Abstract
Highly efficient genetic transformation protocols and the regeneration of transgenic plants of Sugraone and Crimson Seedless grapevines (Vitis vinifera L.) were achieved from embryogenic calli co-cultured with low Agrobacterium tumefaciens densities. The sensitivity of embryogenic cultures to kanamycin, as well as the effect of Agrobacterium strains, C58(pMP90) or EHA105, and the bacterial concentration (0.06 or 0.2 at Optical Density OD600) on transformation efficiency were studied. Embryogenic cultures showed different kanamycin sensitivities and the total suppression of embryo differentiation at 20 and 50 mg/l kanamycin for Crimson Seedless and Sugraone, respectively. sgfp gene expression was evaluated in callus co-cultured with each bacterial strain. Although GFP transient expression was higher with A. tumefaciens EHA105 in both cultivars at the beginning of the culture, there were no significant differences 28 days post-inoculation. However, the concentration of Agrobacterium did affected transformation efficiency: 0.06 OD600 being more effective for the transformation of Crimson Seedless and 0.2 OD600 for Sugraone. By following the optimised procedure, 21 and 26 independent transgenic plants were generated from Sugraone and Crimson Seedless respectively, three to five months post-infection. PCR analyses were carried out to verify the integration of the sgfp and nptII genes into grapevine genome and the stable integration of the sgfp gene was confirmed by Southern blot.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Genetic engineering for grapevine improvement is a powerful tool for introducing disease resistance, product quality and production efficiency traits without altering the essential characters and identity of the cultivar in question (Kikkert et al. 2001). There are two main prerequisites for achieving an efficient Agrobacterium- mediated transformation system: (1) the availability of plant regenerative tissue and (2) an efficient transformation protocol.
Since grapevine embryogenic cultures appear to be the best cell source for transgenic plant regeneration (Martinelli 1997; Martinelli and Mandolino 2001), efforts increasingly focus on improving transformation efficiency using embryogenic calli as starting plant material. The efficiency of the transformation protocol depends on many factors, including the grapevine genotype used (Harst et al. 2000; Torregrosa et al. 2002), the selection strategies (Perl et al. 1996; Spielmann et al. 2000; Yamamoto et al. 2000; Legrand et al. 2003; Wang et al. 2005; Fan et al. 2008), the Agrobacterium strain (Baribault et al. 1989; Berres et al. 1992; Torregrosa et al. 2002), the culture method (Harst et al. 2000; Oláh et al. 2003a) and whether or not antioxidants are used (Mozsár et al. 1998; Perl et al. 1996; Li et al. 2006). The bacterial concentration is also an important factor affecting transformation efficiency although in few cases were comparisons of bacterial density made (Perl et al. 1996; Agüero et al. 2006). A low plant regeneration rate could also be due to a hypersensitivity-like reaction of the plant tissue to Agrobacterium in combination with the treatment used for selection (Perl et al. 1996; Bornhoff et al. 2005). The transformation and regeneration of transgenic grapevine plants from somatic embryos remains a problem (Iocco et al. 2001) at least for some economically important cultivars. Previously we developed an efficient method for somatic embryo recovery and plant regeneration from cvs Crimson Seedless and Sugraone (López-Pérez et al. 2005, 2006).
Crimson Seedless and Sugraone (Superior Seedless®) are two seedless table grape developed by David Ramming and Ron Tarailo (USDA Fruit Genetics and Breeding Research Unit, Fresno, CA) and Sun World International respectively. These two seedless grapes are among the most important and widely appreciated table grape cultivars for supermarkets worldwide because to its excellent eating characteristics. In many areas, young vines of Crimson Seedless and Sugraone have developed a disease condition caused by the presence of some viruses like Grapevine fanleaf virus (GFLV), Grapevine leafroll-associated virus 1, Grapevine leafroll-associated virus 2, Grapevine leafroll-associated virus 3 (GLRaV-1, GLRaV-2, GLRaV-3), Grapevine virus A (GVA), Grapevine virus B (GVB), and Grapevine fleck virus (GFkV) (Prodan et al. 2003; Ahmed et al. 2004; Digiaro et al. 2006). Moreover, nearly all Crimson Seedless vineyards in warm regions require Ethrel (ethephon) in the véraison for optimum color development, while the application of GA at bloom reduces fruitset and increases berry length and berry weight but also reduces berry color, increases berry shatter, and decreases return fruitfulness (Dokoozlian and Peacock 2001; Avenant and Avenant 2006). The development of specific genetic transformation systems would allow the introduction of genes that confer resistance to biotic or abiotic stresses (Baldoni and Rugini 2002). Moreover, efficient genetic transformation systems are valuable tools for functional genomic studies, now that grape genome has been sequenced by the French-Italian Public Consortium for Grapevine Genome Characterization (Jaillon et al. 2007).
