Abstract
The influence of the developmental stage of microspores on establishing isolated microspore cultures of three Hungarian (‘Szegedi 80’, ‘Szegedi 178’, and ‘Remény’) and three Spanish (‘Jeromin’, ‘Jariza’, and ‘Jaranda’) pepper genotypes was investigated. Donor anthers containing 80% uninucleated and 20% binucleated microspores yielded the highest frequency of successful microspore cultures. Co-cultures with wheat, line ‘CY-45’, ovaries exhibited enhanced frequency of embryoid production than those with pepper ovaries. Differences in efficiency of isolated pepper microspore culture establishment were observed among different pepper genotypes. Green plantlets were regenerated from microspore-derived embryoids, but some were exhibited abnormal growth habits, such as leaf rosetting. A total of seven fertile microspore-derived plants were obtained, including three ‘Jariza’, three ‘Jaranda’, and a single ‘Szegedi 80’ plant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Capsicum is one of the most variable genera amongst horticultural species (Derera et al. 2005). Fruits range from tiny hot chillies to long cayenne chillies. Common names of Capsicum genotypes are also varied with synonyms such as pepper, paprika, chilli, pimiento, Spanish pepper, and condiment paprika, among others, depending on the continent and local traditions (Derera et al. 2005).
Anther culture is one of the best-known methods for the production of doubled-haploid (DH) lines. The first reports on pepper anther culture-derived haploid plants were published simultaneously by three different laboratories (George and Narayanaswamy 1973; Kuo et al. 1973; Wang et al. 1973). Sibi et al. (1979) introduced the two-step anther culture system, which was further optimized by Dumas de Vaulx et al. (1981). This method has been subsequently modified and enhanced by different laboratories (Mittykó et al. 1995; Dolcet-Sanjuan et al. 1997; Gémes Juhász et al. 1988, 2006; Bárány et al. 2005; Kim et al. 2004) and sporadically applied in different breeding programs (Thomas et al. 2003; Gémes Juhász et al. 2006; Mitykó and Gémes Juhász 2006). The application of DH lines is becoming increasingly important in genetic studies since the use of uniform lines can furnish essential information on the genetic background of qualitative and quantitative traits associated, for example, with pathogen and disease resistance or anthocyanin biosynthesis (Gyulai et al. 2000; Chaim et al. 2003; Sugita et al. 2006).
Despite early promising efforts in integrating anther culture into plant breeding, there are several factors, including manual work, low efficiency, and genotype dependence, that have limited its use. Haploid induction is highly dependent on the tissue culture response of the genotype used (Mittykó et al. 1995), and amenability of anthers to respond to induction is highly influenced by the age of donor plants (Kristiansen and Andersen 1993; Ercan et al. 2006).
Microspore culture offers an alternative means of developing haploid and DH plants. Isolated microspores are cultured in a liquid medium without somatic tissues, and thus embryos and regenerated plants are presumably haploids. Embryogenesis is more easily monitored in isolated microspore cultures than in anther cultures. Direct observation of microspore embryogenesis aids in rapid optimization of culture conditions.
For some species, such as rapeseed (Coventry et al. 1988; Custers et al. 1994), tobacco (Touraev et al. 1996), barley (Davies and Morton 1998; Kasha et al. 2001), and maize (Nageli et al. 1999), establishing isolated microspore cultures has been readily achieved. For some monocots, including wheat (Mejza et al. 1993), durum wheat (Cistue et al. 2006), triticale (Eudes and Amundsen 2005), and barley (Li and Devaux 2001), ovary co-culture has a significant effect on the efficiency of microspore-derived plant production.
Among Solanaceae, tobacco (Nicotiana tabacum L.) is readily amenable for microspore culture (Touraev et al. 1996); whereas, pepper (Capsicum annuum L.) has long been reported to be recalcitrant to haploid induction. Recently, Supena et al. (2006a, b) reported on the first successful microspore culture-derived haploids in Indonesian hot pepper, but without providing experimental details. Later, Kim et al. (2008) reported on establishing haploids from the hot pepper ‘Milyang-jare’ using microspore culture, and optimized plating density of microspores (8 × 104 − 10 × 104) as well as source and amount of added carbon (9% w/v sucrose) in the medium.
In this study, successful establishment of isolated microspore cultures of three Hungarian and three Spanish pepper genotypes is reported using a modified cereal microspore culture protocol (Pauk et al. 2003; Lantos et al. 2005). Microspore-derived embryoids were obtained using wheat ovaries as feeders, and both haploid and DH plants were successfully developed from four different pepper genotypes.
