Abstract
Pogonatherum paniceum (Poaceae) is a perennial plant with good potential for eco-recovery and ornamental function. This study presents in vitro culture systems of simple hormonal regulation of somatic embryogenesis and shoot organogenesis from mature caryopses. Mature caryopses of P. paniceum were grown on Murashige and Skoog medium with 3% sucrose (w/v) and various concentrations or combinations of 2,4-dichlorophenoxyacetic acid (2,4-D), α-naphthaleneacetic acid (NAA) and 6-benzylaminopurine (BAP). Morphological development was analyzed by light microscope after histological sectioning. Four types of callus were induced by different concentrations of 2,4-D. Type I callus was regenerated via somatic embryogenesis; type II callus failed to produce any regeneration; type III callus had both somatic embryogenesis and shoot organogenesis capacities; and type IV callus only displayed shoot organogenesis capacity. Regarding hormone combinations used in this study, NAA only induced type IV callus and BAP only induced direct multiple shoot formation. The combinations of 2,4-D and NAA induced type III callus. Several of the regeneration pathways were simply controlled by one or two kinds of plant hormones. The established systems will be helpful for further research on the developmental mechanism of switch between somatic embryogenesis and shoot organogenesis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The capacity of cultured plant cells, tissues and organs to undergo somatic embryogenesis or shoot organogenesis has provided opportunities for numerous applications of plant development and transformation (Zimmerman 1993; Hicks 1994; Sugiyama 1999; Phillips 2004). Although these two processes have been known for 50 years, plant scientists still understand only a little about what actually controls them. The search for answers might shed light on how cells’ fates become determined during the development process (Vogel 2005). Plant hormones are most likely the candidates in the regulation of developmental switches (Bhaskaran and Smith 1990; Fehér et al. 2003). Studying the relationships between plant hormones and explants leading to morphogenesis will be a practicable approach for this issue.
Auxins and cytokinins are the two main growth regulators in plants involved in the regulation of division and differentiation (Fehér et al. 2003) and have been proved to play an important role in the induction of somatic embryogenesis and shoot organogenesis (Gaspar et al. 1996). Generally, in dicots, for the same kinds of explants, auxins with or without low levels of cytokinins have often been reported to induce somatic embryogenesis. Elevated cytokinin levels or cytokinin alone are usually required for the induction of shoot organogenesis (Matsuoka and Hinata 1979; Tabei et al. 1991; Tang et al. 2000; Nikolić et al. 2006). In some studies, the potential mechanisms of these two types of plant hormones controlling the switch between embryogenic and organogenic pathways have been further studied (Charriére et al. 1999; Thomas et al. 2004). However, in monocots, especially in graminaceous species, the plantlet regeneration was considered mainly performed via somatic embryogenesis (Vasil 1982). Evidences of morphogenic pathways in the same kind of explants under the control of auxins and/or cytokinins were only observed in few species, such as sorghum (Bhaskaran and Smith 1989), Bouteloua gracilis (Aguado-Santacruz et al. 2001), minor millet (Paspalum scrobiculatum) (Vikrant 2002, 2003), sugarcane (Lakshmanan et al. 2006), Neyraudia arundinacea (Ma et al. 2007). Although combinations of plant hormones required for different morphogenic pathway were similar to dicots in those studies, the regulation processes were more complicated and the studies on regulation mechanisms were much fewer.
Pogonatherum paniceum (Poaceae) is a perennial plant distributed in temperate, tropical and subtropical regions of Asia, Oceania and Africa (Clayton et al. 2002). Due its attractive appearance and strong adaptation to various ecosystems, it has being exploited as an eco-recovery and ornamental pot plant (Wang et al. 2007). Hassan and Debergh’s study (1987) and our (Wang et al. 2006) previous works have proved that P. paniceum explants can regenerate plantlets via somatic embryogenesis or direct shoots formation. This paper further presents somatic embryogenesis and/or shoot organogenesis from four types of callus and direct shoot organogenesis from mature caryopsis in vitro culture of P. paniceum and their simple regulation by 2,4-dichlorophenoxyacetic acid (2,4-D), α-naphthaleneacetic acid (NAA) and 6-benzylaminopurine (BAP).
Materials and methods
Plant material
The spikes of P. paniceum were collected from the field in Renshou, Sichuan Province, China and air dried for a week at room temperature (25 ± 2°C). After husking, relatively large and healthy-looking caryopses were selected for experiments.
