in the callus obtained on low concentrations of cytokinins (Table 1). Higher concentration of cytokinins in the medium was inhibitory for growth of callus as well as accumulation of PH and HPH. The levels of PH and HPH were observed to be higher in the leaf-derived callus raised on BA-containing medium, followed by TDZ- and Kin-containing media. The callus derived from leaf explant showed significantly higher levels of PH and HPH as compared to node derived callus on BA containing medium (Table 1).
The calluses raised on MS medium incorporated with NAA showed higher levels of PH and HPH compared to IAA (Table 3), whereas lower levels were observed in the calluses obtained on 2,4-D-containing medium. These results are in conformity to earlier reports (Ramawat and Merrilion 1999), where auxins, particularly 2,4-D, was shown to promote de-differentiation and primary metabolism and suppress secondary metabolism. However, incorporation of auxins in the MS medium resulted in inverse relationship between accumulation of PH and HPH in callus and growth of callus. Possibly, the diversion of carbon flux from the secondary metabolic pathway, low activity of key enzymes, lack of appropriate storage sites or transport mechanism or unregulated catabolism of the product are responsible for such effects (Dicosmo and Misawa 1996).
Incorporation of auxins together with cytokinins reduced the accumulation of PH and HPH in the calluses derived from leaf as well as nodal explant (Table 4). The most potent inhibitor was 2, 4-D, followed by IAA and NAA. Among the explants, callus derived from leaf explant contained higher levels of PH and HPH compared to that of callus derived from nodal explant (Table 4). This trend remained the same irrespective of type and concentration of growth regulator. However, the dry weight of the callus and accumulation of PH and HPH in the callus produced on MS medium containing 2.22 μM BA were the highest among the calluses produced on cytokinin and auxin, alone and in combination (Tables 1, 2, 3 and 4). Cytokinins have been demonstrated to stimulate production of alkaloids in cultures of Catherantus roseus (Decendit et al. 1992); catechins, proanthocyanidins and lignins in callus cultures of Pseudotsuga menziesii (Zaprometov 1988). In contrast, cytokinins were found to inhibit napthaquinone formation in Lithospermum erythrorhizon (Fujita et al. 1981) and secoiridoid production in Blackstonia perpuriata (Sabovljevic et al. 2006). The results of the present investigation are in agreement with the assertion that the change in the type and concentration of growth regulator may drastically reduce or increase product accumulation (Constabel 1987).
Regeneration of shoot and root from callus
The calluses derived from the nodal and leaflet explants and sub-cultured on the media containing IAA (0.57–28.54 μM) or NAA (0.5–26.85 μM) proliferated and produced white thin roots in the second week of culture. Maximum number of roots (11.6 ± 0.3/culture) was produced from nodal explant derived callus on MS medium fortified with 5.71 μM IAA (Table 3). Rajasubramaniam and Saradhi (1994) reported formation of roots from the surface of hypocotyl-derived callus in P. fraternus on B5 medium fortified with 10–6M BAP + 10–6M NAA. In the present study, the calluses developed on 2,4-D-containing media did not respond to rooting (Table 3). 2,4-D generally blocks morphogenesis and favors de-differentiation and callus development (Dicosmo and Misawa 1996). The leaf callus developed on medium with 2.26 μM 2,4-D and 2.32 μM Kin, upon transfer to MS medium with TDZ (0.45–9.08 μM) responded for shoot regeneration. The regenerative callus first turned dark green and nodular, and then these nodular structures further developed into small shootlets which attained the height of about 3–4 cm in 4 weeks after the initiation of culture (Fig. 1b, c). The medium supplemented with 0.45 μM TDZ usually induced 8–12 shoots per culture, whereas raising the level up to 2.27 μM TDZ resulted in an increase in the percentage of cultures responding for shoot formation (69.2%) and mean number of shoots per culture (maximum 32.4 ± 1.3 shoots per culture) (Table 5; Fig. 1c). At higher levels (4.54–9.08 μM), however, reduction in number of shoots per culture was observed. Addition of IAA and NAA (2.5–10 μM) to medium containing 2.27 μM TDZ completely inhibited shoot regeneration (data not presented). The fresh as well as sub-cultured calluses derived from leaf explants cultured on all the media except MS medium with 2.26 μM 2 4-D: 2.32 μM Kin, when transferred onto the medium with different levels of BA, Kin and TDZ, alone or together with auxins IAA, NAA or 2,4-D, did not show shoot regeneration. None of the calluses derived from the nodal explant responded to shoot regeneration.