Our aim, therefore, was to determine the most suitable kanamycin concentration as well as the Agrobacterium strain and bacterial concentration best suited in order to establish efficient transformation protocols in the seedless table grapevine cultivars Crimson Seedless and Sugraone. To our knowledge, this is the first report that transgenic plants have been regenerated from Crimson Seedless.
Materials and methods
Plant material
Two seedless table grapevine cultivars (Sugraone and Crimson Seedless) were used. Embryogenic calli were induced from immature anthers as previously described by López-Pérez et al. (2005) and were maintained by monthly transfers onto fresh 4/1.3 culture medium.
Kanamycin sensitivity of embryogenic cultures
In order to establish a kanamycin concentration that completely inhibits somatic embryo differentiation, embryogenic calli were cultured onto petri dishes (50 × 15 mm) with 10 ml ½ MSAC medium (López-Pérez et al. 2005) with different concentrations of this antibiotic (5, 10, 20, 30, 40 and 50 mg/l). As controls, embryogenic calli cultured onto ½ MSAC medium, with or without cefotaxime (300 mg/l) were used. Twenty calli were used for each treatment and experiment was repeated twice. Cultures were maintained in the dark at 25°C. After 15 and 30 days, the percentage of calli that differentiated somatic embryo was scored for each treatment.
Bacterial strains and plasmid
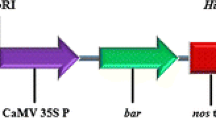
Agrobacterium tumefaciens strains C58(pMP90) (Koncz and Schell 1986), a disarmed derivative of C58, and EHA105 (Hood et al. 1993), a disarmed derivative of A281, both carrying the binary plasmid pBin19-sgfp (Chiu et al. 1996), were used for in vitro transformation of grapevine embryogenic callus. The T-DNA of pBin 19-sgfp contains the NOSpro-nptII-NOSter cassette, which is used as a selectable marker, and the 35Spro-sgfp-35Ster cassette, which is used as a reporter marker (Ghorbel et al. 1999).
Effect of Agrobacterium tumefaciens strain and bacterial concentration
Bacteria were cultured overnight in an orbital shaker at 240 rpm and 28°C in LB medium with 100 μM aceto-syringone (AS), 25 mg/l kanamycin and containing 25 mg/l nalidixic acid for EHA105/pBin19-sgfp or 20 mg/l gentamicin sulphate for C58(pMP90)/pBin19-sgfp. Cells were centrifuged at 3,500 rpm for 10 min and resuspended to a final OD600 of 0.2 or 0.06, in liquid MS medium plus AS (100 μM). Twelve millilitre of Agrobacterium suspension were added to 0.08 g of embryogenic callus cultured on ½ MSAC medium and incubated for 10 min. Excess bacteria were removed and calli were co-cultured for 48 h in dark at 26°C, washed with 12 ml of MS with cefotaxime (900 mg/l) for 20 min and cultured in a thin layer on sterile filter paper (Whatman Nº 2) onto ½ MSAC medium with cefotaxime (300 mg/l). At 10 d, the calli were transferred to the same culture medium with kanamycin. Six calli per table grapevine genotype and treatment were used and the experiments were repeated twice. The number of GFP spots (individual cells or multicellular aggregates) were counted periodically during a month and expressed as number of GFP spots per gram of callus (López-Pérez 2006).
Genetic transformation and plant regeneration
Embryogenic calli from the two cvs. were transformed with Agrobacterium tumefaciens EHA105/pBin19-sgfp at an OD600 of 0.2 for Sugraone, and 0.06 for Crimson Seedless, following the protocol described above. Ten days post co-cultivation, calli were transferred to ½ MSAC with 20 mg/l kanamycin for Crimson Seedless or 50 mg/l kanamycin for Sugraone. The cultures were maintained in the dark at 26°C and subcultured to fresh medium every 30 days.
