Abstract
Historical data indicate that approximately 10% of acute coronary syndrome patients have no obstructive coronary artery disease (CAD) but contemporary incidence of non-obstructed coronary arteries in ST-segment elevation myocardial infarction (STEMI) is not clear. We aimed both to identify the contemporary incidence of MI without obstructive CAD (MINOCA)—using the ESC definition—and assess clinical outcomes. We assessed consecutive unselected STEMI patients presenting to the cardiac catheterisation laboratory with a view to undergoing primary percutaneous coronary intervention (PPCI). MINOCA was defined according to ESC criteria. Electronic patient records, blood results, angiographic and echocardiographic data were interrogated to determine final diagnosis, as well as 30-day and 1-year mortality rate. Of 2521 patients with full electronic dataset, 2158 (85.6%) underwent PPCI for obstructive CAD (angiographic stenosis > 70%). A further 167 (6.6%) with obstructive CAD were treated medically or surgically. The remaining 196 (7.8%) patients had absence of obstructive CAD at angiography, of whom 167 had no stenosis (< 30%) and 29 had mild coronary atheroma (stenosis > 30% but < 50%). A total of 110 (4.4%) patients met diagnostic criteria for MINOCA. All-cause mortality at 30-days and 1-year were 3.6% and 4.5%, respectively. In our cohort, 1 in 20 patients presenting with STEMI had MINOCA. This is the first description of the relatively high incidence of MINOCA in a STEMI cohort using current ESC definition and diagnostic criteria and could help power future trials in this area. Mortality rate was relatively high in our study and similar to that in large meta-analyses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
The incidence of MINOCA amongst patients presenting with STEMI is unclear.
-
Our study shows that 4% of patients presenting with STEMI for PPCI have MINOCA as defined by the ESC.
-
Mortality of patients with MINOCA is relatively high with 3.6% at 30 days and 4.5% at 1 year.
-
The heterogenous aetiology highlights the need to actively seek the underlying diagnosis using diagnostic algorithms recommended by the ESC and the COVADIS group.
-
The relatively high incidence of MINOCA in a STEMI cohort using current ESC definition and diagnostic criteria and could help power future trials in this area for novel and targeted therapies.
Introduction
Approximately 90% of patients with myocardial infarction have angiographic evidence of obstructive coronary artery disease (CAD) based on registry studies published more than 30 years ago [1, 2]. The realisation that obstructive CAD was causative in the majority of patients with ST-segment elevation myocardial infarction (STEMI), led to the development of current management strategies including primary percutaneous coronary intervention (PPCI). In contrast to the clear aetiology and guidelines for the management of STEMI with CAD, in the 10% of patients who experience myocardial infarction in the absence of obstructive CAD, the aetiology is often obscure and optimal management unclear [3]. Until recently, myocardial infarction with non-obstructive coronary arteries (MINOCA) was a “Cinderella” condition: little known, little understood and under-appreciated. In 2015, Pasupathy et al. published a comprehensive systematic review of patients with suspected myocardial infarction without obstructive CAD [4] and in 2017, the European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacotherapy published a position paper which was, arguably, the first authoritative statement on definition, clinical features and recommended investigations in patients with MINOCA [5].
Although a large registry of patients with non-ST-elevation myocardial infarction published 10 years ago showed the incidence of non-obstructive CAD to be 10% [6], another registry around the same time showed the incidence of angiographically normal coronaries in acute coronary syndrome to be only 2.8% [7]. These studies did not define the incidence of MINOCA in STEMI, and MINOCA definition was not based on contemporary criteria.
MINOCA is not a benign condition; a meta-analysis indicated that 1-year all-cause mortality was 4.7% [4]. More recently, data from 2003 to 2013 SWEDEHEART registry revealed that over a 4-year follow-up, 23.9% patients with MINOCA experienced a major cardiac event [8].
Establishing the true incidence of MINOCA among patients presenting with STEMI is important as these patients may require specific emergency investigation and ad hoc treatments that may differ from those currently recommended in conventional CAD STEMI. Future trials of new treatments for MINOCA STEMI patients will need to be adequately powered based on contemporary incidence, aetiology and outcomes.
It was our aim to identify the contemporary incidence of MINOCA amongst patients with ST-segment elevation, delineate the underlying diagnoses using the ESC definition [5] and assess 1-year clinical outcomes.