TDZ is a potent bioregulant of in vitro morphogenesis (Murthy et al. 1998). Studies confirm the effective use of TDZ as the sole growth regulating compound for the induction of regeneration in many species like Tylophora indica (Thomas and Philip 2005), Glycine max (Kaneda et al. 1997) and Fragaria sp. (Landi and Mezzetti 2006). In the present study, only TDZ (2.27 μM) was effective for shoot regeneration in leaf-derived callus on MS medium with 2,4-D (2.26 μM) together with Kin (2.32 μM). These results revealed that the source of explant as well as growth regulator in the medium were critical for regenerative response in terms of induction of callus and differentiation of shoots in P. amarus.
Accumulation of PH and HPH
Biosynthesis and accumulation of metabolites in relation to organ development like root and shoot is well established and is often bound to a specific stage of plant development. Even within a single cell, a high degree of organization is observed (Wink 1987). In this study, the accumulation of PH and HPH was low in root-forming calluses of both nodal and leaf explants grown on IAA- and NAA-containing MS medium and in the roots of naturally grown plants, suggesting that roots are not a good source of PH and HPH.
The production of PH and HPH from the node-derived calluses on 2,4-D:Kin containing media was comparatively much less and remained in the range of 10–20 μg/g DW and 5–10 μg/g DW, respectively (data not presented). However, only the leaf-derived calluses grown on 2,4-D- and Kin-containing media upon transfer to TDZ-containing medium showed shoot regeneration. Different quantities of PH and HPH were detected in shoot biomass obtained through TDZ treatments (Table 5). Two-fold increased production of PH (456.4 ± 0.3 μg/g DW) and HPH (332.7 ± 0.2 μg/g DW) was observed in the shoots obtained on 2.27 μM TDZ-containing medium compared to that of leaflets of naturally grown plants (Tables 5 and 6; Fig. 2b). The differentiation, organ development and presence of TDZ together might be responsible for the creation of favorable cytoplasmic conditions for enhanced production of PH and HPH in the in vitro regenerated shoots. It is generally considered that plant growth regulators do not react with intermediate compounds of biosynthetic pathways but appear to change cytoplasmic conditions for product formation (Ramawat and Merrilion 1999).
TDZ has been well established as a potent cytokinin for several plants. Smith et al. (2002) reported significantly increased gland number and hypericin accumulation in shoot cultures of Hypricum perforatum on medium fortified with TDZ. Likewise, Liu et al. (2007) also observed increased hypericin and clustering shoots in H. perforatum on medium supplemented with 2.27 μM TDZ. In contrast, in H. sampsonii, TDZ (0.23– 0.91 μM) incorporation decreased hypericin levels and resulted in malformed leaves and poor shoot growth (Liu et al. 2007). The results of current study on P. amarus and earlier reports on H. perforatum and H. sampsonii indicate that plant growth and developmental stage are important factors in the accumulation of secondary metabolites.
Rooting of shoots and acclimatization
The success of in vitro regeneration depends on efficient rooting of regenerated shoots and survival of the plantlets under field conditions. The regenerated shoots of approximately 2–3 cm length failed to root on auxin free medium, whereas those cultured on MS medium fortified with either IAA or NAA (1.5–22 μM) rooted with variable frequency (Table 7). Catapan et al. (2002) reported spontaneous rooting in microcuttings of P. urinaria on medium lacking plant growth regulators. In the present study, defoliation was observed in the shoots upon transfer to root induction medium, though new leaves were produced within 4 weeks of culture. Root initiation was observed within 10–12 days in shoots cultured on IAA- or NAA (1.5–22 μM)-containing media, but with different rooting percentage, number of roots per shoot and average length of roots. The best response was 12.7 roots per shoot, obtained on medium supplemented with 1.5 μM IAA (Fig. 1d). Shoots from this treatment had the mean longest root length of 5.3 cm (Table 7) and the highest percentage (98%) of rooting shoots. Inclusion of higher levels of IAA reduced the percentage of shoots producing roots, number of roots per shoot and root length. The shoots transferred on NAA-containing medium produced callus interspersed very thin roots. Such shoots failed to survive in soil during hardening. Callus formed at the shoot base can interfere with the connection between the shoot and root, resulting in poor root formation and on transfer, such plantlets do not survive field conditions (Quraishi et al. 1996).