After surface sterilized with 1% solution of sodium hypochlorite (v/v) for 5 min and five times washed in sterile deionized water, the caryopses were cultured on induction medium.
Media and induction
Explants of sterilized caryopses were cultured on solid Murashige and Skoog (1962) basal medium (MS) containing 3% sucrose (w/v) and 0.8% agar (w/v) with different plant hormone combinations. The medium was adjusted to pH 5.8 before autoclaving at 121°C for 20 min. The culture protocols are presented in Fig. 1.
For callus induction, various concentrations of 2,4-D, NAA were used alone and in combinations (Tables 1, 3).
For direct shoot formation, various concentrations of BAP were used (Table 4).
Plant regeneration
For the plant regeneration, the callus induced by high levels of 2,4-D and the combinations of 2,4-D and NAA were put on the MS basal medium without any plant hormones.
The shoots induced by BAP, NAA or low levels of 2,4-D were separated and rooted on MS or half strength MS basal medium with different concentrations of NAA when they were about 1 cm in height.
Plants reaching about 10 cm in height were acclimatized in greenhouse conditions for 1 week. Then they were washed in water to remove agar and transferred to pots containing garden soil and vermiculite (2:1).
Culture conditions
Cultures were incubated in the dark at 26 ± 1°C for callus induction and under 12/12 h (light/dark) photoperiod conditions with a light intensity of approximately 40 μmol m−2 s−1 provided by cool, white fluorescent tubes (40 W, Phillips, China) for shoots induction and plant regeneration and growth. The cultures incubated with NAA alone and low concentrations of 2,4-D (less than 0.1 mg l−1) were under dark conditions for callus induction, and when the callus developed into shoots, they were transferred to 12/12 h (light/dark) photoperiod conditions.
Histological observation
The calli were vacuum-infiltrated for 20 min with FAA (formalin:acetic acid:ethanol:water in a ratio of 5:5:45:45 v/v) and fixed for 7 days at room temperature (25 ± 2°C). Fixed tissues were washed in running water for 10 min and dehydrated in a gradual ethanol/xylene series of solutions (ethanol: 70, 85, 95, 100%, and ethanol: xylene in a ratio of 50:50 and 100% xylene). Sections (10 μm) of paraffin wax embedded material were obtained using a sliding microtome, affixed to glass slides with albumin, stained with hematoxylin, mounted in Canada balsam, and covered with a cover glass for microscopic observations.
Statistical analysis
All the experiments were laid out in completely randomized design with at least five repetitions; in each replicate 20–30 caryopses were used.
Induction percentage is expressed as the average percentage of caryopses that developed into callus, shoots or normal seedlings divided by the number of total caryopsis explants. Rooting percentage is expressed as the average percentage of shoots that rooted divided by the number of total shoots tested. Germination percentage is expressed as the average percentage of caryopses that germinated divided by the number of total caryopses tested. All percentage values were arcsine-transformed prior to analysis to normalize the data and reduce heteroscedasticity, while untransformed values are presented in the tables.
Counts of shoots per explants and length of shoots are presented as the mean ± standard error. The data were subjected to a standard analysis of variance (ANOVA). The LSD test was used to estimate significant differences among mean values of the treatments.
Results
After inoculation on hormone-free MS medium for about 5 days, caryopses germinated readily and eventually developed into normal plants. The germination rate was of 50% (Tables 2, 4). One caryopsis only resulted in one seedling.
Callus induction
After 9–11 days of incubation, the primary callus appeared on the micropylar end of the caryopsis on MS medium with various combinations of plant hormones. One month later, all of the calli were between 0.5 and 0.8 mm in diameter. The calli were then classified under the following four types (Fig. 2a–f):
Callus induction and plantlet regeneration from mature caryopses of Pogonatherum paniceum. (a–f) The four types of callus induced by auxin: (a, b) Type I; (c) Type II; (d, e) Type III; (f) Type IV. (g) A developing somatic embryo on hormone-free MS medium. (h) Plantlets with shoots and roots from type I callus. (i) Detached plantlets from somatic embryo with roots on base of shoots. (j) Rooted-shoots from type IV. (k) Regenerated plants in pots. Bars in a, d = 1 mm; b, c, f, G = 0.5 mm; e = 0.2 mm; h = 5 mm; i, j = 1 cm; k = 5 cm
The first type of callus (embryogenic callus, type I; Fig. 2a, b) was compact, highly nodular and white or yellow-white in appearance. Histological studies showed that there were a lot of fuscous meristemoid in the callus, which could develop into bipolar mature somatic embryo through globule stage, pear-shaped stage and scutiform stage. This bipolar somatic embryo had no vascular connection with maternal tissue (Fig. 3b–e).