Materials and methods
Plant material and donor plant growth conditions
Three Hungarian, ‘Szegedi 80’, ‘Szegedi 178’, and ‘Remény’, and three Spanish, ‘Jeromin’, ‘Jariza’, and ‘Jaranda’ (obtained from Junta de Extremadura, Servicio de Investigación Agraria, Finca La Orden, Badajoz, Spain), pepper genotypes were used. Seeds were germinated in PVC bags (100 × 180 mm) containing 1:1 peat and sandy soil mix. After ~1 month, seedlings (200 mm in length) were transferred into plastic pots containing 1:1 peat and sandy soil mix, and grown in the greenhouse (Henssler, Beilstein, Germany) under a 16 h photoperiod with a temperature of 25–32°C in the daylight and 15–19°C during the dark. Plants were fertilized once every 2 weeks with Volldünger® [N:P:K:Mg/14:7:21:1, plus 1% microelements: B, Cu, Fe, Mn, and Zn; Magyar Kwizda Ltd., Budapest, Hungary].
Donor bud collection and pretreatment of anthers
Flower buds were collected at four stages of microspore development (tetrads, late uninucleated stage, 80% late uninucleated and 20% early binucleated stages, and pollen grains). The developmental stage of microspores was determined microscopically at 200× magnification under an Olympus CK2 inverted microscope (Olympus Ltd., UK). Flower buds were sterilized for 20 min in an Erlenmeyer flask containing 50 ml 2% NaOCl solution plus one drop of Tween-20, and then rinsed three times with sterile distilled water (Millipore Elix 5). Anthers were isolated directly into 55 mm diameter glass Petri dishes containing 5 ml 0.3 M mannitol solution and 200 mg l−1 cefotaxime. These anthers then underwent heat pretreatment at 32°C in the dark for 7 days.
Isolation protocol and culture of microspores
Microspores were isolated using a protocol previously used for cereal (Pauk et al. 2003; Lantos et al. 2005), but with one modification whereby 30% maltose (Sigma-Aldrich Inc., St. Louis, USA, Cat. M5895) solution was used instead of 21% along with 0.3 M mannitol. Pretreated anthers of a single flower bud contained ~8 × 104 microspores.
Isolated microspores were cultured in 35 mm diameter plastic Petri dishes (Sarstedt Inc., USA, Cat. 83.1800) containing 1.5 ml modified W14 (Ouyang et al. 1989) liquid medium (W14mi) containing 9% maltose, 1,000 mg l−1 glutamine, 0.5 mg l−1 kinetin, 0.5 mg l−1 2,4-Dichlorophenoxyacetic acid, and 200 mg l−1 cefotaxime. The concentration of the microspore suspension was determined using Burker chamber and estimated at 3 × 104 microspores ml−1.
For the Foreign species ovary co-culture (FSOC) method, spikes of wheat (‘CY-45’) ovaries were collected 2 days before pollination, and seven isolated wheat ovaries were added to each Petri dish containing freshly isolated pepper microspore cultures. Petri dishes were incubated at 28°C and 80% humidity, and maintained in the dark for 2 months.
Plantlet regeneration and ploidy level determination
When microspore-derived embryoids were at the bipolar stage of development, 8–10 individual embryoids were transferred to 55 mm diameter Petri dishes containing 8 ml of R1 regeneration medium, consisting of KNO3 2150 mg l−1, (NH4)2SO4 34 mg l−1, Ca(NO3)2 × 4H2O 50 mg l−1, KH2PO4 142 mg l−1, MgSO4 × 7H2O 412 mg l−1, NH4NO3 1238 mg l−1, CaCl2 × 2H2O 313 mg l−1, NaH2PO4 × H2O 38 mg l−1, KCl 7 mg l−1, FeSO4 × 7H2O 27.85 mg l−1, Na2EDTA 37.25 mg l−1, MS micro elements, glycine 0.1 mg l−1, thiamine-HCl 0.55 mg l−1, pyridoxine-HCl 5 mg l−1, nicotinic acid 0.75 mg l−1, meso–inositol 50 mg l−1, Ca–panthotenate 0.5 mg l−1, biotin 0.005 mg l−1, kinetin 0.5 mg l−1, sucrose 30,000 mg l−1, pH 6.1, and Oxoid agar 5.0 6,800 mg l−1 (Dumas de Vaulx et al. 1981). Cultures were maintained at 24°C under a 16 h photoperiod at 100 μmol m−2 s−1 light intensity.
