Abstract
In an attempt to improve Agrobacterium-mediated transformation frequency of American chestnut somatic embryos, a novel method of inoculation/co-cultivation was developed. Plate flooding is a simple method where the Agrobacterium inoculum is poured onto the embryos while they remain on multiplication medium. This method tested the hypothesis that wounding tissues prior to co-cultivation was unnecessary or counterproductive. Two clones, WB296 and P1-1, were tested for differences in transformation efficiency as measured by the number of transformed embryogenic cell lines per Petri dish, the total number of transformed cell lines (embryos plus callus) and percentage of transformants that remained embryogenic. Plate flooding using clone WB296 produced significantly more transformed embryo cell lines and had a higher percentage of transformants remain embryogenic. The number of total transformed cell lines (embryos plus callus) was the same as obtained by other methods (desiccation, blot dry, sand abrasion, sonication and vacuum infiltration). With clone P1-1 there were no significant differences among the inoculation/co-cultivation treatments tested. Polymerase chain reaction and Southern hybridizations confirmed that the transgene of interest had been stably integrated into both American chestnut clones. Whole plants were regenerated from clone P1-1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It has been more than 20 years since the introduction of the leaf-disk transformation method (Horsch et al. 1985). Wounding of leaves by cutting them into discs is an important step in the transformation process. Several changes in methodology, however, may make wounding unnecessary for Agrobacterium-mediated transformation of somatic embryos. First, acetosyringone is now commonly added to the Agrobacterium inoculation medium to induce the virulence genes (de la Riva et al. 1998). Second, somatic embryos are usually grown on media that promote rapid cell proliferation. Finally, the wounding step may cause embryogenic tissue to generate callus rather than regenerate new somatic embryos.
For species that cannot be easily regenerated from leaf discs or callus, some researchers have turned to somatic embryos. Various modifications to the leaf disc transformation protocol have been made for the transformation of somatic embryos and PEMs. Mango (Magnifera indica) PEMs were macerated before inoculation (Mathews et al. 1992). Peach (Prunus persica) and European chestnut (Castanea sativa Mill.) somatic embryos were blotted dry after inoculation without wounding (Scorza et al. 1990; Corredoira et al. 2004). Tea (Camellia sinensis L.) somatic embryos were blotted dry, wounded with sand by abrasion and pricked with a hypodermic needle (Mondal et al. 2001). Somatic embryo suspension cultures of soybean (Glycine max (L.) Merr.), Ohio buckeye (Aesculus glabra Willd.), cowpea (Vigna minima (Roxb.) Ohwi & H. Ohashi), white spruce (Picea glauca (Moench) Voss), wheat (Triticum aestivum L.), and maize (Zea mays) were sonicated during inoculation (Trick and Finer 1997). American chestnut (Castanea dentata (Marsh.) Borkh) somatic embryos were wounded by microprojectile bombardment prior to Agrobacterium inoculation, achieving transformed callus (Merkle and Carraway 1994). Finally, wheat somatic embryos were desiccated during co-cultivation (Cheng et al. 2003). The first study to show wounding was not necessary for Agrobacterium-mediated transformation used walnut (Juglans nigra L.) somatic embryos (McGranahan et al. 1988). Despite this wide range of alternative treatments, wounding continues to be included in many Agrobacterium-mediated transformation protocols using somatic embryos.
To date, regeneration of American chestnut leaf segments and callus into whole plants has not been accomplished. American chestnut somatic embryos have been developed (Merkle et al. 1991) and regenerated into whole plants (Carraway and Merkle 1997; Xing et al. 1999), therefore transformation has primarily focused on this tissue. Recent success has been achieved in the transformation and regeneration of American chestnut somatic embryos (Polin et al. 2006) using a desiccation method first described for wheat somatic embryos (Cheng et al. 2003). In our attempts to find other transformation methods for American chestnut somatic embryos, we developed a method we call plate flooding. This method involves pouring Agrobacterium inoculum onto somatic embryos while still on the multiplication medium, waiting a short time, drawing off the excess liquid and then co-cultivating in place.