The regeneration protocol was as described previously by López-Pérez et al. (2005). Putative transgenic somatic embryos (1–2 mm) from different transformation events, were isolated and transferred individually onto ½ MSAC with cefotaxime (300 mg/l) but without kanamycin and maintained in the dark at 25°C for 20 to 30 days. Somatic embryos were then transferred onto germination medium (López-Pérez et al. 2006) with cefotaxime (300 mg/l) and maintained at 25°C and 16 h photoperiod with a photon flux density of 45 μmol m−2 s−1 provided by Grolux fluorescent tubes (Sylvania). Germinated somatic embryos were cultured into test tubes with half strength MS medium and cefotaxime (300 mg/l) to regenerate plants. Plants were micropropagated on the same culture medium in 300 ml glass pots without cefotaxime.
Calli were examined periodically under a Leica MZ16F fluorescence stereomicroscope with a GFP2 filter. The light source was provided by a HBO 100.W high-pressure mercury lamp. The red autofluorescence from chlorophyll was not blocked with any interference filter. Photographs were taken using a digital camera Canon Power Shot S50.
For each condition and cultivar, six calli (0.08 g fresh weight per callus) were used. Number of GFP spots (expressed as number of GFP positive spots per gram of callus) and the number of differentiated transgenic somatic embryos were counted for each condition. The experiment was repeated twice. Data was transformed using √x before statistical analysis to stabilize the variance.
Acclimatization of plants: Well rooted and elongated plants were transplanted into 10 cm pots with a mixture of 50% peat and 50% perlite, covered with a plastic bag and incubated in a chamber under constant conditions: 16 h photoperiod, 27 ± 1°C for 2 weeks and then, the plastic bag was gradually raised before being completely removed.
PCR and Southern blot analyses
Genomic DNA was isolated from young leaves according to Lodhi et al. (1994). Standard PCR techniques were employed to detect the presence of the sgfp and nptII genes in leaf samples from the regenerated putative transgenic plantlets. The primer pairs used were 5′-ATGGTGAGCAAGGGCGAGGA and 5′- GGACCATGTGATCGCGCTTC that amplified a 650 bp fragment of the sgfp gene and 5′-GACGAGGCAGCGCGGCTAT and 5′-AAGAAGGCGATAGAAGGCGA that amplified a 600 bp fragment of the nptII gene (Ghorbel et al. 1999). PCR reactions were performed in 12 μl volume containing 4 ng DNA, 200 μM dNTPs, 3 mM MgCl2, 50 mM KCl, 20 mM Tris–HCl pH: 9.0, 0.25 μM of each primer, and 0.24U of Taq DNA polymerase (Ecogen). Reactions were subjected to 35 cycles of 30 s at 95°C, 30 s at 55°C and 1 min at 72°C. Amplified DNA was detected by UV after electrophoresis on agarose gel (1% w/v) with ethidium bromide.
Southern blot analyses were performed to confirm the stable integration of the sgfp gene and to determine the number of integration events in the transgenic plants. DNA samples (20 μg) were digested with BamHI, separated on 1% (w/v) agarose gels and blotted onto nylon membranes positively charged (Hybond, Roche). Following the transfer, the DNA was UV cross-linked to the membrane prior to overnight hybridization with a DNA DIG-labelled probe (20 ng/ml) from the coding region of the sgfp gene obtained after a PCR with the above described primers according to the manufacturer instructions.
Results and discussion
This report focuses on the effect of the Agrobacterium strain and bacterial concentration on the transformation efficiency and the optimum kanamycin concentration for selecting the transgenic cells using embryogenic calli of two table grape cultivars as starting material.
Sensitivity of embryogenic cultures to kanamycin
Different selection strategies have been followed to improve transgenic plant regeneration efficiency. Kanamycin is commonly used to select grapevine transformed cells and plants but the sensitivity of grape tissues is highly variable to this antibiotic. Thus, it is important to determine the sensitivity of each particular species, cultivar or explant to this antibiotic to develop a new efficient transformation system.
In the present work, clearly differentiated somatic embryos for both cultivars could be observed in both control media (C and C + cef) after 15 and 30 d of culture with no differences among them. It was observed that inhibitory concentration of kanamycin depended on the cultivar. As Fig. 1 shows, Crimson Seedless embryogenic tissue was much more sensitive to kanamycin than Sugraone. Total suppression of embryo differentiation was achieved with 20 mg/l of kanamycin in calli of Crimson Seedless and with 50 mg/l in calli of Sugraone. These results are in contrast with those previously reported by Perl et al. (1996) who observed that 50–500 mg/l kanamycin was unable to inhibit embryo regeneration from Superior Seedless (Sugraone). Colby and Meredith (1990) inhibited callus formation, root initiation, and adventitious shoot formation from leaves and stem segments by using 20, 10 and 7 mg/l kanamycin, respectively but no differences were observed between the different genotypes. Vidal et al. (2003) used 15 mg/l kanamycin to select transgenic plants of V. vinifera Chardonnay. In contrast, it has been reported that only high concentrations of kanamycin (80–100–150 mg/l) were clearly lethal for somatic embryos from V. rupestris (Martinelli and Mandolino 1994), from seedless grapes (Gölles et al. 1997) or from other V. vinifera cvs. (Torregrosa et al. 2000).