Regenerated plantlets, with 1–2 leaves and with roots, were transferred to glass tubes containing growth regulator-free half-strength MS (Murashige and Skoog 1962) medium with 2% sucrose.
Ploidy of well-rooted plantlets, with 3–4 leaves, was determined by flow cytometry using a ‘PARTEC I’ flow cytometer (Partec GmbH, Münster, Germany). Nuclei were isolated from in vitro-grown leaves of 15 plantlets using the protocol described by Doležel et al. (1989). DNA content of each sample was measured in triplicate. Diploid control samples (cell nucleus isolates) were prepared from leaves of a seed-derived pepper plant (Gémes Juhász et al. 2006).
Chromosome doubling and transfer of plantlets to soil
Those haploid in vitro plantlets, as determined by flow cytometric analysis, were transferred to a growth regulator-free medium containing 400 mg l−1 colchicine for 6 days at 24°C (Gémes Juhász et al. 2006). Following colchicine treatment, roots were washed with tap water for 30 min, and plantlets were then transferred to plastic pots containing 1:1 mixture of non-sterilized peat and sandy soil mix. During the following 2 weeks, plantlets were acclimatized in a growth chamber at a relative humidity of 80%.
Newly-developed leaves from plantlets were subjected to flow cytometric analysis. Prior to flowering, plants were transferred to separate wooden framed boxes covered with mesh cloth, self-pollination was conducted using a sterilized brush, and fruits were later harvested.
Microscopic investigations
To study the developmental stages of isolated pepper microspores, anthers were stained with fluorescein diacetate (FDA) at a final concentration of 1 μg ml−1 for 10 min at room temperature to assess viability of microspores.
Data collection and statistical evaluation
Data on microspore viability were recorded using an Olympus FV1000 Confocal Laser Scanning Microscope (Heslop-Harrison and Heslop-Harrison 1970). The number of viable microspores in different stages was counted in randomly selected visual areas of the microscope in four replications per sample. Each experiment was performed using at least three replicates. ANOVA was conducted using a Minitab® Release 14 statistical software (Minitab Inc., USA), and means were compared using T-tests.
Results
Effect of microspore developmental stage on efficiency of androgenesis induction in isolated pepper microspore cultures
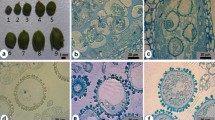
The donor flower buds of two pepper genotypes, ‘Remény’ and ‘Szegedi 80’, were collected, and then grouped into four different developmental stages, based on size of buds and colour of anthers as these served as good indicators of microspore development (Fig. 1). For group 1, primordial petals were fully covered by sepals, anthers were white in color containing tetrad microspores, and buds were dark-green in color. For group 2, petals were already partially visible, and anthers were partially (along one-fourth top surface) purplish in color and containing microspores at late uninucleated stage. For group 3, petals were already well visible, but the size of sepals was twice as long as that of the petals, anthers were purplish in coloration over two-thirds of the surface, and microspores were either at late uninucleated (80%) or early binucleated stages (20%). For group 4, the size of the sepals and petals was equal, anthers were fully purple in color, and contained mature pollen grains.
Description of flower bud/anther morphology and developmental stages of pepper microspores. Collected flower buds were divided into four different groups based on anther morphology and microspore stage of development, (1a, 2a, 3a, 4a; bars = 5 mm). Coloration of anthers are deemed good indicators of stage of microspore development (1b, 2b, 3b, 4b; bars = 1 mm). Stages of microspore development in anthers were determined using a stereo microscope, (1c, 2c, 3c, 4c; bars = 5 μm). Group 1 flower buds consisted of anthers with tetrads (1a–c); group 2 flower buds consisted of anthers with unicellular microspores (2a–c); group 3 flower buds consisted of anthers with 80% uni-nucleate and 20% bi-nucleate microspores (3a–c); and group 4 flower buds consisted of anthers with pollen grains (4a–c)
Following heat pretreatment of anthers, microspores were isolated from the four different groups of buds using maltose/mannitol gradient centrifugation. Viable microspores could not be isolated from groups 1 and 4; while viable microspores were isolated from both groups 2 and 3, at ~8 × 104 viable microspores/bud from each group. Following isolation, developmental stages of pretreated microspores were checked directly by FDA staining (Fig. 2). Pretreated microspores belonging to group 2 consisted mainly of uninucleated, ~90% uninucleated and 10% binucleated, microspores. Pretreated microspores of group 3 were equally divided between uninucleated and binucleated stages of development.