The well-rooted plants were hardened for 2 weeks in the glasshouse (light 60 μmol m2s−1, humidity 80–90% and temperature 25 ± 4°C), of which almost 98% plantlets survived when transferred to field conditions (light 320 μmol m2 s−1, temperature 25 ± 5°C and humidity 70–80%) (Fig. 1e). The acclimatized plants were healthy and exhibited normal growth and completed the life cycle within 4 months.
PH and HPH contents in in vitro explants and in vivo plant parts
Secondary metabolites are usually not distributed uniformly in different parts of the plant. Some are restricted to specific organs or tissue (Wink 1987). Among the different parts of naturally grown plants of P. amarus, higher content of PH (223.6 ± 0.2 μg/g DW) and HPH (113.2 ± 0.3 μg/g DW) was recorded in leaf (Fig. 2a), followed by fruit and comparatively much less content in the stem and roots (Table 6). A similar trend was observed for content of PH and HPH in root, stem, leaf and fruit of in vitro raised field acclimatized plants and the contents were not significantly different as compared to the parts of naturally grown plants (Table 6). The results indicate that the in vitro raised plantlets on acclimatization to the natural conditions were as good as the naturally grown plants for production of PH and HPH. The shoots raised on TDZ-containing medium showed two-fold higher contents of PH and HPH. However, after rooting, these shoot-derived plantlets showed decline in PH and HPH which was almost equal to that of naturally grown plants. It is possible that IAA present in the rooting medium counteracted the effect of TDZ and inhibited the production of PH and HPH. Sabovljevic et al. (2006) reported that, though there was no qualitative difference between the plants of Blackstonia perfoliata growing in the wild and those cultured in vitro, the levels of secondary metabolites secoiridoids, gentiopierin, swertiamarin and sweroside differed in them.
To our knowledge, this is the first report on comprehensive analysis of the effects of auxins and cytokinins on the production of phyllanthin and hypophyllanthin in callus cultures, shoots and roots in comparison to in vitro raised—field acclimatized plants and naturally grown plants of P. amarus. As indicated by the variables tested in this study, the shoots regenerated from leaf-derived callus on TDZ containing MS medium can be one of the best sources of hepatoprotective phyllanthin and hypophyllanthin. This protocol may be useful for their scaling up using bioreactor-based production.