Materials and methods
Our transformation experiment used American chestnut clones P1-1 (in late globular stage) and WB296 (in PEM stage) as well as Agrobacterium strain EHA105 containing the plasmid pVspB-OxO (GFP, BAR and OxO). The OxO gene was included because it had been shown to enhance fungal resistance in woody plant species (Liang et al. 2001). Somatic embryos were cultured on E1 medium, a modified Woody Plant Media adopted from Merkle et al. (1991). Agrobacterium growth protocols and plasmid construction are described in Polin et al. (2006). Briefly, Agrobacterium inoculum was made by growing a culture in Luria-Bertani broth (Sambrook et al. 1989) with 60.75 μM rifampicin and 85.8 μM kanamycin to an optical density (OD650) of 1.0. The culture was pelleted by centrifugation, suspended in virulence-induction medium and incubated for three hours on a shaker at 28°C. Each treatment consisted of one Petri dish containing approximately 0.5 g of clone P1-1 somatic embryos or approximately 1.0 g of clone WB296 somatic embryos. At the time of inoculation, all embryos had been growing on E1 medium for 12 days since the last subculture in 60 ×15 mm Petri plates. Three non-wounding treatments (plate flooding, desiccation and blot dry) and three wounding treatments (sand, sonication and vacuum) were tested. The virulence-induction medium was inoculated with Agrobacterium prior to plant tissue inoculation for all treatments except the “no-agro” control.
The plate flooding method consisted of pouring 2 ml of Agrobacterium inoculum onto the embryos while still on E1 medium, removing the liquid after 1 h using a sterile disposable pipette and co-cultivating for 2 days. The desiccation treatment involved transferring the embryo clumps into a 15 ml sterile disposable culture tube with 2 ml of virulence-induction medium for 1 h. The embryos were then removed from the tube and placed on a moistened, sterile filter paper in a Petri dish for 2 days of co-cultivation. For the blot dry treatment, the embryos were inoculated in the same fashion as the desiccation treatment then blotted dry on sterile filter paper and placed onto fresh E1 medium for 2 days of co-cultivation. For the three wounding treatments, the embryos were transferred into 15 ml sterile disposable culture tubes containing 2 ml of Agrobacterium inoculum, wounded and then incubated for 1 h before being placed onto E1 medium for 2 days of co-cultivation. The embryos assigned to the sand treatment were wounded with 100 mg of sterile sand by vortexing the embryos with the sand for 5 s. The embryos assigned to the sonication treatment were sonicated for 60 s in a FS20 Ultrasonic Cleaner (Fisher Scientific). The embryos assigned to the vacuum infiltration treatment were placed in a vacuum chamber for 15 min followed by a rapid return to normal air pressure.
After 2 days of co-cultivation, all embryos were transferred to agro-kill medium (E1 with 474 μM carbenicillin) for 2 weeks and then to selection medium (E1 with 474 μM carbenicillin and 5 μM PPT). All transformed tissues for clone WB296 were isolated from non-transformed tissues 4 weeks post inoculation and callus tissue was separated from the PEMs 2 weeks later. For clone P1-1, embryos were transferred to fresh selection medium every 2 weeks and multiplying transformed embryos were isolated 8 weeks post inoculation. Transformed cell lines were identified by their ability to grow uniformly in the presence of 5 μM PPT as well as the presence of uniform green fluorescence seen under a Nikon Stereoscopic Zoom Microscope SMZ1500 with an En GFPLP 83458M filter (Nikon Instruments Inc., Melville, NY). All treatments were analyzed for variation in the mean number of T-events, number of T-embryos and percentage of T-events that remained embryogenic using SAS 8.2 (SAS Institute Inc., Cary, NC). The experiment was performed a total of four times.
Results
A sample of eight putatively transgenic cell lines was chosen for molecular characterization. DNA was isolated using the CTAB extraction method (Lodhi et al. 1994) from approximately 0.5 g of embryo tissue from each cell line. Southern hybridization reactions and polymerase chain reaction (PCR) were used to detect the gfp gene and the OxO gene, respectively, as per Polin et al. (2006).
The “no-agro” control (somatic embryos that were not inoculated with Agrobacterium) were uniformly GFP negative, stopped growing after 2 weeks and died after 6 weeks on selection medium. Putatively-transformed somatic embryo cell lines were selected each transfer cycle on the basis of vigorous growth on selection medium and GFP expression. It was observed that only embryos that were GFP positive multiplied and, after 2 months, only these GFP positive cell lines remained.
PCR analysis using primers for the OxO gene gave a positive result for all eight lines tested (data not shown) indicating that transformation had occurred. All eight lines also tested positive for the Southern hybridization reactions, showing stable integration of one or more copies of the gfp transgene (mgfp5-ER). Six lines with the strongest signal are shown in Fig. 1.