All isolated microspores were cultured in W14mi medium in the presence of wheat ovaries. The number of cell divisions of isolated microspores of group 3 was significantly higher for both ‘Remény’ (60%) and ‘Szegedi 80’ (40%) than that for group 2 ‘Remény’ (50%) and ‘Szegedi 80’ (25%). These microspores produced significantly higher multicellular structures/Petri dish as well as embryoids/Petri dish (Table 1).
Adventitious embryogenesis in isolated microspores of pepper with FSOC
Isolated microspores of ‘Remény’ and ‘Szegedi 80’were incubated in W14mi liquid medium in the presence of either pepper ovaries or wheat ovaries, following the FSOC protocol, or in the absence of any ovaries. First cell divisions were observed inside microspore walls by the end of the first week of culture. In the presence of either wheat or pepper ovaries, multicellular colonies emerging from microspore walls were observed during the second week of culture, but no colonies were observed in microspores incubated in the absence of ovaries.
In the presence of pepper ovaries, multicellular structure development stopped by the third week of culture, and only multicellular structures were detected in these cultures. Whereas in the presence of wheat ovaries, multicellular structures continued to develop (Fig. 3a), and after 5–6 weeks, well-developed embryoids were observed (Fig. 3b). Microspore-derived structures and embryoids were obtained in isolated microspore cultures of all six pepper genotypes with FSOC; while, none produced multicellular structures in the absence of ovaries.
Development of microspore-derived embryoids in the presence of wheat ovaries. a Embryoid-like structures (five in the colony) detected in the presence of wheat ovaries (FSOC protocol); and b influence of wheat ovaries on the development of embryoids; structures are visible to the naked eye in a Petri dish. Wheat ovaries (arrows) in a microspore culture at 6 weeks following culture. Bars = 100 μm for (a), 10 mm for photograph (b)
Effect of genotype on embryoid production and regeneration of pepper plantlets
Significant differences were observed among pepper genotypes for number of embryoids (Table 2). Microspore-derived embryoids were then transferred to the regeneration medium. For well-developed embryoids, apical tissues turned greenish in color; while root-tips along with a few hairy roots were readily detected with the naked eye by the second week following transfer (Fig. 4a). By the third week, most embryoids (73.9%) exhibited either distorted leaf rosettes with abnormal green leaves or did not germinate; however, few embryoids (26.1%) germinated and developed normal shoots.
a Well-developed microspore-derived embryos. b Rooting of shoots in glass tubes. c In vitro plantlets acclimatized to greenhouse conditions. d A microspore-derived DH plant setting fruit. e Fruits containing seeds of fertile plants. Bars = 1 mm for photograph (a), 10 mm for photograph (b), 50 mm for photographs (c–d), 10 mm for photograph (e)
Successful embryoid germination and shoot regeneration was achieved for five genotypes, and there was a significant genotype influence on embryoid germination and number of shoots/Petri dish (Table 2). Regenerated shoots subsequently developed roots on growth regulator-free half-strength MS medium (Fig. 4b). Whole plantlets were obtained from four genotypes, including ‘Jariza’, ‘Jaranda’, ‘Szegedi 80’, and ‘Remény’.
Ploidy analysis revealed that three regenerated plantlets of each of ‘Jariza’, ‘Jaranda’, ‘Szegedi 80’ and ‘Remény’ were spontaneous diploids; while two ‘Jariza’ and one ‘Jaranda’ plantlets were haploid.
The chromosome number of the three haploid plantlets was subsequently doubled using colchicine. Three weeks following colchicine treatment, in vitro plantlets were analyzed for ploidy, and all were diploid. All 12 well-developed spontaneous diploids and the three di-haploid (DH) plantlets were transplanted into pots, and grown in the greenhouse. From all the diploid and DH plantlets, the three plantlets of ‘Jaranda’ and ‘Jariza’ along with a single ‘Szegedi 80’ plantlet survived (Fig. 4c). Following assisted self-pollination in separate cloth frames, all plantlets set fruits with seeds (Fig. 4d, e) were evaluated and checked in the DH1 generation. No detected observed phenological or morphological variation was detected between mother donor plants and DH1 plants.
