Abstract
Tranexamic acid (TXA) can reduce blood loss and transfusion rates in orthopaedic surgery. In this regard, a new viscoelastometric test (TPA-test, ClotPro), enables the monitoring of TXA effects. This prospective observational study evaluated and correlated TXA plasma concentrations (cTXA) following intravenous and oral administration in patients undergoing elective orthopaedic surgery with lysis variables of TPA-test. Blood samples of 42 patients were evaluated before TXA application and 2, 6, 12, 24 and 48 h afterwards. TPA-test was used to determine lysis time (LT) as well as maximum lysis (ML) and cTXA was measured using Ultra-High-Performance-Liquid-Chromatography/Mass-Spectrometry. Data are presented as median (min–max). LTTPA-test and MLTPA-test correlated with cTXA (r = 0.9456/r = 0.5362; p < 0.0001). 2 h after intravenous TXA administration all samples showed complete lysis inhibition (LTTPA-test prolongation: T1: 217 s (161–529) vs. T2: 4500 s (4500–4500);p < 0.0001), whereas after oral application high intraindividual variability was observed as some samples showed only moderate changes in LTTPA-test (T1: 236 s (180–360) vs. T2: 4500 s (460–4500); p < 0.0001). Nevertheless, statistically LTTPA-test did not differ between groups. MLTPA-test differed 2 h after application (i.v.: 9.0% (5–14) vs. oral: 31% (8–97); p = 0.0081). In 17/21 samples after oral and 0/21 samples after intravenous administration cTXA was < 10 µg ml−1 2 h after application. TPA-test correlated with cTXA. MLTPA-test differed between intravenous and oral application 2 h after application. Most patients with oral application had TXA plasma concentration < 10 µg ml−1. The duration of action did not differ between intravenous and oral application. Additional studies evaluating clinical outcomes and side-effects based on individualized TXA prophylaxis/therapy are required.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Viscoelastometry now enables bedside monitoring of tranexamic acid effects
-
Viscoelastometric variables correlate well with tranexamic acid plasma concentrations in orthopaedic patients
-
Oral administration of tranexamic acid shows high variability in lysis inhibition measured by viscoelastometry
-
New viscoelastometric tests will monitor tranexamic effects and can help to drive antifibrinolytic therapy
Introduction
Administration of tranexamic acid (TXA) is an accepted standard-treatment and prophylaxis of hyperfibrinolysis during major surgery, as TXA has been shown to reduce blood loss and transfusion-rates in total knee and hip arthroplasty [1,2,3,4]. Surprisingly little is known about dosage adaptation in conditions which influence TXA effect duration and plasma peak concentrations, like impaired kidney function [5, 6]. Therefore, monitoring of TXA effects and effect duration is of interest, as underdosed TXA can increase blood loss and an overdosage can extensively inhibit fibrinolysis (fibrinolytic shutdown) which can be associated with thromboembolic complications and organ failure [7].
Several studies and meta-analyses give recommendations regarding dosages, route of application and side-effects [1,2,3, 8]. To date, some data implicate that perioperative reduction of blood loss is independent of the route of application although TXA plasma concentrations are higher with intravenous administration [3]. Nevertheless, oral administration is increasingly used to reduce side effects which might occur with high peak concentrations following intravenous application like seizures or thromboembolic events. However, differences between intravenous and oral application on lysis variables have not yet been analysed.
Therefore, we evaluated a newly available viscoelastic test (TPA-test, ClotPro, enicor GmbH, Munich, Germany) to determine fibrinolytic responses over time after TXA application in patients undergoing orthopaedic surgery. We hypothesized that the variables provided by the TPA-test correlate with TXA plasma concentrations (cTXA) enabling an evaluation of the individual fibrinolytic response. Secondary we investigated differences on lysis variables and TXA plasma concentrations between oral and intravenous administration over time.
Material and methods
The study was approved by the Ludwig-Maximilians-University ethics committee (No. 17–525-4), registered in the German clinical trials database (DRKS00015269), and performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients prior to study inclusion.