Histological observation of somatic embryo development of Pogonatherum paniceum. (a) Shoots (sh) with vascular strains (vs) connected to the type III callus and fuscous meristemoid (fm). (b) Global embryo (ge) in type I callus. (c) Somatic embryo in pear-shaped stage. (d, e) Somatic embryo with scutellum (sc), shoot meristem (sm) and root meristem (rm). Bars in a, d = 200 μm; b, c, e = 100 μm
The second type of callus (non-regeneration callus, type II; Fig. 2c) looked non-nodular, soft, friable, mucilaginous, and yellow or brown-yellow, which occurred along with the embryogenic callus or the third type (type III) described below in most of the explants. The type II callus did not give rise to any organogenesis.
Type III callus looked hard and pale yellow, with both nodules and bud-like structures on the surface (Fig. 2d, e). The meristemoid in the outer layer of callus developed into unipolar shoot rudimental organ without root apical meristem which connected to callus tissue through vascular strains. Subsequently, they regenerated into shoots after being transferred to hormone-free MS medium (Fig. 3a). At the same time, lots of fuscous meristemoid occurred in inner layer of callus and could develop into the bipolar somatic embryo as type I callus.
The fourth type of callus (organogenic callus, type IV) was yellowish and hard (Fig. 2f). Because the callus could directly differentiate into multiple shoots on the induction medium immediately, the period of callus phase (3–5 days) was short.
Effects of 2,4-D on callus types and in vitro morphogenesis
The four types of callus described above from P. paniceum mature caryopses were induced by 2,4-D as the sole plant hormone. The callus type changed from type I to type IV with the decreasing concentrations of 2,4-D. Concentrations of 2,4-D higher than 1 mg l−1 favoured type I and type II callus, but inhibited type III and IV callus; when the concentrations were over 2 mg l−1, the induction rate of type I callus decreased with the increasing concentrations of 2,4-D. The optimal concentration of 2,4-D for somatic embryogenesis was 2 mg l−1. When 2,4-D concentrations went down from 1 to 0.1 mg l−1, the rate of type III callus increased from 20.84 to 32.36%. But when 2,4-D concentrations were less than 0.05 mg l−1, only type IV callus occurred (Table 1).
Effects of NAA on indirect multiple shoot formation
Unlike 2,4-D, all the NAA concentrations tested in this study only induced the type IV callus (Fig. 2f, Table 2). At low concentrations of NAA, few caryopses produced callus. The callus induction rates increased along with the increasing NAA concentrations. When the concentrations were more than 2 mg l−1, the induction rates maintained at 50%. But the number of shoots per explant was also affected by the NAA concentrations; the highest number happened at 1 mg l−1. These results suggested the optimal NAA concentration for type IV callus induction and shoot regeneration was 1 mg l−1 (Table 2).
Effects of 2,4-D and NAA combinations on in vitro morphogenesis
No matter that the ratios of 2,4-D to NAA were low or high, in the five combinations we tested, type I and IV callus vanished and only type II and type III callus appeared. The total callus induction rates of each treatment maintained at 50%, but the rates of type III callus decreased along with increasing 2,4-D concentrations in the five combinations (Table 3). The induction rates of type III callus in the medium with the NAA and 2,4-D combinations were higher than with 2,4-D alone in corresponding concentrations, whereas the rates of type II callus were lower than 2,4-D alone (Tables 1, 3).
Effects of BAP on direct shoots organogenesis
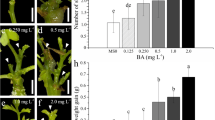
After 3–5 days’ culture on MS medium containing BAP as the sole plant hormone, the caryopses germinated normally (Fig. 4a). The germination rates (50%) at different concentrations were no significantly different except the ones with more than 10 mg l−1 (Table 4).