Discussion
The developmental stage of microspores has a significant effect on androgenesis induction, both in isolated microspore cultures and in anther cultures. For pepper, the recommended developmental stages are different for anther culture, shed, and isolated microspores. For anther cultures, the late uninucleated microspore developmental stage is recommended as the donor stage (Gyulai et al. 2000; Buyukalaca et al. 2004; Ercan et al. 2006). However, Kim et al. (2004) have reported that early binucleated (less than 75%) microspores are most amenable for androgenesis induction. For microspore cultures, Supena et al. (2006a) have used late uninucleated microspores. More recently, Kim et al. (2008) have reported that both late uninucleated and early binucleated microspores enhanced androgenesis in isolated microspore cultures of hot pepper. In this study, optimal developmental stages for establishing isolated microspore cultures, included those anthers containing 80% uninucleated and 20% binucleated microspores. Moreover, a 1 week heat pretreatment in the presence of 0.3 M mannitol is important for harvesting of viable microspores, and similar to findings reported by Supena et al. (2006a). Moreover, this treatment promoted synchronized development of late uninucleated- and binucleated-stage microspores (~50% of each). These microspores were successfully used for androgenesis induction in isolated pepper microspore cultures. Thus, later developmental stages of microspores are recommended for successful induction of androgenesis in pepper (Gyulai et al. 2000; Buyukalaca et al. 2004; Ercan et al. 2006).
Previous reports have indicated that presence of ovaries in the induction medium was not necessary for embryoid production for barley (Davies and Morton 1998; Kasha et al. 2001), rapeseed (Coventry et al. 1988; Custers et al. 1994), tobacco (Touraev et al. 1996), and maize (Nageli et al. 1999), among others. However, for other plant species, including wheat (Mejza et al. 1993), durum wheat (Cistue et al. 2006), and triticale (Eudes and Amundsen 2005), presence of ovaries of the same species in the induction medium enhanced efficiency of microspore culture. Similarly, Li and Devaux (2001) successfully improved embryoid production of isolated barley microspore cultures by co-culturing barley ovaries for those genotypes exhibiting low androgenic response. Adding foreign species-derived ovaries to isolated microspore cultures was also reported to enhance androgenesis in several studies. Microspore-derived wheat embryos were produced through the use of barley ovaries as nurse agents (Bruins et al. 1996); while, co-culture of barley ovaries increased viability of maize microspores (Szarka et al. 2001). Zheng et al. (2003) observed that wheat ovaries significantly improved the efficiency of maize microspore cultures, and Coronado et al. (2005) reported that wheat ovary co-cultures increased efficiency of androgenesis in recalcitrant barley genotypes. In this study, when pepper microspores were incubated in an induction medium in the absence of ovaries, the development of isolated microspores of pepper was arrested by the second week of culture, and no further cell division was observed. Whereas, in the presence of pepper ovaries, some proembryoids were detected during the third week of culture, but these embryoids failed to develop further. However, well-developed microspore-derived embryoids were produced only in the presence of wheat ovaries. Therefore, while ovary co-culture has been successfully used to induce androgenesis in monocots, this is the first report on successful induction of androgenesis using ovary co-culture, and in particular of FSOC, in a dicotyledonous crop, pepper.
Although various studies have reported on the positive effects of various types of ovaries on androgenesis, little is known on the reason(s) for these effects on microspore cultures. Letarte et al. (2006) have reported that arabinogalactans and arabinogalactan proteins excreted by wheat ovaries likely played key roles in inducing androgenesis in wheat microspores. By replacing ovaries with supplemented arabinogalactans and arabinogalactan proteins to wheat microspore cultures, androgenesis is induced in wheat microspores, although the efficiency is lower than that observed for ovaries (Letarte et al. 2006). Therefore, it is likely that arabinogalactans and arabinogalactan proteins of wheat ovaries are also promoting androgenesis in pepper, but further studies should be conducted to elucidate the role of these and other organic compounds and proteins that are influencing the androgenic response in pepper microspore cultures.
