Abstract
The application of the droplet vitrification cryopreservation technique to taro accessions from a range of Asia Pacific countries is presented. The optimum protocol involves excision of about 0.8 mm shoot-tips from in vitro plants, 20–40 min PVS2 exposure at 0°C followed by rapid plunge into liquid nitrogen. Thawing was done at room temperature (25°C) and shoot-tips inoculated on MS medium with 0.1 M sucrose regenerated into plantlets 4–6 weeks later. This new droplet vitrification protocol improved the mean post-thaw regeneration rates to 73–100% from 21–30% obtained with the previous cryo-vial vitrification protocol.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Taro (Colocasia esculenta var. esculenta (L.) Schott), a herbaceous, vegetatively propagated tuber crop, is a very important crop in the Pacific Island countries with great cultural, dietary and economical importance. Traditionally, taro germplasm has been conserved in situ and/or field genebanks and through an in vitro collection of 758 taro cultivars from Asia-Pacific countries at the Regional Germplasm Centre (RGC) of the Secretariat of the Pacific Community (SPC) in Suva, Fiji. Cryopreservation would be the most cost effective, secure and sustainable method of long term conservation of taro germplasm.
Thinh (1997) developed a tissue paper envelope vitrification protocol for Asian taro (C. esculenta var. esculenta) cultivars with 67–96% survival rates. Sant et al. (2006) applied this method for Pacific taro cultivars resulting in lower regeneration rates of 21–30%. Furthermore, this protocol was successful for only three out of eight cultivars investigated.
The droplet freezing method, first developed for cryopreservation of potato by Schäfer-Menuhr et al. (1997), is proving to be an efficient method across many species and gave the highest regeneration rates in comparative studies with different vitrification methods for yam (Leunufna and Keller 2003) and banana (Panis et al. 2005). This study attempts to verify that the droplet vitrification is an efficient and easy to execute protocol resulting in high regeneration rates across a wide range of taro genotypes.
In vitro stock plants of taro (C. esculenta var. esculenta) were obtained from the taro collection maintained at the SPC RGC, Suva. It comprises of taro varieties from several countries in the Asia-Pacific region. Eighteen varieties were selected from the collection, representative for its diversity. Shoots were multiplied according to reported methods (Tuia 1997; Sant et al. 2006). When sufficient material was generated, the accessions were cultured in liquid Murashige and Skoog (1962) medium (MS) with no growth regulators for one month to reduce any carry-over effects of the plant growth regulators. From these plants, 2 cm explants, including some basal corm tissue and an adjoining length of leaf cluster base were excised and grown on 15 ml of MS in 100 ml glass jars (Cospak) for 3 months without subculture. The MS medium contained 3% sucrose and was solidified with 7.5 g l−1 Agar Type A (Sigma Chemical Co., Germany) at pH 5.8 ± 0.1. Cultures were incubated at 25°C under 30 μEm−2 s−1 photon flux density (cool white fluorescent lamps) with a photoperiod of 16/8 h day/night. After 3 months, 2 cm explants were sampled from these plants and conditioned on MS medium supplemented with 90 g l−1 (0.26 M) sucrose for 4–8 weeks.
Individual apical meristems were excised under a binocular microscope under aseptic conditions. Leaves were removed one by one with scalpel blade until the shoot-tip (ST) consisting of the apical dome covered with two young leaf primordia was isolated. This ST, 0.8–1.0 mm in size, was cut out with 1.0 mm cube leaf base (corm tissue) (Fig. 1a). The dissected ST was placed on solid MS medium containing 0.3 M sucrose until all STs were excised. The STs were immersed in a McCartney bottle containing 5 ml of filter sterilized loading solution that contained 2 M glycerol and 0.4 M sucrose dissolved in MS medium (pH 5.8) for 20 min at 25°C (Sakai et al. 1990).