The seedlings were 0.5 to 0.8 cm in height after 3 weeks. Then, the basal part of some seedlings began swelling (Fig. 4b) and some bud-like structures appeared on the surface, which developed into multiple shoots (Fig. 4c–e) 1 month later. The seedlings without swelling (about 5 to 20%) did not develop further and died after 2 months (Fig. 4f).
The percentage of caryopses producing multiple shoots increased with the concentrations of BAP within a range of 0 to 1 mg l−1; but decreased along with the higher BAP concentrations (more than 1 mg l−1). Average shoot length decreased from 4.5 cm to 1 cm with the increased BAP concentration (0.2 to 15 mg l−1) on MS medium (Table 4).
Plant regeneration and rooting of shoots
On hormone-free MS basal medium, the nodular structure on the surface of type I and III callus developed into mature embryos (Fig. 2g) and further into plantlets by elongating and rooting shoots (Fig. 2h, i). For the initiation and development of somatic embryo were not synchronous, about 100–200 shoots grew from a piece of callus between 5 mm and 8 mm in diameter after 1 month. When the plantlets reached about 1 to 2 cm after 1 month, they were transferred to fresh MS basal medium for growth. The bud-like structure on surface of the type III callus developed into shoots without roots. In contrast, no plant regeneration occurred in callus type II on hormone-free MS basal medium after more than 2 months.
The multiple shoots (about 2–3 cm) induced by NAA, BAP (Fig. 4e) or from type III callus were excised and transferred to the rooting medium. The shoots rooted after 1 week and the roots were about 3 to 5 cm in length 2–3 weeks later. The regenerated roots were from the nodes of shoots (Fig. 2j), unlike the roots of normal seedlings from caryopses germination or plantlets derived from somatic embryo, which are from the root apical meristem (Fig. 2i). All the media tested induced rooting, among which the half-strength basal MS media with 0.5 mg l−1 NAA induced more than 90% of rooting (Table 5).
About 90.25% of the regenerated plants survived when they were acclimatized for 7 days and transferred to soil (Fig. 2k).
Discussion
The high frequency plantlets regeneration has been reported from different vegetative parts (leaves, seeds, seedlings, and stems) of P. paniceum in previous papers (Hassan and Debergh 1987; Wang et al. 2006, 2007). In this study, we further observed that somatic embryogenesis and/or shoot organogenesis occurred not only in the same piece of callus but also in different types of callus from mature caryopses explants and the direct shoots organogenesis form seedlings germinated on induction medium.
Caryopses explants of P. paniceum in our culture conditions produced four types of callus with different appearances and inner structures. These calli developed obeying three basic developmental patterns. First, the type I callus conducted somatic embryogenesis program. In this developmental pattern somatic embryo has two meristems, viz. shoot meristem and root meristem. Second, the type IV callus exerted unipolar organ redifferentiation (shoot organogenesis); in this pattern only shoot rudimental organ appeared. And the third, the type II callus only proliferated but did not further regenerate into plantlets, viz. development-termination. The type III callus displayed mixed developmental pathways with both somatic embryogenesis and shoot organogenesis. Although, each of the three programs have been observed in other graminaceous species, however, each of those researches only presented one or two pattern from same kind of explants in one species (Hassan and Debergh 1987; Kothari and Varshney 1998; Yemets et al. 2003; Ncanana et al. 2005). Therefore, we can suggest that diversity of developmental programs exist in cultured plant explants and that the developmental orientation in P. paniceum mature caryopsis explants is more easily reprogrammed than in other graminaceous species studied previously.
Auxin and cytokinin, the very important plant hormones that regulate many aspects of growth and development (Mok 1994; Woodward and Bartel 2005; Sakakibara 2006), are usually employed for dedifferentiation and redifferentiation of explants during plant tissue cultures. Generally, auxin is used for callus induction and proliferation, and both cytokinin and auxin are required for redifferentiation of callus (Gaspar et al. 1996). Shoots were produced at high cytokinin to auxin ratios, while roots were formed when the ratios were reversed (Che et al. 2006). However, P. paniceum caryopses explants here presented some distinct characters, all four types of callus with different developmental fates could be induced by synthetic auxin 2,4-D as the sole plant hormone. High concentrations of 2,4-D induced type I callus and low levels of 2,4-D stimulated type IV callus formation for shoot development (Table 1). On the other hand, NAA, another important synthetic auxin used as the sole hormone in induction medium only induced type IV callus. If 2,4-D supplemented with NAA was added into callus induction medium, mid-fated development pathways appeared, viz. type III and type II callus were induced.