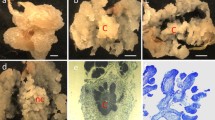
Southern hybridization analysis. (a) Six transgenic cell lines of American chestnut somatic embryos digested with ScaI enzyme and probed with a DNA fragment from the gfp gene. All six lines were derived from clone P1-1 and based on the unique banding patterns, each RR number represents a different transformation event. (b) Left—Non-transformed American chestnut DNA digested with ScaI enzyme and probed with a DNA fragment from the gfp gene. Right—pCambia3301/mgfp5-er plasmid DNA probed with a DNA fragment from the gfp gene
Clone WB296 proved to be difficult to regenerate into whole plants. After three attempts, five shoots were regenerated, but when transferred to shoot multiplication medium they did not multiply and died after a few weeks. Two transformed cell lines of P1-1 were successfully regenerated into phenotypically normal shoot cultures, multiplied for more than 6 months, rooted (Fig. 2), transferred to potting mix and grown in a growth chamber.
Analysis of the data showed a significant difference in clonal homogeneity of variance for T-events, T-embryos and percent embryos, therefore all subsequent statistical analysis was performed separately for each clone. For clone P1-1, there were no significant differences among treatments for T-events (P = 0.067), T-embryos (P = 0.427) or percent embryos (P = 0.536) (Figs. 3a, 4).
(a, b) The effect of six Agrobacterium inoculation/co-cultivation treatments on the mean number of transformation events per Petri dish of American chestnut somatic embryos clones P1-1 (in torpedo stage) and WB296 (in PEM stage). Each column represents the mean of four replications with standard error bars. Each replication is one Petri dish containing approximately 0.5 g (P1-1) and 1.0 g (WB296) of tissue. Desiccation treatment was slowly dried for 2 days, sand treatment was vortexed for 5 s with 100 mg of sterile sand and the sonication treatment was sonicated for 60 s
Percent of transformed cell lines for two American chestnut clones (P1-1 in torpedo stage and WB296 in PEM stage) that remained embryogenic after various Agrobacterium-mediated transformation treatments. Each column represents the mean of four replications with standard error bars. Desiccation treatment was slowly dried for 2 days, sand treatment was vortexed for 5 s with 100 mg of sterile sand and the sonication treatment was sonicated for 60 s
For clone WB296, there were significant differences in the mean number of T-embryos (P = 0.039) and percent embryos (P = 0.001). Single-degree-of-freedom comparisons demonstrated that plate flooding induced significantly higher transformation rates than all other treatments for T-embryos. Plate flooding also had a significantly higher percentage of transformants remain embryogenic (Fig. 4). The mean number of T-embryos induced by plate flooding was more than six-fold higher than blot dry, the next best treatment (Fig. 3b). When the treatments were grouped and the three wounding treatments were compared with the three non-wounding treatments, the percentage of transformants that remained embryogenic were significantly higher (P = 0.002) for the non-wounding treatments but the mean number of T-embryos was not significantly different (P = 0.102). There were no significant differences among treatment means for T-events (P = 0.052) (Fig. 3b).
Discussion
The results of this experiment show that plate flooding is at least as effective as other Agrobacterium-mediated transformation methods and significantly better for one of the two clones evaluated. For clone WB296, plate flooding produced more than a 100-fold higher mean number of transformation events per Petri dish when compared to the desiccation method. For clone P1-1 the desiccation treatment produced a slightly higher mean number of transformation events but a slightly lower mean number of embryogenic events, however, neither difference was statistically significant.
Clearly the two clones responded differently to the various wounding and co-cultivation treatments, particularly to desiccation. The most obvious morphological difference between the two clones is that WB296 multiplies as tiny clumps of cells called proembryogenic masses and P1-1 multiplies as considerably larger clumps of torpedo stage embryos (von Arnold et al. 2002). It may be that the tiny PEMs of clone WB296 are more susceptible to desiccation damage than the relatively massive torpedo-stage clumps of P1-1. Polin et al. (2006) tested multiple clones using the desiccation method and also observed large differences in transformation frequency among clones. Other clones will have to be tested to discern if plate flooding will be a broadly applicable transformation method for American chestnut.
Wounding was found not to be necessary for transforming American chestnut somatic embryos. This agrees with the findings of McGranahan et al. (1988) for walnut and Mondal et al. (2001) for tea. We also found that for clone WB296, wounding lowers the percentage of transformation events that remain embryogenic (Fig. 4). Since American chestnut thus far cannot be regenerated from callus, maintaining the embryo-forming potential is important for regenerating transgenic whole plants.
Plate flooding should also be tested on somatic embryos of other plant species to determine if it can increase transformation rates and reduce the amount of time needed to inoculate the plant tissues.