Comparison of the efficiencies of different in vitro haploid induction methods, including anther, shed-microspore, and isolated microspore culture, are difficult due to differences in culture conditions. Depending on the plant species and genotype, 1.0–178.2 embryos are produced per 100 anthers in anther cultures (Mittykó et al. 1995; Gyulai et al. 2000; Buyukalaca et al. 2004; Ercan et al. 2006; Kim et al. 2004). For shed-microspore cultures a maximum of 40.8 embryos were induced from a single flower bud (carrying six anthers) in Indonesian hot pepper genotypes (Supena et al. 2006a). Kim et al. (2008) obtained 54 embryos from 10 × 104 microspores isolated from a single donor flower bud. These findings are likely to correspond with the efficiency with haploid plant production. In this study, differences in genotypic responses were detected. Among six responding pepper genotypes, the highest frequency of embryoid production from microspore cultures was observed in ‘Jariza’ (65.75 embryoids/Petri dish); while, the lowest frequency of embryoid production was observed in ‘Szegedi 178’ (3.75 embryoids/Petri dish). This is similar to other studies on induction of androgenesis in anther as well as shed microspore cultures (Mittykó et al. 1995; Gyulai et al. 2000; Ercan et al. 2006; Supena et al. 2006a). In this study, genotypic differences influenced both number of embryoids and regenerated shoots from isolated microspores, and similar to those findings reported in anther cultures (Mittykó et al. 1995; Gyulai et al. 2000; Ercan et al. 2006; Supena et al. 2006a).
Plant regeneration from microspore-derived embryos is one of the most critical steps in pepper microspore culture. In this study, four of the six tested genotypes produced microspore culture-derived diploid plants (0.5–1.25 plants/Petri dish). These results were higher than those reported by Supena et al. (2006a) (0.1 plant/flower bud), but the efficiency of regeneration was lower than that reported by Kim et al. (2008) (4 plants/flower bud). For anther cultures, frequency of plant regeneration is highly variable, 0.29–75.8 plants/100 anthers, depending on the genotype (Mittykó et al. 1995; Gyulai et al. 2000; Ercan et al. 2006). Frequency of plant regeneration for shed microspores ranges between 0.7 and 7.1 plants/bud (Supena et al. 2006a). Additional studies should be conducted to enhance regeneration efficiency of microspore-derived embryoids in pepper, and overcome genotypic observed genotypic differences. In this study, successful chromosome doubling was achieved using colchicine treatment of in vitro-grown plantlets. A total of three dihaploids were obtained, along with four additional diploids obtained via spontaneous doubling of chromosomes. All seven regenerants, derived from ‘Jariza’, ‘Jaranda’, and ‘Szegedi 80’, were successfully acclimatized, and grown in the greenhouse to set fruit and seed. The DH progeny did not exhibit any observed morphological and phenological differences when compared to their mother plants. These new microspore culture-derived DH lines have been integrated into the Hungarian pepper breeding program, and used as parent lines for hybrid seed production.
Abbreviations
- FSOC:
-
Foreign species ovary co-culture
- FDA:
-
Fluorescein diacetate
- DH:
-
Doubled-haploid
References
Bárány I, González-Melendi P, Fadón B, Mitykó J, Risueno MC (2005) Microspore-derived embryogenesis in pepper (Capsicum annuum L.): subcellular rearrangements through development. Biol Cell 97:709–722. doi:10.1042/BC20040142
Bruins MBM, Rakoczy-Trojanowska M, Snijders CHA (1996) Isolated microspore culture in wheat (Triticum aestivum L.): the effect of co-culture of wheat or barley ovaries on embryogenesis. Cereal Res Commun 24:401–408
Buyukalaca S, Comlekcioglu N, Abak K, Ekbic E, Kilic N (2004) Effects of silver nitrate and donor plant growing conditions on production of pepper (Capsicum annuum L.) haploid embryos via anther culture. Eur J Hortic Sci 69:206–209
Chaim AB, Borovsky Y, De Jong W, Paran I (2003) Linkage of the A locus for the presence of anthocyanin and fs10.1, a major fruit-shape QTL in pepper. Theor Appl Genet 106:889–894