Effect of Agrobacterium tumefaciens strain and bacterial concentration on the transformation efficiency
Differences in the transient GFP expression were observed for each Agrobacterium strain, bacterial concentration and cultivar (Figs. 2, 3a shows GFP transient expression in calli of Crimson Seedless, 2–5 days post co-cultivation).
Number of GFP spots per gram of callus in Crimson Seedless (a) and Sugraone (b) embryogenic calli transformed with two Agrobacterium tumefaciens strains (EHA105-pBin19-sgfp and C58(pMP90)-pBin19-sgfp) at two bacterial concentrations (OD600: 0.06 and 0.2). Per each bacterial strain and concentration the same letter means no statistical difference at P < 0.05 by LSD test
Crimson Seedless and Sugraone transgenic plants. (a) Transient GFP expression 2–5 d post co-cultivation. (b–d) Somatic embryos showing stable GFP expression at different stages of development. (e) Transgenic Crimson Seedless plant. Left: illuminated by incident light, Right: illuminated by blue light
In general, GFP transient expression (number of spots expressing GFP) was higher in calli infected with EHA105 than with C58(pMP90) for both cultivars. Nearly 2,000 and 4,000 GFP spots per gram of callus being obtained 8–12 days post co-cultivation in Crimson Seedless and Sugraone, respectively. The number of GFP spots increased during the first 8–12 days and then gradually decreased. This could be correlated with the culture of calli on selective medium with kanamycin at 10 d post-infection as previously described with other reporter genes (Vidal et al. 2003).
For Crimson Seedless, a bacterial concentration of 0.06 at OD600 (independently of strain used) increased the number of GFP spots, while for Sugraone a concentration of 0.2 was the most suitable. This suggests that Crimson Seedless is a much more sensitive cultivar to the Agrobacterium concentration than Sugraone. At 28 d post co-cultivation (stable expression of GFP), the best results were observed with EHA105 at an OD600 of 0.06 for Crimson Seedless (424.4 GFP spots/g) and at an OD600 of 0.2 for Sugraone (349.7 GFP spots/g).
The effect of the Agrobacterium tumefaciens strain on the grapevine transformation has been described previously (Baribault et al. 1989; Berres et al. 1992). Torregrosa et al. (2002) reported that the supervirulent strain EHA105 increased transformation efficiency (6.0 to 137.08 number of positive units/plate, 5wk after co-cultivation, depending on the grapevine cultivar used) compared to the widely used strain LBA4404 (2.42 to 37.0 positive units). These observations are in accordance with our results, since strain EHA105, showed better transformation efficiency than strain C58(pMP90) (although differences were not significant). The difference between both strains may be due to the different genetic background of the Ti plasmid from the Agrobacterium strains employed (pTiBo542 vs. pTiC58). Similarly, Torregrosa et al. (2002) also observed differences among V. vinifera cultivars.
Bacterial concentration is also an important factor affecting the transformation efficiency. Concentrations from 0.4, 0.6 or 1.8 measured as O. D. to 550, 600 or 630 nm (Torregrosa et al. 2002; Bornhoff et al. 2005; Wang et al. 2005) or 108 cfu/ml (Oláh et al. 2003b; Nakajima et al. 2006) have been used, although in few cases were made comparisons of bacterial density. Perl et al. (1996) observed that bacterial cultures of A. tumefaciens with different optical densities (0.1–0.7 at O.D.630) resulted in plant tissue necrosis and subsequent cell death and only after the inclusion of antioxidant mixtures in the transformation protocols it was possible to regenerate stable transgenic grape plants of Superior Seedless. Li et al. (2006) minimized Agrobacterium-induced tissue browning/necrosis by preculturing somatic embryos for 7 d in fresh medium or by adding 1 g/l of dithiothreitol to the post co-cultivation wash media. In our case, the use of such low bacterial densities as 0.06 or 0.2 at OD600 did not result in tissue necrosis and transgenic plants of both cvs. Sugraone (Superior Seedless) and Crimson Seedless could be regenerated. In preliminary experiments (data not shown) we observed necrosis by co-culturing embryogenic calli with higher concentrations of bacteria (0.5–0.6 at OD600).