Therefore, from the experiments described here we deduce the following hypotheses: (1) 2,4-D concentrations controlled the developmental pathways. Low levels of 2,4-D initiate shoot development, and only 2,4-D rising to an opportune concentration, cause root development programs to be initiated. (2) Except for the positive effect on stimulating development of explants, 2,4-D is a two-edged regulator since it inhibits further embryonic development (Steward et al. 1958; Charriére et al. 1999; Che et al. 2006). It is necessary that the type I and type III callus should be transferred to auxin-free MS medium for regeneration. And much higher levels of 2,4-D inhibited the embryonic callus formation, induced more type II callus without plantlets regeneration. (3) Compared with 2,4-D, NAA is less active on embryogenic callus induction, in this study it only stimulated shoot development. Similar results were reported in pennisetum (Talwar and Rashid 1990) and carrot (Ribnicky et al. 1996). (4) There may exist negative interactions in some extent between 2,4-D and NAA in morphogenesis of P. paniceum callus. 2,4-D weakens or erases the functions of NAA, and vice versa.
Many studies have confirmed that auxins like 2,4-D and NAA can activate auxin response factors (ARF) (Inukai et al. 2005) and auxin signal pathways such as Rac-like GTPases (Tao et al. 2002) and regulate a lot of genes related to growth and development (Che et al. 2002, 2006; Pischke et al. 2006), such as the LEC1 gene (Yazawa et al. 2004), prha gene (Plesch et al. 1997).
On the other hand, the cytokinin usually used in plant tissue culture for plant regeneration from callus (Rout et al. 2006) was not necessary for the in vitro regeneration of P. paniceum, in which callus could directly regenerate on plant hormone-free MS basal medium. If cytokinin BAP replaced 2,4-D or NAA in callus induction medium, it markedly stimulated multiple shoot formation of P. paniceum caryopses cultures (no visible callusgenesis) while not affecting the caryopses germination (except concentration of BAP more than 10 mg l−1). Also, BAP inhibited shoot elongation (Table 4, Fig. 4f), which is in accordance with the previous research result in plant Dendrocalamus asper (Arya et al. 1999) and could be understood with decreasing apical dominance or lateral bud dormancy (Gaspar et al. 1996; Mok and Mok 2001).
As a conclusion, indirect somatic embryogenesis and/or indirect shoot organogenesis and direct organogenesis were simply controlled by one or two kinds of plant hormones. As the sole plant hormone in induction medium, the most suitable concentration of 2,4-D for indirect shoot organogenesis was 0.05 mg l−1and for somatic embryogenesis was 2 mg l−1; the optimal concentration for type III callus with both indirect somatic embryogenesis and for indirect shoot organogenesis was 0.1 mg l−1. But in the whole studies, 1 mg l−1 NAA was the optimal concentration for inducing indirect shoot organogenesis and 1 mg l−1 BAP was best for direct multiple shoot formation. The combination of 2 mg l−1 NAA and 1 mg l−1 2,4-D was most suitable for type III callus. The simple regulation system of morphogenesis pathways will be helpful for further research works to study the relationship between plant hormones and in vitro explants development.