42 patients undergoing elective orthopaedic surgery (primary total hip or primary/partial knee replacement surgery/shoulder surgery and spine surgery) were included in this study. Exclusion criteria were age < 18 years, coagulation disorders (e.g. pre-existing coagulation disorder, oral anticoagulation, myelodysplastic syndrome), convulsive disorders, intake of TXA within the last 2 weeks prior to surgery or refusal of the patient. All patients obtained standardized general anaesthesia according to institutional standards. In detail, after transfer to the anaesthesia induction room, patient’s vital signs were monitored, and general anaesthesia was initiated using sufentanil 0.5–1 µg kg−1 body weight (BW), propofol 2 mg kg−1 BW and rocuronium 0.6 mg kg−1 BW.
Patients were included between 10/01/2018 and 03/01/2019. Until end 12/31/2018 patients received TXA intravenously. Becoming effective on 01/01/2019 standard operating procedure (SOP) had been changed to oral administration based on a meta-analysis implicating that reduction of perioperative blood loss is independent of way of application to an oral application. Thus, patients obtained either 1 gr TXA intravenously or orally, depending on time of study inclusion. Measurements were done before TXA application (T1), after 2 h (T2), 6 h (T3), 12 h (T4), 24 h (T5) and 48 h (T6). TXA was administered only once in all patients.
At every time point coagulation analyses (standard laboratory and viscoelastometric tests) were performed within 2 h after blood withdrawal and plasma was immediately stored at −80 °C for further investigations.
Viscoelastometry
Viscoelastometric tests were performed using the point of care device ClotPro (enicor GmbH, Munich, Germany) and its consumables following manufacturer’s instructions [9]. ClotPro is a newly introduced viscoelastometric system based on the original technique introduced by Hartert et al. [10]. Briefly summarized, clot formation is measured using a cup and a pin. The pin is stationary in this system whereas the cup rotates during the measurement. During the progress of clot formation, the rotation of the cup is restricted. The change is detected by the analyzer and is then graphically transformed to the viscoelastometric amplitude. ClotPro has six independent channels for parallel testing. All tests are heparin insensitive due to the addition of polybrene, and all reagents are provided in a ready-to use dry reagent format. All samples were analysed under standardized conditions (temperature 37 °C and maximum run time 4500 s). All viscoelastometric tests were started within two hours after blood withdrawal and done in a randomized order throughout the study. Samples were stored in heat bench of the ClotPro device (37 °C). Quality controls and round robin tests were routinely performed as recommended by the manufacturer.
The following tests were performed at all time points and for all blood samples [11]: 1. TPA-test: Coagulation is initiated by recalcification and recombinant tissue factor. TPA is added to induce fibrinolysis within the sample. TPA-test was performed twice for every sample and the mean was used for analysis. 2. EX-test: This test represents the extrinsic pathway. Coagulation is again initiated by recalcification and recombinant tissue factor. 3. FIB-test: FIB-test is similar to EX-test but with cytochalasin D added to block platelet function. Thus, FIB-test provides information on the contribution of functionally active fibrinogen to clot firmness.
The following variables were analysed throughout the study: Clotting time (CT; time from initiation of the test to 2 mm clot amplitude), clot formation time (CFT; CT until clot amplitude of 20 mm), A5, A10 (clot amplitude 5 and 10 min after CT), maximum clot firmness (MCF; maximum amplitude of the clot), maximum lysis (ML; maximum lysis in relation to MCF at any time point of the measurement), lysis time (LT; time from CT until 50% lysis of MCF; in case a 50% lysis did not occur, i.e. due to lysis inhibition, LT was set to maximum run time of the test, which is 4500 s) and lysis onset time (LOT; time from CT until 15% lysis of MCF).
Standard laboratory tests
Standard laboratory tests were performed by the LMU Munich institute for laboratory medicine. These tests included international normalized ratio (INR) (Thromborel S, Siemens Healthcare GmbH, Erlangen, Germany), activated partial thromboplastin time (aPTT) (Actin FSL, Siemens Healthcare GmbH, Erlangen, Germany) and blood count. Fibrinogen plasma concentrations were measured by Clauss method (optical measurement, Multifibren U, Siemens Healthcare GmbH, Erlangen, Germany). Tests were performed on BCS XP (Siemens Healthcare GmbH, Erlangen, Germany). Furthermore, serum creatinine (creatinine OSR6178 (Beckman Coulter); AU 5800 / AU 680 (Beckman Coulter)) was measured.