Agüero et al. (2006) reported that inoculation with 109 cells ml−1 Agrobacterium (EHA101) strain resulted in a higher number of selected calli than cultures inoculated with 107 or 108 cells ml−1, independently of the cv. used (Chardonnay and Thompson Seedless). In our case, we observed an interaction between bacterial density and grape cultivar: the highest number of GFP spots was achieved with an optical density of 0.06 (approx. 0.3 × 108 cells ml−1) in Crimson Seedless and 0.2 (approx. 2.2 × 108 cells ml−1) in Sugraone.
Genetic transformation and plant regeneration
Taking into account the optimal bacterial concentration of Agrobacterium tumefaciens strain and optimum kanamycin concentration for each grapevine cultivar, we developed two cultivar-specific genetic transformation protocols.
One month after calli co-cultivation with EHA105-pBIN19-sgfp Agrobacterium strain at 0.06 OD600 for Crimson Seedless and 0.2 for Sugraone, independent white somatic embryos of 5–8 mm (Fig. 3b) and with clearly visible apical roots (Fig. 3c, d) were isolated on medium with 20 or 50 mg/l kanamycin, respectively, although not all of them were GFP-positive. In each Petri dish, many brown and smaller somatic embryos without apical roots were observed, indicating that kanamycin selection was effective. The number of white somatic embryos isolated on kanamycin during the selection phase (1–2 months) was 204 for Sugraone and 422 for Crimson Seedless (Table 1). In the germination medium, 142 (69.6%) and 72 (17.1%) embryos from Sugraone and Crimson Seedless, respectively, were able to germinate. Torregrosa et al. (2002) observed that 28% of the isolated gfp-expressing embryos developed shoots. In our case, somatic embryos that failed germination, showed morphological abnormalities and no further development was observed as described by Bornhoff et al. (2005) and López-Pérez et al (2006). From germinated embryos, 22 and 28 independent regenerated plants (15.5 and 38.9% plant regeneration) were obtained for Sugraone and Crimson Seedless respectively, in a period of 3–5 month after transformation (Fig. 3e).
Putative transgenic plants were initially analysed by PCR in order to check for the insertion of both sgfp and nptII genes (Fig. 4). Most of the regenerated plants were PCR positive for both sgfp and nptII genes (Table 1) while only 3 plants (one of Sugraone and two of Crimson Seedless) tested positive for the nptII gene but not for the sgfp gene. This result suggests that the integration of the T-DNA (two linked cassettes) was not always achieved, as mentioned by Yamamoto et al. (2000). Another three plants were negative for both genes and are considered as escapes. Several reasons could explain these results. As Iocco et al. (2001) described, non-transgenic embryos developing before 10–12 weeks could be attributed to the presence of some advanced multicellular embryos at the time of Agrobacterium inoculation, giving rise to untransformed or partly transformed multicellular embryos which may be resilient to the effects of kanamycin. In our case, it was possible that the starting material was a mixture of proembryogenic callus in various stages of early development. Torregrosa et al. (2002) reported that most of the putative structures isolated did not produce gfp-expressing shoots, probably because of their chimeric status or because the expression of nptII gene was ineffective.
PCR analysis of regenerated plants for positive signals of sgfp and nptII genes. Left samples 1–9 correspond to Sugraone regenerated plants and right samples 1–16 correspond to Crimson Seedless regenerated plants. M: molecular weight marker VI (Roche), C+: positive control pBin19-sgfp plasmid, C−: negative control plant
Efficiency of transgenic plant regeneration (measured as the number of PCR positive plants/regenerated plants) was achieved at high levels: in Sugraone, 21 independent transgenic plants (95.5%) were PCR positive (20 with both genes and 1 plant with only nptII gene), and in Crimson Seedless 26 independent transgenic plants (92.8%) were positive (24 with both genes and 2 plants with only nptII gene).
The stable insertion of sgfp gene was confirmed by Southern Blot analysis (Fig. 5). Digestion with BamHI, which has an unique restriction site between the 35S promoter and sgfp gene (Fig. 5b), revealed up to three integration events in three Sugraone transgenic plants (Fig. 5a, lanes 3, 4 and 5) and only one integration in the two Crimson Seedless transgenic plants analysed (Fig. 5a, lanes 8 and 9). No hybridization signals were detected in non-transgenic control plants. To our knowledge, this is the first time that transgenic plants of Crimson Seedless have been generated.