Abbreviations
- WB296:
-
American chestnut somatic embryo clone WB296-10A-2
- BAR:
-
Bialaphos (and PPT)-resistance
- GFP:
-
Green fluorescent protein
- OxO:
-
Oxalate oxidase
- P1-1:
-
American chestnut somatic embryo clone Pond1-1
- PEM:
-
Pro-embryogenic mass
- PPT:
-
Phosphinothricin
- T-embryos:
-
Transformed embryogenic cell lines
- T-events:
-
Transformed cell lines (embryos plus callus)
References
Carraway DT, Merkle SA (1997) Plantlet regeneration from somatic embryos of American chestnut. Can J Forest Res 27:1805–1812
Cheng M, Hu T, Layton J, Liu C, Fry JE (2003) Desiccation of plant tissues post-Agrobacterium infection enhances T-DNA delivery and increases stable transformation efficiency in wheat. In Vitro Cell Dev Biol Plant 39:595–604
Corredoira E, Montenegro D, San-Jose MC, Vieitez AM, Ballester A (2004) Agrobacterium-mediated transformation of European chestnut embryogenic cultures. Plant Cell Rep 23:311–318
de la Riva GA, Gonzalez-Cabrera J, Vazquez-Padron R, Ayra-Pardo C (1998) Agrobacterium tumefaciens: a natural tool for plant transformation. Electron J Biotechnol 1:1–16
Horsch RB, Fry JE, Hoffmann NL, Eichholtz D, Rogers SG, Fraley RT (1985) A simple and general method for transferring genes into plants. Science 227:1229–1231
Liang H, Maynard CA, Allen RD, Powell WA (2001) Increased Septoria musiva resistance in transgenic hybrid poplar leaves expressing a wheat oxalate oxidase gene. Plant Mol Biol 45:619–629
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994) A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol Biol Rep 12:6–13
Mathews H, Litz RE, Wilde HD, Merkle SA, Wetzstein HY (1992) Stable integration and expression of β-glucuronidase and NPTII genes in mango somatic embryos. In Vitro Cell Dev Biol 28P:172–178
McGranahan GH, Leslie CA, Uratsu SL, Martin LA, Dandekar AM (1988) Agrobacterium-mediated transformation of walnut somatic embryos and regeneration of transgenic plants. Biotechnology 6:800–804
Merkle SA, Wiecko AT, Watson-Pauley BA (1991) Somatic embryogenesis in American chestnut. Can J Forest Res. 21:1698–1701
Merkle SA, Carraway DT (1994) Somatic embryogenesis and gene transfer in American chestnut. Biotechnology of trees. Madrid: INIA, 199–209
Mondal TK, Bhattacharya A, Ahuja PS, Chand PK (2001) Transgenic tea [Camellia sinensis (L.) O Kuntze cv Kangra Jat] plants obtained by Agrobacterium-mediated transformation of somatic embryos. Plant Cell Rep 20:712–720
Polin LD, Liang H, Rothrock RE, Nishii M, Diehl DL, Newhouse AE, Nairn CJ, Powell WA, Maynard CA (2006) Agrobacterium-mediated transformation of American chestnut (Castanea dentata (Marsh.) Borkh.) somatic embryos. Plant Cell Tiss Org Cult 84:69–78
Sambrook J, Fritsch EE, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd ed, Cold Spring Harbor, NY
Scorza R, Morgens PH, Cordts JM, Mante S, Callahan AM (1990) Agrobacterium-mediated transformation of peach (Prunus persica L. Batsch) leaf segments, immature embryos, and long-term embryogenic callus. In Vitro Cell Dev Biol 26:829–834
Trick HN, Finer JJ (1997) SAAT: sonication-assisted Agrobacterium-mediated transformation. Trans. Res. 6:329–336
von Arnold S, Sabina I, Bozhkov P, Dayachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tiss Org Cult 69:233–249
Xing Z, Powell WA, Maynard CA (1999) Development and germination of American chestnut somatic embryos. Plant Cell Tiss Org Cult 57:47–55
Acknowledgements
The authors thank the New York State Chapter of The American Chestnut Foundation for financial support and donation of somatic embryo cell line P1-1, ArborGen LLC for financial support and technical advice, Joyce Fry and John Dougherty for technical advice, the Monsanto Fund for financial support and finally Dr. Scott Merkle at The Warnell School of Forest Resources at The University of Georgia for the donation of somatic embryo cell line WB296.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rothrock, R.E., Polin-McGuigan, L.D., Newhouse, A.E. et al. Plate flooding as an alternative Agrobacterium-mediated transformation method for American chestnut somatic embryos. Plant Cell Tiss Organ Cult 88, 93–99 (2007). https://doi.org/10.1007/s11240-006-9170-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-006-9170-7