Abbreviations
- BA:
-
6-benzyladenine
- Kin:
-
Kinetin 6-furfurylaminopurin
- TDZ:
-
Thidiazuron(1-phenyl-3-(1,2,3-thiadiazol-5-yl) urea)
- 2,4-D:
-
2,4-dichlorophenoxyacetic acid
- NAA:
-
α-naphthalene acetic acid
- IAA:
-
Indol acetic acid
- HPLC:
-
High performance liquid chromatography
- TLC:
-
Thin-layer chromatography
- NMR:
-
Nuclear Magnetic resonance
References
Abhyankar G, Suprasanna P, Pandey BN, Mishra KP, Rao KV, Reddy VD (2010) Hairy root extract of Phyllanthus amarus induces apoptotic cell death in human breast cancer cells. Innov Food Sci Emerg Tech 11:526–532
Banerjee A, Chattopadyay S (2009) Genetic transformation of hepatoprotective plant, Phyllanthus amarus. In Vitro Cell Dev Biol Plant 45:57–64. doi:10.1007/s11627-008-9160-z
Bhattacharyya R, Bhattacharyya S (2001) High frequency in vitro propagation of Phyllanthus amarus Schum and Thonn. by shoot tip culture. Indian J Exp Biol 39(11):1184–1187
Bhattacharyya R, Bhattacharya S, Wenzel-Mathers M, Buckwold VE (2003) Phyllanthus amarus root clone with significant activity against bovine viral diarrhoea virus—a surrogate model of hepatitis C virus. Curr Sci 84(4):529
Catapan E (1999) In vitro culture and phytochemical analysis of Phyllanthus spp. MSc thesis, Federal University of Santa Catarina, Florianopolis, SC, Brazil (in Brazilian Portuguese with English summary)
Catapan E, Otuki MF, Viana A (2000) In vitro culture of Phyllanthus corolinienis (Euphorbiaceae). Plant Cell Tiss Org Cult 62:195–202
Catapan E, Otuki MF, Viana AM (2001) In vitro culture of Phyllanthus stipulatus (Euphorbiaceae). Revta Brasil Bot 24(1):25–34
Catapan E, Marcio L, da Busi S, Fabio NM, Ana M (2002) Micropropagation, callus and root culture of Phyllanthus urinaria (Euphorbiaceae). Plant Cell Tiss Org Cult 70:301–309
Constabel F (1987) Cell culture in phytochemistry. In: Constable F, Vasil IK (eds) Cell culture and somatic cell genetics in plants, vol 4. Academic, New York, pp 3–13
Decendit A, Liu D et al (1992) Cytokinins enhanced accumulation of indol alkaloids in Catheranthus roseus cell culture-the factor affecting the cytokinins response. Plant Cell Rep 11:400–403
Dicosmo F, Misawa M (1996) Plant cell culture secondary metabolism towards industrial application. CRC, Boca Raton, New York, pp 139–166
Duncan DB (1955) Multiple range and multiple F-test. Biometrics 11:1–42
Fujita Y, Hara Y, Morimoto T, Misawa M (1981) Production of shikonin derivatives by cell cultures of Lithospermum erythrorhizon II: a new medium for the production of shikonin derivatives. Plant Cell Rep 1:61–63
Ghanti K, Govindraju B, Venugopal R, Rao R, Kaviraj C, Jabeen F (2004) High frequency shoot regeneration from Phyllanthus amarus Schum and Thonn. Ind J Biotechnol 3:103–107
Govindarajan R, Singh DP, Rawat AK (2007) High-performance liquid chromatographic method for the quantification of phenolics in ‘Chyavanprash’ a potent ayurvedic drug. J Pharm Biomedical Anal 43:527–532
Islam A, Selvan T, Mazumdar UK, Gupta M, Ghosal S (2008) Antitumor effect of phyllanthin and hypophyllanthin from Phyllanthus amarus against ehrlich ascites carcinoma in mice. Pharmacology Online 2:796–807
Kaneda Y, Tabei Y, Nishimura S, ltarada K, Akihama T, Kitamura K (1997) Combination of thidiazuron and basal media with low salt concentrations increases the frequency of shoot organogenesis in soybeans (Glynine max L. Merr.). Plant Cell Rep 17:8–12
Kiemer AK, Hartung T, Huber C, Vollmar AM (2003) Phyllanthus amarus has anti-inflammatory potential by inhibition of iNOS, COX2, and cytokines via the NF-κB pathway. J Hepatol 38(3):289–297
Kumar KBH, Kuttan R (2005) Chemoprotective activity of an extract of Phyllanthus amarus against cyclophosphamide induced toxicity in mice. Phytomed 12(6–7):494–500
Landi L, Mezzetti B (2006) TDZ, auxin and genotype effects on leaf organogenesis in Fragaria. Plant Cell Rep 25:281–288