Southern blot hybridisation for the sgfp gene. (a) Right: three transgenic Sugraone plants (3, 4, 5); left: two transgenic Crimson Seedless plants (8, 9). NT: non-transgenic plant. (M) Molecular weight marker II (Roche). (b) Map of the T-DNA of pBin19-sgfp with the restriction site for BamHI used to digest plant DNA, and the position of the sgfp probe
For each condition and cultivar, a total of 12 calli, with 0.08 g fresh weight per callus were used for transformation. In preliminary experiments, we used between 0.2 and 1 g fresh weight per embryogenic callus for co-cultivation but the degree of necrosis during the selection phase was high and very few plants were recovered (data not shown). In additional preliminary experiments (data not shown) we cultured the calli grouped in the centre of the Petri plate or spread in a thin layer all over the Petri plate surface. For the first case the necrosis was higher and the recoveries of somatic embryos lower than for the second case. Therefore the amount of tissue used in these experiments spread in a thin layer onto the filter paper over the culture medium was enough to reduce the necrosis and to efficiently recover transgenic somatic embryos. Thus, the efficiency of regenerated transgenic plants was high and ranged between 21 and 26 plants per gram of callus for Sugraone and Crimson Seedless respectively. These results were slightly higher than those obtained by Perl et al. (1996) who using 3 g of fresh weight embryogenic callus and an infection density of 0.6 at OD630, were able to regenerate 14–20 plants per gram of embryogenic callus of Superior Seedless (on basta or hygromycin selective medium), but only after the inclusion of antioxidant mixtures in the transformation protocol. In our case, it was not necessary to add antioxidants because decreasing the amount of callus and the bacterial density at OD600 to 0.2 or 0.06, for Sugraone and Crimson Seedless respectively, greatly reduced the necrotic effects of Agrobacterium, as it was also observed by Iocco et al. (2001). Recently, the transformation procedures developed by Fan et al. (2008) based on an appropriate selection method with hygromycine, allow the production of 72% GFP-positive germinated embryos and 38% of transformed embryos regenerated into normal plantlets, achieving 19 PCR-HPT II-positive transgenic lines, from which eleven were also positive for STS (stilbene synthase gene).
Transgenic plants were micropropagated, well rooted and elongated plantlets were transplanted to soil and acclimated progressively to phytotron conditions, where they grew normally. In our conditions, the percentage of acclimatization was high, up to 90–95%.
In conclusion, we present specific and highly efficient transformation protocols for two seedless table grapevine cultivars, Crimson Seedless and Sugraone, involving (1) the establishment of a suitable selective kanamycin concentration for each cultivar, 20 mg/l for Crimson Seedless and 50 mg/l for Sugraone; and (2) the use of the appropriate Agrobacterium strain (EHA105) and the optimal bacterial concentration for each grapevine cultivar: OD600 at 0.06 or 0.2, respectively. Moreover, the use of a small amount of calli for co-cultivation and their culture on a thin layer reduced the necrosis of embryogenic tissues allowing a higher recovery of plants (data not shown). Following these protocols, in our experimental conditions, we achieved 21 (95.5%) and 26 (92.8%) independent transgenic Sugraone and Crimson Seedless plants from a total of 22 and 28 regenerated plants, respectively.