Cistue L, Soriano M, Castillo AM, Valles MP, Sanz JM, Echavarri B (2006) Production of doubled haploids in durum wheat (Triticum turgidum L.) through isolated microspore culture. Plant Cell Rep 25:257–264
Coronado MJ, Hensel G, Broeders S, Otto I, Kumlehn J (2005) Immature pollen-derived doubled haploid formation in barley cv. Golden Promise a tool for transgene recombination. Acta Physiol Plant 27(4B):591–599. doi:10.1007/s11738-005-0063-x
Coventry J, Kott L, Beversdorf WD (1988) Manual for microspore culture technique for Brassica napus. University of Guelph, Guelph, pp 1–35
Custers JBM, Cordewener JHG, Nollen Y, Dons HJM, Campagne MMV (1994) Temperature controls both gametophytic and sporophytic development in microspore cultures of Brassica napus. Plant Cell Rep 13:267–271. doi:10.1007/BF00233317
Davies PA, Morton S (1998) A comparison of barley isolated microspore and anther culture and the influence of cell culture density. Plant Cell Rep 17(3):206–210. doi:10.1007/s002990050379
Derera FN, Nagy N, Hoxha A (2005) Condiment paprika research in Australia. J Bus Chem 2:4–18
Dolcet-Sanjuan R, Claveria E, Huerta A (1997) Androgenesis in Capsicum annuum L. Effect of carbohydrate and carbon dioxide enrichment. J Am Soc Hortic Sci 122:468–475
Doležel J, Binorova P, Lucretti S (1989) Analysis of nucler-DNA content in plant-cells by flow-cytometry. Biol Plant 31:113–120. doi:10.1007/BF02907241
Dumas de Vaulx R, Chambonet D, Pochard E (1981) Culture in vitro d’anthères du piment (Capsicum annuum L.): amélior des taux d’obtention de plantes chezdifférents génotypes par des traitments ŕ + 35 C. Agronomie 1:859–864. doi:10.1051/agro:19811006
Ercan N, Sensoy FA, Sensoy S (2006) Influence of growing season and donor plant age on anther culture response of some pepper cultuvars (Capsicum annuum L.). Scientia Hortic 110:16–20. doi:10.1016/j.scienta.2006.06.007
Eudes F, Amundsen E (2005) Isolated microspore culture of Canadian 6× triticale cultivars. Plant Cell Tissue Org 82:233–241. doi:10.1007/s11240-005-0867-9
Gémes Juhász A, Sági Zs, Salamon P, Somogyi N, Zatykó L, Vencel G (1988) Experiences and results of in vitro haploid methods application in pepper breeding programme. Xth EUCARPIA Meeting on Genetics and Breeding of Capsicum and Eggplant Avignon, France, September 7–11. Proceedings:201–203
Gémes Juhász A, Vencel G, Sági Zs, Gajdos L, Kristóf Z, Vági P, Zatykó L (2006) Production of doubled haploid breeding lines in case of paprika, eggplant, cucumber, zucchini and onion. Acta Hortic 725:845–854
George L, Narayanaswamy S (1973) Haploid capsicum through experimental androgenesis. Protoplasma 78:467–470. doi:10.1007/BF01275781
Gyulai G, Gémesné JA, Sági Z, Vencel G, Pintér P, Kristóf Z, Törjék O, Heszky L, Bottka S, Kiss J, Zatykó L (2000) Doubled haploid development and PCR analysis of F-1 hybrid derived DH-2-R paprika (Capsicum annuum L.) lines. J Plant Physiol 156:168–174
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence; intracellular hydrolysis of fluorescein diacetate. Stain Technol 45:115–120
Kasha KJ, Simion E, Oro R, Yao QA, Hu TC, Carlson AR (2001) An improved in vitro technique for isolated microspore culture of barley. Euphytica 120:379–385. doi:10.1023/A:1017564100823
Kim M, Kim J, Yoon M, Choi DI, Lee KM (2004) Origin of multicellular pollen and pollen embryos in cultured anthers of pepper (Capsicum annuum L.). Plant Cell Tissue Org 77:63–72. doi:10.1023/B:TICU.0000016506.02796.6a
Kim M, Jang IC, Kim JA, Park EJ, Yoon M, Lee Y (2008) Embryogenenesis and plant regeneratio of hot pepper (Capsicum annuum L.) through isolated microspore culture. Plant Cell Rep 27:425–434. doi:10.1007/s00299-007-0442-4
Kristiansen K, Andersen SB (1993) Effect of donor plant-temperature, photoperiod, and age on anther culture response of Capsicum annuum L. Euphytica 67:105–109. doi:10.1007/BF00022732
Kuo JS, Wang ZZ, Chien NF, Ku SJ, Kung ML, Hsu HC (1973) Investigation on the anther culture in vitro of Nicotiana tabacum L. and Capsicum annuum L. Acta Bot Sin 15:43–47
Lantos C, Jancsó M, Pauk J (2005) Microspore culture of small grain cereals. Acta Physiol Plant 27(4B):631–639. doi:10.1007/s11738-005-0067-6
Letarte J, Simion E, Miner M, Kasha K (2006) Arabinogalactans and arabinogalacta-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell Rep 24:691–698. doi:10.1007/s00299-005-0013-5