Tranexamic acid plasma concentration
Tranexamic acid plasma concentration was assessed by Ultra-High Performance Liquid Chromatography/ Mass Spectrometry (UHPLC-MS/MS) as described by Barreiros et al. [12].
Statistics
Data are presented as median ± range (minimum – maximum) unless indicated otherwise. Statistical analysis was performed using GraphPad Prism eight (La Jolla, USA). Statistical differences between timepoints or conditions (oral or intravenous administration) were analysed using two-way-ANOVA and Tukey’s multiple comparison test. Alpha error was adjusted for multiple testing (p = 0.05/n). A priori sample size calculation had been performed using G*Power 3.1 (https://www.gpower.hhu.de/; Düsseldorf, Germany) and revealed 21 patients per group (Two-way-ANOVA, power = 0.8, effect size = 0.25).
Results
Patients’ characteristics and preoperative medication are displayed in Table 1. All patients obtained TXA upon arrival at the operating area following institutional standards. All patients had elective orthopaedic surgery, with a median intraoperative blood loss of 375 ml (100–2000).
First, we analysed cTXA plasma concentrations: Following intravenous administration of TXA, at 2 h (T2) plasma concentration increased compared to baseline (T1: 0 µg ml−1 vs. T2: 20.8 µg ml−1 (10.7–43.0); p < 0.0001) whereas after oral administration median plasma concentration was low (7.8 µg ml−1 (1.0–76.0)) and not statistically different from baseline (p = 0.1147). In detail, 2 h after application 17/21 (81%) patients with oral intake but none of the patients (0/21; 0%) with intravenous administration had TXA plasma concentrations below the recommended plasma concentration of 10 µg ml−1. Of note, at later timepoints (T3–T6) cTXA did not differ between i.v. and oral application but was still increased up to 48 h after application (see Fig. 1a). Blood loss did not differ between patients with intravenous and oral administration (i.v.: 400 ml (100–1200) vs. oral: 350 ml (150–2000); p = 0.4676).
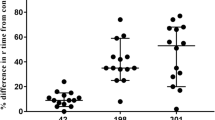
TXA plasma concentrations and viscoelastometric variables (n = 42) a TXA plasma concentration over time b Lysis time (LTTPA-test) = time span between clotting time (CT) and 50% lysis of MCF c Lysis Onset Time (LOTTPA-test) = time span from CT to 15% lysis of MCF d Maximum Lysis (MLTPA-test) = difference between Maximum Clot Firmness (MCF) and lowest amplitude in % of MCF Data presented as scatter dot plot with median and interquartile range. *p< 0.05, **p< 0.0001vs. baseline (before tranexamic acid) for intravenous application §p< 0.05, §§p< 0.0001vs. baseline (before tranexamic acid) for oral application; #p< 0.05, ##p< 0.0001vs. baseline (before tranexamic acid) for intravenous vs. oral application TXA: tranexamic acid
Second, we analysed whether cTXA and thromboelastometric variables correlate, and report a strong association between TXA plasma concentration and LTTP-test (i.v.: r = 0.9454; p < 0.0001/ oral: r = 0.9455; p < 0.0001) and LOTTPA-test (i.v.: r = 0.8786; p < 0.0001/ oral: r = 0.8612; p < 0.0001). Of note correlation with MLTP-test (i.v.: r = 0.5483; p < 0.0001/ oral: r = 0.5181; p < 0.0001) was intermediate, as even endogenous factors can influence MLTP-test.
Finally, we analysed the impact of TXA administration on different thromboelastometric variables:
Maximum lysis (ML)
MLTPA-test decreased from baseline to T2 (i.v.: T1: 96%; (93–97) vs. T2: 9% (3–20); p < 0.0001 / oral: T1: 96% (93–97) vs. T2: 31% (16–89); p < 0.0001; Fig. 1e). However, most important, at T2 MLTPA-test was different between i.v. and oral application (i.v.: 9.0% (5–14) vs. oral: 31% (8–97); p = 0.0081). During the time course and with decreasing TXA plasma concentrations, MLTPA-test again increased and returned to baseline values at T6 (48 h) and did not differ between i.v. and oral application at any other point of measurement (Fig. 1d).