Abbreviations
- 2,4-D:
-
2, 4-Diclorophenoxiacetic acid
- BA:
-
6-Benzyladenine
- IAA:
-
Indol-3-acetic acid
- sgfp :
-
Green fluorescent protein gene
- nptII :
-
Neomycine phosphotransferase II gene
- PCR:
-
Polymerase chain reaction
- NOSpro/ter:
-
Nopaline synthase promoter and terminator
- 35Spro:
-
35S Cauliflower mosaic virus promoter
- OD:
-
Optical density
References
Agüero CB, Meredith CP, Dandekar AM (2006) Genetic transformation of Vitis vinifera L. cvs Thompson Seedless and Chardonnay with the pear PGIP and GFP encoding genes. Vitis 45(1):1–8
Ahmed HMH, Digiaro M, Martelli GP (2004) Viruses and virus diseases of grapevine in Egypt. EPPO Bull 34:395–398
Avenant JH, Avenant E (2006) The effect of ethephon on berry colour of Crimson Seedless and Ebony Star table grapes. Acta Hortic 727:381–388
Baldoni L, Rugini E (2002) Genetic modification of agronomic traits in fruit crops. In: Valpuesta V (ed) Fruit and vegetable biotechnology. Woodhead Publishing Ltd., Cambridge, England, pp 23–113
Baribault TJ, Skene KGM, Scott NS (1989) Genetic transformation of grapevine cells. Plant Cell Rep 8:137–140
Berres R, Otten L, Tinland B, Malgarini-Clog E, Walter B (1992) Transformation of Vitis tissue by different strains of Agrobacterium tumefaciens containing the T-6B gene. Plant Cell Rep 11:192–195
Bornhoff BA, Harst M, Zyprian E, Töpfer R (2005) Transgenic plants of Vitis vinifera cv. Seyval blanc. Plant Cell Rep 24:433–438
Chiu W, Niwa Y, Zeng W, Hirano T (1996) Engineered GFP as a vital reporter in plants. Curr Biol 6:325–330
Colby SM, Meredith CP (1990) Kanamycin sensitivity of cultured tissues of Vitis. Plant Cell Rep 9:237–240
Digiaro M, Fiore N, Tarricone L, Prodan S, Elbeaino T (2006) Influence of viruses on the performance and quality of cv. Crimson seedless. 15th Meeting of the International Council for the Study of Virus and Virus-like Diseases of the Grapevine (ICVG Conf), Stellenbosch, South Africa, pp. 186–188
Dokoozlian N, Peacock B (2001) Gibberellic acid applied at bloom reduces fruit set and improves size of ‘Crimson Seedless’ table grapes. HortScience 36:706–709
Fan C, Pu N, Wang X, Wang Y, Fang L, Xu W, Zhang J (2008) Agrobacterium-mediated genetic transformation of grapevine (Vitis vinifera L.) with a novel stilbene synthase gene from Chinese wild Vitis pseudoreticulata. Plant Cell Tiss Organ Cult 92:197–206
Ghorbel R, Juárez J, Navarro L, Peña L (1999) Green fluorescent protein as a screenable marker to increase the efficiency of generating woody fruit plants. Theor Appl Genet 99:350–358
Gölles R, da Câmara-Machado A, Tsolova V, Bouquet A, Moser R, Lopes MS, Mendonca D, Katinger H, Laimer da Câmara-Machado M (1997) Transformation of somatic embryos of Vitis sp. (grapevine) with different constructs containing nucleotide sequences from nepovirus coat protein genes. Acta Hortic 447:265–272
Harst M, Bornhoff BA, Zyprian E, Töpfer R (2000) Influence of culture technique and genotype on the efficiency of Agrobacterium-mediated transformation of somatic embryos (Vitis vinifera) and their conversion to transgenic plants. Vitis 39:99–102
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Iocco P, Franks P, Thomas MR (2001) Genetic transformation in mayor wine grape cultivars of Vitis vinifera L. Transgenic Res 10:105–112
Jaillon O, Aury JM, Noel B et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449(7161):463–467
Kikkert JR, Thomas MR, Reisch BI (2001) Grapevine genetic engineering. In: Roubelakis-Angelakis KA (ed) Molecular biology & biotechnology of the grapevine. Kluwer Academic Publishers, The Netherlands, pp 393–410
Koncz C, Schell J (1986) The promotor of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol Gen Genet 204:383–396
Legrand V, Dalmayrac S, Latché A, Pech JC, Bouzayen M, Fallot J, Torregrosa L, Bouquet A, Roustan JP (2003) Constitutive expression of Vir-ERE gene in transformed grapevines confers enhanced resistance to eutypine, a toxin from Eutypa lata. Plant Sci 164:809–814
Li ZT, Dhekney S, Dutt M, Van Aman M, Tattersali J, Kelley KT, Gray DJ (2006) Optimazing Agrobacterium-mediated transformation of grapevine. In Vitro Cell Dev Biol Plant 42:220–227
Lodhi MA, Ye G-N, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars, Vitis species and Ampelopsis. Plant Mol Biol Rep 12:6–13