Li H, Devaux P (2001) Enhancement of microspore culture efficiency of recalcitrant barley genotypes. Plant Cell Rep 20:475–481. doi:10.1007/s002990100368
Mejza SJ, Morgant V, DiBona DE, Wong JR (1993) Plant regeneration from isolated microspores of Triticum aestivum. Plant Cell Rep 12:149–153. doi:10.1007/BF00239096
Mittykó J, Andrásfalvy A, Csilléri G, Fáry M (1995) Anther-culture response in different genotypes and F1 hybrids of pepper (Capsicum annuum L.). Plant Breed 114:78–80. doi:10.1111/j.1439-0523.1995.tb00764.x
Mitykó J, Gémes Juhász A (2006) Improvement in the haploid technique routinely used for breeding sweet and spice pepper in Hungary. Acta Agron Hung 54:203–219. doi:10.1556/AAgr.54.2006.2.8
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cutures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nageli M, Schmid JE, Stamp P, Büter B (1999) Improved formation of regenerable callus in isolated microspore culture of maize: impact of carbohydrates, plating density and time of transfer. Plant Cell Rep 19:177–184. doi:10.1007/s002990050730
Ouyang JW, Jia SE, Zhang C, Chen X, Fen G (1989) A new synthetic medium (W14) for wheat anther culture. Annual report. Institute of Genetics, Academia Sinica, Beijing, pp 91–92
Pauk J, Mihály R, Monostori T, Puolimatka M (2003) Protocol of triticale (x Triticosecale Wittmack) microspore culture. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants, a mannual. Kluwer Academic Publishers, Dordrecht, pp 129–134
Sibi M, Dumas de Vaulx R, Chambonnet D (1979) Obtention de plantes haplod’des par androgeňcse in vutro chez le piment (Capsicum annuum L.). Ann Amclior Plantes 29:583–606
Sugita T, Yamaguchi K, Kinoshita T, Yuji K, Sugimura Y, Nagata R, Kawasaki S, Todoroki A (2006) QTL analysis for resistance to phytophthora blight (Phytophtora capsici Leon.) using an intraspecific doubled-haploid population of Capsicum annuum. Breed Sci 56:137–145. doi:10.1270/jsbbs.56.137
Supena EDJ, Suharsono S, Jacobsen E, Custers JBM (2006a) Successful development of a shed-microspore culture protocol for doubled haploid production in Indonesian hot pepper (Capsicum annuum L.). Plant Cell Rep 25:1–10. doi:10.1007/s00299-005-0028-y
Supena EDJ, Muswita W, Suharsono S, Custer JBM (2006b) Evaluation of crucial factors for implementing shed-microspore culture of Indonesian hot pepper (Capsicum annuum L.) cultivars. Scientia Hortic 107:226–232. doi:10.1016/j.scienta.2005.08.006
Szarka B, Dévényi M, Mórocz S (2001) Fertile maize lines obtained from isolated microspores. Euphytica 122:53–60. doi:10.1023/A:1012699332546
Thomas WTB, Forster BP, Gertsson B (2003) Doubled haploids in breeding. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants, a manual. Kluwer Academic Publishers, Dordrecht, pp 95–102
Touraev A, Ilham A, Vicente O, HeberleBors E (1996) Stress-induced microspore embryogenesis in tobacco: an optimized system for molecular studies. Plant Cell Rep 15:561–565. doi:10.1007/BF00232453
Wang YY, Sun CS, Wang CC, Chien NF (1973) The induction of the pollen plantlets of triticale and Capsicum annuum from anther culture. Scientia Sin Vol. XVI 1:147–151
Zheng MY, Weng Y, Sahibzada R, Konzak CF (2003) Isolated microspore culture in maize. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants a manual. Kluwer Academic Publishers, Dordrecht, pp 95–102
Acknowledgments
This work was supported by the Agency for Research Fund Management and Research Exploitation (KPI) and the National Office for Research and Technology (NKTH), project number DA_TECH_05-FPFTT005. The authors thank Erzsébet Juhász Fehér, Éva Kótai, Petra Majer, Mária Olasz, Zsuzsanna Kun and Zsuzsanna Ábrahám Táborosi for their conscientious work and Maria Isabel Garcia Pomar, Council of Extremadura Region Agricultural Research Service, “La Orden” Experimental Station, Badajoz, Spain, for the Spanish pepper genotypes.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lantos, C., Juhász, A.G., Somogyi, G. et al. Improvement of isolated microspore culture of pepper (Capsicum annuum L.) via co-culture with ovary tissues of pepper or wheat. Plant Cell Tiss Organ Cult 97, 285–293 (2009). https://doi.org/10.1007/s11240-009-9527-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9527-9