Methods
We assessed all consecutive patients with ST-elevation admitted with a view to PPCI, to East and North Hertfordshire NHS Trust and Norfolk and Norwich University Hospital, United Kingdom. These Heart Attack Centres (HAC) serve a population of 1.5 million, supported by East of England Ambulance Service NHS Trust. According to standard protocol, all patients who meet the criteria for STEMI are brought directly to the HAC for emergency PPCI. Criteria for PPCI protocol activation are symptoms compatible with an acute myocardial infarction within 12 h with any of the following electrocardiographic (ECG) criteria: ST-segment elevation ≥ 1 mm in contiguous limb leads, > 2 mm in contiguous chest leads, bundle branch block believed to be new in the context of acute cardiac-sounding chest pain, or patients resuscitated from cardiac arrest with ECG criteria as above. All patients who met these criteria were included.
Patients with MINOCA according to the ESC position paper and InterTAK Diagnostic Criteria [5, 9] were identified by review of all cases in the HAC activation database.
Initial MINOCA screening diagnosis required the presence of all of the following criteria:
-
(1)
Meet Universal Definition of Acute Myocardial Infarction criteria [10].
-
(2)
No obstructive CAD at angiography, defined as no stenosis (diameter reduction) ≥ 50% in any potential infarct-related artery.
-
(3)
No other clinically-overt cause for the specific presentation.
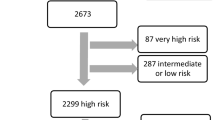
Patients were identified using a predefined flowchart (Fig. 1) to ensure a standardised approach. Electronic patient records, blood results, angiographic data and echocardiographic data were used to determine diagnosis, based on ESC recommendations for diagnostic work-up [11].
Flowchart for identification of patients. For the purposes of this registry, the criteria for definition of myocardial infarction included a positive cardiac biomarker defined as a rise and/or fall in serial levels with at least 1 value above the 99th percentile upper reference limit and clinical evidence of myocardial infarction as evidenced by ischaemic symptoms and/or ischaemic changes manifesting in new ST-segment changes or left bundle branch block. Patients with fixed ST-segment changes without elevation of cardiac enzymes and subsequently labelled as having “normal variant” ECGs, were excluded from analysis
Results
We assessed 2521 consecutive unselected patients with ST-elevation fulfilling criteria for PPCI, with full electronic dataset (Fig. 2). Of these, 85.6% underwent PPCI for obstructive CAD. A further 6.6% patients with obstructive CAD were treated medically or with surgical revascularisation. Angiographically-significant CAD was absent in 7.8% patients. A total of 110 patients (4.4% of all STEMIs) met diagnostic criteria for MINOCA, 54% were male, with mean age 63.5 ± 13.9 years.
The aetiology of MINOCA was determined to be a coronary cause in 28%, non-coronary cardiac cause in 61% and non-coronary extra-cardiac cause in 11% of patients. Coronary causes included plaque disruption (39%), coronary spasm (10%), spontaneous coronary artery dissection (19%), coronary embolism (23%) and aortic dissection (10%).
Non-coronary cardiac causes included myocarditis (36%), Takotsubo syndrome (30%) and type 2 myocardial infarction (34%)—the latter comprising patients with cardiomyopathy, anaemia, valvular disease and arrhythmia.
Non-coronary (extra-cardiac) causes included pulmonary embolism (50%), cerebrovascular event (8%), and other causes included sepsis, gallstone pancreatitis and extracardiac tumour compressing the heart.
In the remaining 86 patients with absence of angiographically-significant CAD, the final diagnoses were predominantly pericarditis, myocarditis and normal-variant ECG.
In MINOCA patients, 30-day all-cause mortality was 3.6% and 1-year mortality was 4.5%.
Discussion
The main findings of our study are that the incidence of MINOCA in a contemporary cohort of patients presenting with STEMI is 4.4%, and that 30-day and 1-year mortality rate are 3.6% and 4.5%, respectively.