(a) Dissected shoot-tip of cultivar CPUK consisting of meristematic dome and first two leaf primodia (Bar = 0.5 mm). Growth of cryopreserved shoot-tips after (b) 2 weeks and (c) 5 weeks of thawing (the STs in the two rows on the left were exposed to 20 min PVS2 and those on the right were exposed to 30 min) (Bar = 1 cm)
After loading, the solution was replaced with 5 ml of chilled PVS2 solution. The filter sterilized PVS2 solution consisted of 30% (w/v) (3.26 M) glycerol, 15% (w/v) (2.42 M) ethylene glycol and 15% (w/v) (1.9 M) dimethylsulphoxide in liquid MS medium containing 0.4 M sucrose at pH 5.8 (Sakai et al. 1990). Just before the end of the PVS2 treatment time, STs were sucked up in a sterile disposable pipette and placed on a strip of sterile aluminium foil (5 × 20 mm) with a droplet of PVS2 solution. Five minutes prior to use, the strip was placed on a plastic Petri dish resting on top of a frozen cooling element to maintain the temperature around 0°C. After the PVS2 treatment, the aluminium strip was plunged directly into liquid nitrogen with a fine forceps until bubbling stopped then quickly transferred to a 2 ml cryo-vial filled with liquid nitrogen. Two replications with four to five shoot-tips per replication were executed for each length of PVS2 exposure. As a first step, shoot-tips of the cultivars CPUK and TNS were investigated for their PVS2 sensitivity at 0°C for exposure times of 5, 10, 20, 30, 40, 50, 60, 90 and 120 min. The resulting optimum PVS2 exposure times were further tested with 16 other cultivars (Table 1).
Rapid thawing was executed by removing the aluminium strip from liquid nitrogen with fine forceps and quickly plunging in 5 ml of filter sterilized unloading solution (liquid MS medium with 1.2 M sucrose at pH 5.8) (Sakai et al. 1990), contained in 15 ml Petri dish, for 15 min at 25°C.
The STs were then plated on two layers of sterile filter paper placed on solid MS with 0.3 M sucrose. These plates were cultured overnight in the dark at 25°C. The following day, the STs were transferred to plates containing solid MS with 0.1 M sucrose without the filter paper. The cultures were maintained in the dark for five days and then transferred to dim light (3.5 μEm−2 s−1) for 10 days before exposure to normal culture conditions as stated above. STs growing into plantlets with proper leaves and roots 4–6 weeks following cryopreservation were scored as regeneration (Fig. 1b, c). Statistical analysis was performed with STATISTICA for Windows 5.1 (StatSoft Inc., Tulsa, USA).
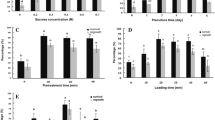
All PVS2 sensitivity trials, even after 120 min of PVS2 exposure, gave 100% recovery for the cultivars CPUK and TNS (data not presented), indicating these taro cultivars’ low sensitivity towards dehydration with PVS2. The optimal PVS2 exposure range for successful vitrification of shoot-tips of two cultivars was 20–40 min (Fig. 2).
Post-thaw regeneration of shoot-tips of taro cultivars CPUK and TNS (%). In vitro shoot-tip donor plants were grown on solid MS + 90 g l−1 sucrose medium for 7 weeks; shoot-tips dissected and cryopreserved the same day. Each data point is the mean of two replicates with five shoot-tips per replicate (n = 10). Vertical bars represent standard error of regeneration
According to Tukeys HSD test (P < 0.05) there were no significant differences in post-thaw regeneration rates for 20, 30 and 40 min PVS2 exposure times for any of the cultivars. Some genotype specific responses were observed as cultivars such as PNG 165 and MAL 09 had significantly lower post-thaw regeneration rates than PNG 17 and VAN 77. Although there are genotypic differences in regeneration percentages, even the lowest rate of 73.3% is sufficient for practical purposes of in vitro conservation of germplasm.
This study shows a dramatic increase in average post-thaw regeneration rates (73–100%) compared to the previously reported paper-envelope/cryo-vial method that was applied to the same cultivars (21–30%) (Sant et al. 2006). A possible reason for this could be the ultra-rapid cooling rate of 130°C/min obtained with PVS2 droplet on aluminium strip compared to cryo-vial freezing cooling rates of about 6°C/min (Towill and Bonnart 2003).
A second reason for the enhanced success of this new method could be the exposure of shoot-tips to PVS2 at 0°C instead of 25°C. Most injury effects caused by the dehydration process are reduced or eliminated by the loading step and optimizing PVS2 exposure at 0°C (Sakai et al 1990). PVS2 exposure at 0°C allows good recovery for a range of exposure times as opposed to the critical exposure time of 12 min at 25°C (Sant et al. 2006). Not only PVS2 exposure at 0°C gives better replication of results, but also a broad range of optimum PVS2 exposure time offers the practical advantage of cryopreserving larger numbers of shoot-tips in a day. This was also observed for banana where optimal length of PVS2 treatment was 30–50 min at 0°C (Panis et al. 2005).