Lysis time (LT)
At T1 (before TXA application) all samples showed rapid lysis with a median LTTPA-test of 232 s (161–529). Following TXA application at T2 (2 h after TXA administration) all samples after intravenous application showed strong lysis inhibition, and LTTPA-test even exceeded the test run time of 4500 s (T1: 217 s (161–529) vs. T2: 4500 s (4500–4500); p < 0.0001), whereas with oral application LTTPA-test showed higher variability and ranged from 460–4500 s. However, in contrast to cTXA, LTTPA-test at T2 following oral administration was significantly different from baseline (T1: 236 s (180–360) vs. T2: 4500 s (460–4500); p < 0.0001) (Fig. 1b) indicating that TPA-test is a sensitive tool to detect changes in fibrinolytic capacity. Thus, LTTPA-test was more sensitive in detecting changes from baseline then cTXA. Furthermore, LTTPA-test was not different between i.v. and oral application at T2 (p = 0.0921) and all other time (T3–T6; p = n.s). Of note, in 6/21 (29%) patients with oral application LTTPA-test increased until T3, whereas there was no change in the i.v. patients (p = 0.0207). Over the following timepoints, LTTPA-test decreased again, but remained prolonged as long as 48 h after application (i.v.: T1: 217 s (161–529) vs. T6: 353 s (206–1289); p = 0.0025 and oral: T1: 236 s (180–360) vs. T6: 302 s (241–538); p = 0.0004) (see Fig. 1b).
Lysis onset time (LOT)
Again, LOTTPA-test increased after administration of TXA at T2 compared to baseline (i.v.: T1: 74 s (66–116) vs. T2: 210 s (141–314); p < 0.0001 / oral: T1: 77 s (69–100) vs. T2: 185 s (120–246); p < 0.0001; Fig. 1c). After oral application LOTTPAtest further increased compared to T2 (T2: 185 s (120–246) vs. T3: 202 s (134–286; p < 0.0001). There was no difference in LOTTPA-test between i.v. and oral application at any point of measurement (Fig. 1c).
To identify variables affecting cTXA we performed a multiple linear regression which revealed weight and body mass index (BMI) as strong variables influencing cTXA (weight: p = 0.015/ BMI: p = 0.004).
Discussion
This is the first study to evaluate tranexamic acid effects on fibrinolysis using a bedside available TPA-based viscoelastometric test in patients undergoing orthopaedic surgery. First, viscoelastometric lysis variables correlate well with measured TXA plasma concentrations. Second, 2 h after oral application of 1 gr TXA, 81% of patients had TXA plasma concentrations below 10 µg ml−1, but none (0%) after intravenous application. Accordingly, lysis inhibition (maximum lysis) differed between intravenous and oral administration 2 h after intake. Third, thromboelastometric lysis variables were prolonged as long as 48 h after application irrespective of way of application.
It is known, that tranexamic acid (TXA) can reduce blood loss and transfusion rates in orthopaedic surgery [1, 13]. Furthermore, various meta-analyses evaluated different ways of application and dosages of TXA to give recommendations for therapy regimens [2, 4]. Thus, actually it is widely accepted that plasma concentrations of 10–15 µg ml−1 inhibit fibrinolysis without exposing the patient to high risk for adverse effects [14]. Interestingly, 2 h after oral intake 81% of the patients failed to reach the plasma concentration threshold of 10 µg ml−1. This means that after oral application many patients did not reach a recommended TXA plasma concentration for orthopaedic surgery.
Supporting this fact, ML was 31% following oral application and significantly higher than with intravenous administration (9%). Furthermore, following oral intake plasma concentrations and lysis variables highly varied at time of surgery (2 h) and lysis inhibition was more pronounced at 6 h (after surgery), which might put the patient at risk for postoperative thromboembolic events. Additionally, plasminogen activator inhibitor (PAI) is upregulated postoperatively, which further increases clot stability [15]. In contrast intravenous application showed early and effective lysis inhibition at time of surgery. Furthermore, variability in plasma concentrations was lower at 2 h. Duration of action nevertheless was not different between ways of application, and as long as 48 h.