The incidence of MINOCA -using the diagnostic criteria proposed by the Agewall position paper [5] and specifically within the STEMI and PPCI setting- has not been reported previously. Knowing the incidence is important for planning future studies of treatment for this cohort, particularly in the acute phase, as treatments may differ markedly between STEMI patients with CAD and MINOCA patients. In the most recent publication on the incidence of this condition in STEMI, derived from the HORIZONS-AMI trial conducted > 10 years ago, the reported incidence of “apical ballooning syndrome” based on the Mayo Clinic diagnostic criteria was 0.5% in 2648 STEMI patients [12]. In the largest systematic review by Pasupathy et al., the incidence of MINOCA was reported to be 6% amongst patients with acute coronary syndromes [4], but the incidence specifically in patients presenting with STEMI was not defined. This is very much lower than that reported in a retrospective analysis of the PRAGUE studies from pre-2002, in which the incidence of angiographically normal coronary arteries in 1004 emergency angiograms performed for STEMI, was reported as 26% [13]. Although in the systematic review, some 30% of patients with MINOCA were reported to present with ST-segment elevation [4], in the SWEDEHEART registry of MINOCA patients from 2003 to 2013, only 17% had ST-elevation [8]. Neither of these registries applied the diagnostic criteria recently proposed by the ESC MINOCA position paper [5].
The 1-year mortality rate of 4.5% in our cohort is similar to the mortality rate observed in the International Takotsubo Registry, where rate of mortality rate was 5.6% per patient-year [14, 15] and two more recent large registries reporting 1-year mortality rates of 4.2% [16] and 5.3% [17], although these reports did not report specifically on patients with ST-elevation. On the other hand, it contrasts with the absence of major adverse cardiac or cardiovascular events seen over a 2-year follow-up in the HORIZONS-AMI trial in STEMI patients with apical ballooning [12] and the 0.05% mortality rate reported in the Swedish Angiography and Angioplasty Register (SCAAR) [18], although the latter was not confined to STEMI.
The heterogenous aetiology of MINOCA is further highlighted in our cohort, with coronary causes accounting for only 28% of cases. Approximately 10% of our cohort had a final diagnosis of plaque disruption, which is lower than the 40% incidence in MINOCA patients reported by Reynolds et al. [19] using intravascular ultrasound or high resolution optical coherence tomography [20]. Within our cohort, 2 of 6 patients required the use of intracoronary imaging to establish the diagnosis of spontaneous coronary artery dissection. Only 3% of our cohort had angiographically-apparent coronary spasm, although spasm provocation testing was not undertaken. This is much lower than the incidence in previous reports employing provocative spasm testing where coronary spasm was identified as a cause of MINOCA in ~ 50% of patients [21]. The Coronary Vasomotion Disorders International Study Group (COVADIS) [22] has recommended three criteria to diagnose coronary artery vasospastic disorders, that include a clinical history suggestive of vasospasm, documented transient ischaemic ECG changes and presence of coronary artery spasm either spontaneously or in response to provocative stimulation. The gold standard involves the use of provocative stimulus (typically intracoronary acetylcholine) to produce symptoms and signs of spasm. Provocative spasm testing is indicated in MINOCA [22], and has both diagnostic and prognostic importance, as recently shown by Montone et al. [23, 24] The Montone paper showed that provocative spasm testing is safe, even in the acute phases of MINOCA. Non-coronary cardiac-related causes accounted for 61% of cases, the most frequent diagnoses being myocarditis (22%) and Takotsubo syndrome (18%).
The main limitations of our data are the relatively small, single-centre patient sample size and the retrospective nature of the analysis. Furthermore, coronary spasm provocation testing and intravascular imaging were not routinely performed, and therefore the underlying aetiology may have been inaccurately characterised in some patients. The major strength of our paper is the inclusion of all consecutive, unselected patients, who all met the stringent criteria for STEMI.
Conclusion
Approximately 4% of patients presenting with STEMI for PPCI have MINOCA. This is the first description of the relatively high incidence of MINOCA in a STEMI cohort using current ESC diagnostic criteria. The heterogenous aetiology highlights the need to actively seek the underlying diagnosis using diagnostic algorithms recommended by the ESC and the COVADIS group.