Abbreviations
- BAP:
-
6-Benzylaminopurine
- 2, 4-D:
-
2,4-Dichlorophenoxyacetic acid
- NAA:
-
α-Naphthaleneacetic acid
- MS:
-
Murashige and Skoog
References
Aguado-Santacruz GA, Cabrera-Ponce JL, Olalde-Portugal V, Sánchez-González MR, Márquez-guzmán J, Herrera-Estrella L (2001) Tissue culture and plant regeneration of blue grama grass, Bouteloua gracilis (H.B.K.) Lag. ex Steud. In Vitro Cell Dev Biol Plant 37:182–189
Arya S, Sharma S, Kaur R, Arya ID (1999) Micropropagation of Dendrocalamus asper by shoot proliferation using seeds. Plant Cell Rep 18:879–882. doi:10.1007/s002990050678
Bhaskaran S, Smith RH (1989) Control of morphogenesis in sorghum by 2, 4-dichlorophenoxyacetic acid and cytokineins. Ann Bot (Lond) 64:217–224
Bhaskaran S, Smith RH (1990) Regeneration in cereal tissue culture: a review. Crop Sci 30:1328–1336
Charriére F, Sotta B, Miginiac É, Hahne G (1999) Induction of adventitious or somatic embryos on in vitro cultured zygotic embryos of Helianthus annuus: variation of endogenous hormone levels. Plant Physiol Biochem 37:751–757. doi:10.1016/S0981-9428(00)86688-7
Che P, Gingerich DJ, Lall S, Howell SH (2002) Global and hormone-induced gene expression changes during shoot development in Arabidopsis. Plant Cell 14:2771–2785. doi:10.1105/tpc.006668
Che P, Lall S, Nettleton D, Howell SH (2006) Gene expression programs during shoot, root, and callus development in Arabidopsis tissue culture. Plant Physiol 141:620–637. doi:10.1104/pp.106.081240
Clayton WD, Harman KT, Williamson H (2002) World grass species: descriptions, identification, and information retrieval. http://www.kew.org/data/grasses-db.html. Accessed 18 December 2006; 17:10 GMT
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74:201–228. doi:10.1023/A:1024033216561
Gaspar T, Kevers C, Penel C, Greppin H, Reid DM, Thorpe TA (1996) Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell Dev Biol Plant 32:272–289. doi:10.1007/BF02822700
Hassan AAE, Debergh P (1987) Embryogenesis and plantlet development in the bamboo Phyllostachys virdis (Young) McClure. Plant Cell Tiss Org Cult 10:73–77. Corrected in 1988. Plant Cell Tissue Organ Cult 15:93
Hicks GS (1994) Shoot induction and organogenesis in vitro: a developmental perspective. In Vitro Cell Dev Biol Plant 30:10–15. doi:10.1007/BF02632113
Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I et al (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17:1387–1396. doi:10.1105/tpc.105.030981
Kothari SL, Varshney A (1998) Morphogenesis in long-term maintained immature embryo-derived callus of wheat (Triticum aestivum L)-Histological evidence for both somatic embryogenesis and organogenesis. J Plant Biochem Biotechnol 7:93–98
Lakshmanan P, Geijskes RJ, Wang L, Elliott A, Grof CPL, Berding N et al (2006) Developmental and hormonal regulation of direct shoot organogenesis and somatic embryogenesis in sugarcane (Saccharum spp. interspecific hybrids) leaf culture. Plant Cell Rep 25:1007–1015. doi:10.1007/s00299-006-0154-1
Ma G, Wu G, Bunn E (2007) Somatic embryogenesis and adventitious shoot formation in Burma reed (Neyraudia arundinacea Henr.). In Vitro Cell Dev Biol Plant 43:16–20. doi:10.1007/s11627-006-9010-9
Matsuoka H, Hinata K (1979) NAA-induced organogenesis and embryogenesis in hypocotyl callus of Solarium melongena L. J Exp Bot 30:363–370. doi:10.1093/jxb/30.3.363
Mok MC (1994) Cytokinins and plant development-an overview. In: Mok DWS, Mok MC (eds) Cytokinins: chemistry, activity, and function. CRC Press, pp 155–166
Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118. doi:10.1146/annurev.arplant.52.1.89
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Ncanana S, Brandt W, Lindsey G (2005) Development of plant regeneration and transformation protocols for the desiccation-sensitive weeping lovegrass Eragrostis curvula. Plant Cell Rep 24:335–340. doi:10.1007/s00299-005-0940-1
Nikolić R, Mitić N, Miletić R, Nešković M (2006) Effects of cytokinins on in vitro seed germination and early seedling morphogenesis in Lotus cornicelatus L. J Plant Growth Regul 25:187–194. doi:10.1007/s00344-005-0129-4
Phillips GC (2004) In vitro morphogenesis in plants-recent advances. In Vitro Cell Dev Biol Plant 40:342–345. doi:10.1079/IVP2004555