At present, oral application of TXA is widely used in the consideration that it might lead to lower peak plasma concentrations and probably fewer side effects with the same effect of reduced blood loss and transfusion rates [3]. An accepted oral dose prior to elective orthopaedic surgery is 1 g of TXA which was shown not be inferior to multiple dosing or higher doses regarding transfusion rates and blood loss [3]. This single dosing differs to other fields (e.g. gynecology) in which higher daily doses and multiple dosing is common.
Lower peak plasma concentrations may therefore reduce concerns of clinicians for venous (VTE) or arterial thromboembolic events (ATE) when using TXA as prophylactic medication. A recent meta-analysis evaluated 78 studies and concluded that there is no increased risk of VTE in orthopaedic patients receiving total joint arthroplasty [1]. Nevertheless, the group also concluded that there are only few high-quality studies investigating this topic, especially regarding high risk patients (ASA status three and higher). The authors of this meta-analysis recommend an individual consideration for TXA usage in high risk patients. Individual consideration includes pre-existing diseases like chronic renal impairment or history of VTEs. Especially renal impairment influences TXA plasma concentrations due to the reduced renal elimination [16]. To date the lack of bedside monitoring options for TXA effects led to several studies evaluating pharmacokinetic modelling of TXA [5, 6, 17]. This approach helps to predict the individual dosages for every patient. However, these models are theoretical and do not allow to check the individual response, limiting their clinical application. But, based on these models an individual dosing seems preferable. Regardless, current guidelines for acute trauma or perioperative bleeding still recommend standard dosing [18, 19].
We showed that the newly available viscoelastometric TPA-test is feasible in orthopaedic surgery and it allows to evaluate the individual response to TXA. The combined use of pharmacokinetic modeling and viscoelastometric testing might help to reduce under-/overdosing in the future to avoid side effects like seizures, thromboembolic events or increased bleeding [20,21,22,23]. Considering this aspect, the new TPA-test could be useful in situations of re-do surgery, e.g. in situations of bleeding and possible repetition of TXA. Especially, as the TPA-test is feasible to detect inhibition of fibrinolysis, even in patients with low cTXA plasma concentration of < 10 µg ml−1. Thus, one might emphasize, that even lower cTXA plasma concentrations below 10 µg ml−1 might be sufficient to block fibrinolysis during specific surgical conditions. However, this hypothesis has to be proved in further studies with focus on blood loss and transfusion rates.
Our study has some limitations. First of all, our trial was not designed to detect clinical outcome variables like bleeding volumes, transfusion rates or side-effects. The study was conducted to evaluate the feasibility of a bedside-available new viscoelastometric assay and to assess potential differences between oral and i.v. application. The sample size was calculated to show differences of TXA effect duration over the time course. Nevertheless, the study provides implications for further clinical trials investigating clinical outcome variables based on individualized TXA prophylaxis and therapy.
Conclusion
This study is the first to evaluate tranexamic acid effects on fibrinolysis using a bedside available TPA-based viscoelastometric test in patients undergoing orthopaedic surgery.
First, viscoelastometric lysis variables correlate well with measured TXA plasma concentrations. Second, 2 h after oral application of 1 gr TXA, 81% of patients had TXA plasma concentrations below 10 µg ml−1, but none (0%) after intravenous application. Thus, lysis inhibition (maximum lysis) differed between intravenous and oral administration 2 h after intake. Third, cTXA and lysis variables returned to baseline not until 48 h after application irrespective of way of application.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Fillingham YA, Ramkumar DB, Jevsevar DS et al (2018) The safety of tranexamic acid in total joint arthroplasty: a direct meta-analysis. J Arthroplasty 33:3070–3082.e1