References
DeWood MA, Spores J, Notske R et al (1980) Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med 303:897–902
DeWood MA, Stifter WF, Simpson CS et al (1986) Coronary arteriographic findings soon after non-Q-wave myocardial infarction. N Engl J Med 315:417–423
Ibanez B, James S, Agewall S et al (2017) 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 39:119–177
Pasupathy S, Air T, Dreyer RP et al (2015) Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation 131:861–870
Agewall S, Beltrame JF, Reynolds HR et al (2016) ESC working group position paper on myocardial infarction with non-obstructive coronary arteries. Eur Heart J 38:143–153. https://doi.org/10.1093/eurheartj/ehw149
Gehrie ER, Reynolds HR, Chen AY et al (2009) Characterization and outcomes of women and men with non–ST-segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early. Am Heart J 158:688–694
Larsen AI, Galbraith PD, Ghali WA et al (2005) Characteristics and outcomes of patients with acute myocardial infarction and angiographically normal coronary arteries. Am J Cardiol 95:261–263
Lindahl B, Baron T, Erlinge D et al (2017) Medical therapy for secondary prevention and long-term outcome in patients with myocardial infarction with nonobstructive coronary artery disease clinical perspective. Circulation 135:1481–1489
Ghadri J-R, Wittstein IS, Prasad A et al (2018) International expert consensus document on Takotsubo syndrome (part I): clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J 39:2032–2046
Thygesen K, Alpert JS, Jaffe AS et al (2018) Fourth universal definition of myocardial infarction. Eur Heart J 40:237–269. https://doi.org/10.1093/eurheartj/ehy462
Ghadri J-R, Wittstein IS, Prasad A et al (2018) International expert consensus document on Takotsubo syndrome (part II): diagnostic workup, outcome, and management. Eur Heart J 39:2047–2062
Prasad A, Dangas G, Srinivasan M et al (2014) Incidence and angiographic characteristics of patients With apical ballooning syndrome (takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial. Catheter Cardiovasc Interv 83:343–348
Widimsky P, Stellova B, Groch L et al (2006) Prevalence of normal coronary angiography in the acute phase of suspected ST-elevation myocardial infarction: experience from the PRAGUE studies. Can J Cardiol 22:1147–1152
Templin C, Ghadri JR, Diekmann J et al (2015) Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med 373:929–938
Ghadri JR, Kato K, Cammann VL et al (2018) Long-term prognosis of patients with Takotsubo syndrome. J Am Coll Cardiol 72:874–882
Hjort M, Lindahl B, Baron T, Jernberg T, Tornvall P, Eggers KM (2018) Prognosis in relation to high-sensitivity cardiac troponin T levels in patients with myocardial infarction and non-obstructive coronary arteries. Am Heart J 200:60–66
Bainey KR, Welsh RC, Alemayehu W, Westerhout CM, Traboulsi D, Anderson T, Brass N, Armstrong PW, Kaul P (2018) Population-level incidence and outcomes of myocardial infarction with non-obstructive coronary arteries (MINOCA): insights from the Alberta contemporary acute coronary syndrome patients invasive treatment strategies (COAPT) study. Int J Cardiol 264:12–17
Tornvall P, Collste O, Ehrenborg E et al (2016) A case-control study of risk markers and mortality in Takotsubo stress cardiomyopathy. J Am Coll Cardiol 67:1931–1936
Reynolds HR, Srichai MB, Iqbal SN et al (2011) Mechanisms of myocardial infarction in women without angiographically obstructive coronary artery disease. Circulation 124:1414–1425
Niccoli G, Scalone G, Crea F (2015) Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J 36:475–481
Ong P, Athanasiadis A, Hill S et al (2008) Coronary artery spasm as a frequent cause of acute coronary syndrome. J Am Coll Cardiol 52:523–527
Beltrame JF, Crea F, Kaski JC et al (2015) International standardization of diagnostic criteria for vasospastic angina. Eur Heart J 38:ehv351
Montone RA, Niccoli G, Fracassi F et al (2017) Patients with acute myocardial infarction and non-obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J 39:91–98
Kaski JC (2018) Provocative tests for coronary artery spasm in MINOCA: necessary and safe? Eur Heart J 39:99–101
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gue, Y.X., Corballis, N., Ryding, A. et al. MINOCA presenting with STEMI: incidence, aetiology and outcome in a contemporaneous cohort. J Thromb Thrombolysis 48, 533–538 (2019). https://doi.org/10.1007/s11239-019-01919-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-019-01919-5