Pischke MS, Huttlin EL, Hegeman AD, Sussman MR (2006) A transcriptome-based characterization of habituation in plant tissue culture. Plant Physiol 140:1255–1278. doi:10.1104/pp. 105.076059
Plesch G, Stormann K, Torres JT, Walden R, Somssich IE (1997) Development and auxin-induced expression or the Arabidopsis prha homeobox gene. Plant J 12:635–647. doi:10.1046/j.1365-313X.1997.00635.x
Ribnicky DM, Ilić N, Cohen JD, Cooke TJ (1996) The effects of exogenous auxins on endogenous indole-3-acetic acid metabolism. Plant Physiol 112:549–558
Rout GR, Mohapatra A, Jain SM (2006) Tissue culture of ornamental pot plant: a critical review on present scenario and future prospects. Biotechnol Adv 24:531–560. doi:10.1016/j.biotechadv.2006.05.001
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449. doi:10.1146/annurev.arplant.57.032905.105231
Steward F, Mapes M, Mears K (1958) Growth and organized development of cultured cell. Am J Bot 45:705–708. doi:10.2307/2439728
Sugiyama M (1999) Organogenesis in vitro. Curr Opin Plant Biol 2:61–64. doi:10.1016/S1369-5266(99)80012-0
Tabei Y, Kanno T, Nishio T (1991) Regulation of organogenesis and somatic embryogenesis by auxin in melon, Cucumis melo L. Plant Cell Rep 10:225–229. doi:10.1007/BF00232563
Talwar M, Rashid A (1990) Factors affecting formation of somatic embryos and embryogenic callus from unemerged inflorescences of a graminaceous crop Pennisetum. Ann Bot (Lond) 66:17–21
Tang H, Ren Z, Krczal G (2000) Somatic embryogenesis and organogenesis from immature embryo cotyledons of three sour cherry cultivars (Prunus cerasus L.). Sci Hortic (Amsterdam) 83:109–126. doi:10.1016/S0304-4238(99)00073-4
Tao LZ, Cheung AY, Wu HM (2002) Plant Rac-Like GTPases are activated by auxin and mediate auxin-responsive gene expression. Plant Cell 14:2745–2760. doi:10.1105/tpc.006320
Thomas C, Meyer D, Himber C, Steinmetz A (2004) Spatial expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol Biochem 42:35–42. doi:10.1016/j.plaphy.2003.10.008
Vasil IK (1982) Somatic embryogenesis and plant regeneration in cereals and grasses. In: Fujiwara A (ed) Plant tissue culture. Japanese Association of Plant Tissue Culture, Tokyo, p 107
Vikrant RA (2002) Induction of multiple shoots by thidiazuron from caryopsis cultures of minor millet (Paspalum scrobiculatum L.) and its effect on the regeneration of embryogenic callus cultures. Plant Cell Rep 21:9–13. doi:10.1007/s00299-002-0466-8
Vikrant RA (2003) Somatic embryogenesis or shoot formation following high 2, 4-D pulse-treatment of mature embryos of Paspalum scrobiculatum. Biol Plant 46:297–300. doi:10.1023/A:1022875332607
Vogel G (2005) How does a single somatic cell become a whole plant? Science 309:86. doi:10.1126/science.309.5731.86
Wang WG, Wang SH, Zhuang GQ, Chen F (2006) In vitro rapid propagation of rock plant Pogonatherum paniceum (Lam.) Hack. Plant Physiol Commun 42:482
Wang WG, Wang SH, Wu XA, Jin XY, Chen F (2007) High frequency plantlet regeneration from callus and artificial seed production of rock plant Pogonatherum paniceum (Lam.) Hack. (Poaceae). Sci Hortic (Amsterdam) 113:196–201. doi:10.1016/j.scienta.2007.03.006
Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95:707–735. doi:10.1093/aob/mci083
Yazawa K, Takahata K, Kamada H (2004) Isolation of the gene encoding carrot leafy cotyledon1 and expression analysis during somatic and zygotic embryogenesis. Plant Physiol Biochem 42:215–223. doi:10.1016/j.plaphy.2003.12.003
Yemets AI, Klimkina LA, Tarassenko LV (2003) Efficient callus formation and plant regeneration of goosegrass Eleusine indica (L.) Gaertn. Plant Cell Rep 21:503–510
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgements
Financial support of key project of science and technology research of Ministry of Education of China (No.104255). We thank Dr. Shui Wang and Erik Chavez for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Zhao, X., Zhuang, G. et al. Simple hormonal regulation of somatic embryogenesis and/or shoot organogenesis in caryopsis cultures of Pogonatherum paniceum (Poaceae). Plant Cell Tiss Organ Cult 95, 57–67 (2008). https://doi.org/10.1007/s11240-008-9414-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9414-9