Fillingham YA, Ramkumar DB, Jevsevar DS et al (2018) The efficacy of tranexamic acid in total hip arthroplasty: a network meta-analysis. J Arthroplasty 33:3083–3089.e4
Fillingham YA, Ramkumar DB, Jevsevar DS et al (2018) Tranexamic acid use in total joint arthroplasty: the clinical practice guidelines endorsed by the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. J Arthroplasty 33:3065–3069
Fillingham YA, Ramkumar DB, Jevsevar DS et al (2019) Tranexamic acid in total joint arthroplasty: the endorsed clinical practice guides of the American Association of Hip and Knee Surgeons, American Society of Regional Anesthesia and Pain Medicine, American Academy of Orthopaedic Surgeons, Hip Society, and Knee Society. Reg Anesth Pain Med 44:7–11
Grassin-Delyle S, Tremey B, Abe E et al (2013) Population pharmacokinetics of tranexamic acid in adults undergoing cardiac surgery with cardiopulmonary bypass. Br J Anaesth 111:916–924
Yang QJ, Jerath A, Bies RR et al (2015) Pharmacokinetic modeling of tranexamic acid for patients undergoing cardiac surgery with normal renal function and model simulations for patients with renal impairment. Biopharm Drug Dispos 36:294–307
Moore HB, Moore EE, Liras IN et al (2016) Acute fibrinolysis shutdown after injury occurs frequently and increases mortality: a multicenter evaluation of 2,540 severely injured patients. J Am Coll Surg 222:347–355
Lazic I, Haug AT, von Eisenhart-Rothe R (2020) Perioperativer einsatz der tranexamsäure in der endoprothetik. Knie J 2:3–8
Calatzis A, Wittwer M, Leyser H et al (2018) CloPro - a new generation viscoelastic whole blood coagulation analyzer. Hämostaseologie P013, Schattauer, Vienna
Hartert H (1951) Thrombelastography, a method for physical analysis of blood coagulation. Z Gesamte Exp Med 117:189–203
Whiting D, DiNardo JA (2014) TEG and ROTEM: technology and clinical applications. Am J Hematol 89:228–232
Barreiros L, Amoreira JL, Machado S et al (2019) Determination of tranexamic acid in human plasma by UHPLC coupled with tandem mass spectrometry targeting sub-microgram per milliliter levels. Microchem J 144:144–150
Melvin JS, Stryker LS, Sierra RJ (2015) Tranexamic acid in hip and knee arthroplasty. J Am Acad Orthop Surg 23:732–740
Picetti R, Shakur-Still H, Medcalf RL et al (2019) What concentration of tranexamic acid is needed to inhibit fibrinolysis? A systematic review of pharmacodynamics studies. Blood Coagul Fibrinolysis 30:1–10
Griemert E-V, Schwarzmaier SM, Hummel R et al (2019) Plasminogen activator inhibitor-1 augments damage by impairing fibrinolysis after traumatic brain injury. Ann Neurol 85:667–680
Andersson L, Eriksson O, Hedlund PO et al (1978) Special considerations with regard to the dosage of tranexamic acid in patients with chronic renal diseases. Urol Res 6:83–88
Dowd NP, Karski JM, Cheng DC et al (2002) Pharmacokinetics of tranexamic acid during cardiopulmonary bypass. Anesthesiology 97:390–399
Kozek-Langenecker SA, Ahmed AB, Afshari A et al (2017) Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol 34:332–395
Spahn DR, Bouillon B, Cerny V et al (2019) The European guideline on management of major bleeding and coagulopathy following trauma. Crit Care. https://doi.org/10.1186/s13054-019-2347-3
Hulde N, Zittermann A, Deutsch M-A et al (2019) Tranexamic acid and convulsive seizures after off-pump coronary artery bypass surgery: the role of renal insufficiency. Interact Cardiovasc Thorac Surg 29:852–854
Couture P, Lebon J-S, Laliberté É et al (2017) Low-dose versus high-dose tranexamic acid reduces the risk of nonischemic seizures after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 31:1611–1617
Chornenki NLJ, Um KJ, Mendoza PA et al (2019) Risk of venous and arterial thrombosis in non-surgical patients receiving systemic tranexamic acid: a systematic review and meta-analysis. Thromb Res 179:81–86
Benipal S, Santamarina J-L, Vo L, Nishijima DK (2019) Mortality and thrombosis in injured adults receiving tranexamic acid in the Post-CRASH-2 era. West J Emerg Med 20:443–453
Funding
The study was funded by institutional resources. Furthermore, reagents and viscoelastometric machines were provided by enicor GmbH, Munich.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Groene, P., Sappel, S.R., Saller, T. et al. Functional testing of tranexamic acid effects in patients undergoing elective orthopaedic surgery. J Thromb Thrombolysis 51, 989–996 (2021). https://doi.org/10.1007/s11239-020-02272-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02272-8