The droplet vitrification method used in this study is based on the one developed for banana meristems (Panis et al. 2005). Panis et al. (2005) found an increase of 42% regeneration for banana shoot-tips from four genomic groups cryopreserved by droplet vitrification compared to cryo-vial vitrification. They were also able to cryopreserve cultivars that were previously considered recalcitrant. Our results validate these findings as droplet vitrification method has considerably improved plant regeneration rates in a range of taro cultivars from 21–30% in cryo-vial vitrification (Sant et al. 2006) to 73–100% in droplet vitrification method (Fig. 2 and Table 1). Furthermore, only three of the eight cultivars investigated could be successfully cryopreserved with the former method (Sant et al. 2006), whereas all 18 cultivars investigated for droplet vitrification in this study had high post-thaw regeneration rates.
Cryopreservation survival of different cultivars of the same species can depict genotypic dependence, as reported for papaya (Wang et al. 2005), banana (Thinh 1997; Panis et al. 2005), black spruce (Touchell et al. 2002), sugar beet (Vandenbussche et al. 2000), and taro (Takagi et al. 1997; Sant et al. 2006) and seen in this study (Table 1). Despite these differences the droplet vitrification protocol is applicable to a wide range of taro cultivars of different origins as the lowest regeneration rate recorded is also high enough for cryopreservation purposes. The fact that droplet vitrification method has a broad optimal PVS2 exposure range (20–40 min), is equally applicable across various genotypes and is easy to execute, enhances its chances of being an efficient and reliable protocol under different laboratory settings.
With this study, we clearly demonstrate that pacific taro (C. esculenta var. esculenta) cultivars can be successfully cryopreserved using the droplet vitrification method.
References
Leunufna S, Keller ERJ (2003) Investigating a new cryopreservation protocol for yams (Dioscorea spp.). Plant Cell Rep 21:1159–1166
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plant 15:473–497
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. Var. brasiliensis Tanaka) cooled to −196°C. J Plant Physiol 137:465–470
Sant R, Taylor M, Tyagi A (2006) Cryopreservation of in vitro-grown shoot-tips of tropical taro (Colocasia esculenta var. esculenta) by vitrification. Cryo-Lett 27:133–142
Schäfer-Menuhr A, Schumacher HM, Mix-Wagner G (1997) Cryopreservation of potato cultivars: design of a method for routine application in genebanks. Acta Hortic 447:477–482
Takagi H, Thinh NT, Islam OM, Senboku T, Sakai A (1997) Cryopreservation of in vitro-grown shoot tips of taro (Colocasia esculenta (L) Schott) by vitrification.1. Investigation of basic conditions of the vitrification procedure. Plant Cell Rep 16:594–599
Thinh NT (1997) Cryopreservation of germplasm of vegetatively propagated tropical monocots by vitrification. PhD Dissertation, Kobe University, Japan
Touchell DH, Chiang VL, Tsai CJ (2002) Cryopreservation of embryogenic cultures of Picea mariana (black spruce) using vitrification. Plant Cell Rep 21:118–124
Towill LE, Bonnart R (2003) Cracking in a vitrification solution during cooling or warming does not affect growth of cryopreserved mint shoot tips. Cryo-Lett 24:341–346
Tuia VS (1997) In vitro multiplication of taro (Colocasia esculenta var. esculanta L. Schott). MSc Dissertation, The University of the South Pacific, Samoa
Vandenbussche B, Weyens G, De Proft M (2000) Cryopreservation of in vitro sugar beet (Beta vulgaris L.) shoot tips by a vitrification technique. Plant Cell Rep 19:1064–1068
Wang YL, Fan MJ, Liaw SI (2005) Cryopreservation of in vitro-grown shoot tips of papaya (Carica papaya L.) by vitrification. Bot Bull Acad Sinica 46:29–34
Acknowledgments
Funding for this research was provided by AusAID through the Taro Genetic Conservation and Utilization (TaroGen) project. The training on droplet vitrification technique provided by Dr Panis was funded by INIBAP and AusAID.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sant, R., Panis, B., Taylor, M. et al. Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia esculenta var. esculenta) accessions. Plant Cell Tiss Organ Cult 92, 107–111 (2008). https://doi.org/10.1007/s11240-007-9302-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-007-9302-8