Liu X, Zhang X, Sun J (2007) Effects of cytokinins and elicitors on the production of hypericins and hyperforin metabolites in Hypericum sampsonii and Hypericum perforatum. Plant Growth Regul 53:207–214
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay of tabacco tissue cultures. Physiol Plant 15:473–497
Murthy BNS, Murch SJ, Saxena PK (1998) Thidiazuron: a potential regulator of in vitro plant morphogenesis. In Vitro Cell Dev Biol Plant 34:267–275
Quraishi A, Koche V, Mishra SK (1996) In vitro Micropropagation from nodal segment of Cleistanthus collinus. Plant Cell Tiss Org Cult 45:87–91
Rai V, Mehrotra S (2007) Chromium-induced changes in ultramorphology and secondary metabolites of Phyllanthus amarus Schum & Thonn.—an hepatoprotective plant. Environ Monit Assess 147:307–315
Rajasubramaniam S, Saradhi PP (1994) Organic nitrogen stimulates caulogenesis from hypocotyl callus of Phyllanthus fraternus. Plant Cell Rep 13(11):619–622
Ramawat K, Merrilion J (1999). Biotechnology—secondary metabolites. Mohan Primlani for Oxford and IBH, Delhi, pp 243–247
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Row RL, Srinivasulu C (1966) Crystalline constituent of euphorbiaceae-V. New lignin from Phyllanthus niruri Linn—the constitution of Phyllanthin. Tetrahedron 22:2899–2908
Sabovljevic A, Rosic N, Jankovic T, Grubisic D (2006) Secoirdoid content of Blackstonia perfoliata in vivo and in vitro. In vitro Cell Dev Biol Plant 42:427–431
Santos A, Campos R, Miguel O, Filho V, Siani A, Yunes R, Calixto J (2000) Antinociceptive properties of extracts of new species of plants of the genus Phyllanthus (Euphorbiaceae). J Ethnopharmacol 72:229–238
Sharma PC, Yelne MB, Dennis TJ (2001) Database on medicinal plants used in ayurveda. vol 3. Central Council for Research in Ayurveda and Siddha, Delhi, pp 512–536
Shim SB, Kim NJ, Kim DH (2000) β-Glucuronidase inhibitory activity and hepatoprotective effect of 18 β glycyrrhetinic acid from the rhizomes of Glycyrrhiza uralensis. Planta Med 66:40–43
Singh AK, Sharma M, Varshney R, Agrawal SS, Bansal KC (2006) Plant regeneration from alginate-encapsulated shoot tips of Phyllanthus amarus Schum and Thonn, a medicinally important plant species. In Vitro Cell Develop Biol Plt 42:109–113
Smith MAL, Kobayashi H, Gawienowski M, Briskin GP (2002) An in vitro approach to investigate medicinal chemical synthesis by three herbal plants. Plant Cell Tiss Org Cul 70:105–111
Somanabandhu A, Nitayangkura S et al (1993) 1H and 13C-NMR assignments of phyllanthin and hypophyllanthin lignans that enhance cytotoxic responses with cultured multidrug resistant cells. J Nat Prod 56(2):223–239
Thomas TD, Philip B (2005) Thidiazuron-induced high-frequency shoot organogenesis from leaf-derived callus of a medicinal climber, Tylophora indica (burm. f.) Merrill. In Vitro Cell Dev Biol Plant 41:124–128
Unander DW, Webster GL, Blumberg BS (1995) Usage and bioassays in Phyllanthus (Euphorbiaceae). IV. Clustering of antiviral uses and other effects. J Ethnopharmacol 45:1–18
Wink M (1987) Production of plant secondary metabolites by plant cell cultures in relation to the site and mechanism of their accumulation. In: Constable F, Vasil IK (eds) Cell culture and somatic cell genetics in plants, vol 4. Academic, New York, pp 1–35
Zaprometov MN (1988) Proanthocyanidins and catechins. In: Constable F, Vasil IK (eds) Cell cultures and somatic cell genetics of plants, vol 5. Académic, San Diego, pp 77–88
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nitnaware, K.M., Naik, D.G. & Nikam, T.D. Thidiazuron-induced shoot organogenesis and production of hepatoprotective lignan phyllanthin and hypophyllanthin in Phyllanthus amarus . Plant Cell Tiss Organ Cult 104, 101–110 (2011). https://doi.org/10.1007/s11240-010-9796-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-010-9796-3