López-Pérez AJ (2006) Desarrollo de protocolos de regeneración de plantas vía embriogénesis somática y de transformación de vid (Vitis vinifera L.). PhD Thesis. Universidad de Murcia. Spain
López-Pérez AJ, Carreño J, Dabauza M (2006) Somatic embryo germination and plant regeneration of three grapevine cvs: Effect of IAA, GA3 and embryo morphology. Vitis 45:141–143
López-Pérez AJ, Carreño J, Martinez-Cutillas A, Dabauza M (2005) High embryogenic ability and plant regeneration of table grapevine cultivars (Vitis vinifera L.) induced by activated charcoal. Vitis 44:79–85
Martinelli L (1997) Regeneration and genetic transformation in the Vitis Genus. Ph. D. thesis for the award of the degree of Doctor of Agricultural and Enviromental Sciencies at the Agricultural University of Wageningen, The Netherlands
Martinelli L, Mandolino G (1994) Genetic transformation and regeneration of transgenic plants in grapevine (Vitis rupestris S.). Theor Appl Genet 88:621–628
Martinelli L, Mandolino G (2001) Transgenic grapes (Vitis species). In: Bajaj YPS (ed) Biotechnology in Agriculture and Forestry 47: 325–338 Springer-Verlag, Berlin
Mozsár J, Viczián O, Süle S (1998) Agrobacterium-mediated genetic transformation of an interspecific grapevine. Vitis 37:127–130
Nakajima I, Matsuta N, Yamamoto T, Terakami S, Soejima J (2006) Genetic transformation of ‘Kyoho’ grape with a GFP gene. J Jpn Soc Hortic Sci 75(2):188–190
Oláh R, Szegedi E, Ruthner S, Korbuly J (2003a) Optimization of conditions for regeneration and genetic transformation of rootstock- and scion grape varieties. Acta Hortic 603:491–497
Oláh R, Szegedi E, Ruthner S, Korbuly J (2003b) Thidiazuron-induced regeneration and genetic transformation of grapevine rootstock varieties. Vitis 42:133–136
Perl A, Lotan O, Abu-Abied M, Holland D (1996) Establishment of an Agrobacterium-mediated trasnforamtion system for grape (Vitis vinifera L.): The role of antioxidants during grape-Agrobacterium interactions. Nat Biotechnol 14:624–628
Prodan S, Montealegre J, Aballay E, Pino AM, Fernández P, Reyes R, Fiore N (2003) Report of new viral diseases in Chilean grapevines. 14th meeting of the international council for the study of virus and virus-like diseases of the grapevine (ICVG Conf), Locorotondo, p 145
Spielmann A, Krastanova S, Douet-Orhant V, Gugerli P (2000) Analisys of transgenic grapevine (Vitis rupestris) and Nicotiana benthamiana plants expressing an Arabis mosaic virus coat protein gene. Plant Sci 156:235–244
Torregrosa L, Péros JP, Lopez G, Bouquet A (2000) Effect of hygromycin, kanamycin and phosphinothricin on the embryogenic callus development and axillary micropropagation of Vitis vinifera L. Acta Hortic 528:401–406
Torregrosa L, Iocco P, Thomas MR (2002) Influence of Agrobacterium strain, culture medium, and cultivar on the transformation efficiency of Vitis vinifera L. Am J Enol Vitic 53:183–190
Vidal JR, Kikkert JR, Wallace PG, Reisch BI (2003) High-efficiency biolistic co-transformation and plant regeneration of ‘Chardonnay’ (Vitis vinifera L.) containing npt-II and antimicrobial peptide genes. Plant Cell Rep 22:252–260
Wang Q, Li P, Hanania U, Sahar N, Mawassi M, Gafny R, Sela I, Tanne E, Perl A (2005) Improvement of Agrobacterium-mediated transformation efficiency and transgenic plant regeneration of Vitis vinifera L. by optimising selection regimens and utilizing cryopreserved cell suspensions. Plant Sci 168:565–571
Yamamoto T, Iketani H, Ieki H, Nishizawa Y, Notsuka K, Hibi T, Hayashi T, Matsuta N (2000) Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep 19:639–646
Acknowledgements
We would like to thank Dr. L. Peña for providing the C58(pMP90) and EHA105 Agrobacterium strains and to Dr. J.R. Vidal for the critical reading of the manuscript. This research was supported by the Instituto Murciano de Investigación y Desarrollo Agrario y Alimentario (PR06-002) and by a fellowship provided by Fundación Séneca to A.J. López-Pérez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
López-Pérez, AJ., Velasco, L., Pazos-Navarro, M. et al. Development of highly efficient genetic transformation protocols for table grape Sugraone and Crimson Seedless at low Agrobacterium density. Plant Cell Tiss Organ Cult 94, 189–199 (2008). https://doi.org/10.1007/s11240-008-9404-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9404-y