Abstract
Stroke and venous thromboembolism continues to be a major cause of morbidity and mortality worldwide. The use of anticoagulation therapy has proven effective in the prevention of stroke and management of thromboembolism; however, initiating treatment may bear clinical burden given the capacity of these agents to cause bleeding. Originally, warfarin has been primarily used, but with the approval of direct oral anticoagulants, therapeutic recommendations have shifted to direct oral anticoagulants for first line therapy for venous thromboembolism for patients without cancer. As compared to warfarin, direct oral anticoagulants are associated with predictable pharmacokinetic profiles, lower bleeding risks, and minimal drug interactions. Complexities in the medication use process can however heighten the risks of causing adverse events. The purpose of this article is to describe common medication errors associated with direct oral anticoagulants, provide practical guidance on the management of direct oral anticoagulants, and suggest strategies to reduce errors. Efforts to minimize medication errors involve the participation of an interdisciplinary team that has standardized policies, risk reduction strategies, and guiding principles to achieve optimal therapeutic outcomes. Current primary literature is not robust in assessment of clinical impact of medication errors associated with DOACs but reports of adverse drug events have been noted. Future studies should be guided to assess clinical outcomes associated with medication errors and identify potential clinical interventions to optimize therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Highlights
-
Anticoagulants are primarily associated with bleeding adverse events, however other adverse reactions may also occur due to medication errors.
-
Varying indications and complexities in regimens can contribute to the inappropriate use of direct oral anticoagulants.
-
Risk reduction strategies to minimize errors should be identified and implemented into institutional practices.
-
The inclusion of routine monitoring of direct oral anticoagulants may be necessary to ensure therapeutic outcomes.
Introduction
Thromboembolic events associated with atrial fibrillation (AFib) and venous thrombosis (VTE), are leading causes of morbidity and mortality worldwide [1]. Anticoagulants are indicated for the prevention and/or treatment of VTE and thromboembolic complications associated with AFib such as stroke [2,3,4,5,6]. Oral anticoagulants have been utilized for years with the development of vitamin K antagonist, warfarin, in 1954 [6]. It is estimated that more than 6 million patients in the United States (US) are treated with anticoagulants [7]. Use of anticoagulation is an essential treatment modality for patients yet carries substantial risks of adverse events. Clinical use of oral anticoagulant therapy has expanded considerably since the approval of direct oral anticoagulants (DOACs). Among them are apixaban, dabigatran, edoxaban, and rivaroxaban. An additional agent, betrixaban received final US Food and Drug Administration (FDA) approval in June 2017. These agents exert their anticoagulant effects, by directly inhibiting thrombin (dabigatran) or factor Xa (apixaban, rivaroxaban, edoxaban, and betrixaban) [2,3,4,5]. FDA approved indications and doses are included in Table 1 [2,3,4,5].
Historically, warfarin has been considered the “standard” of therapy, but the fixed-dose regimen, absence of drug monitoring, and fewer drug–drug interactions of DOACs has caused a paradigm shift in treatment guidelines and prescribing patterns of healthcare providers [8, 9]. Global registry data indicate that prescriptions for DOACs have surpassed that of VKA [8]. Advantages of using DOACs over VKA include reaching a more rapid anticoagulant effect within hours after first dose, achieving similar (and in some cases superior) effectiveness compared to VKA, and eliminating the need for routine international normalized ratio (INR) testing [9,10,11]. Although highly effective, all anticoagulants are associated with bleeding risks [12]. Quarter Watch estimates 6.3% of patients exposed to an anticoagulant for one year will need to visit the emergency room [13]. Clinical trials have noted variances in bleeding outcomes in patients receiving DOACs versus VKA therapy [14]. The efficacy and safety of DOACs for stroke prevention in AFib have been established in four phase III trials. Dabigatran, apixaban, rivaroxaban, and edoxaban were studied in the following trials: randomization evaluation of long-term anticoagulant therapy (RE-LY), apixaban for the prevention of stroke in subjects with atrial fibrillation (ARISTOTLE), rivaroxaban once daily oral direct factor Xa inhibitor compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation (ROCKET-AF), and effective anticoagulation with factor Xa next generation in atrial fibrillation-thrombolysis in myocardial infarction 48 (ENGAGE AF-TIMI 48), respectively [15,16,17]. A meta-analysis pooling the results of the four pivotal clinical trials in patients with non-valvular atrial fibrillation (RE-LY, ROCKET AF, ARISTOTLE, and ENGAGE AF-TIMI 48) included 42, 411 patients receiving a DOAC and 29, 272 patients receiving a VKA [18].
The analysis confirmed that DOACs had significant reduction rates in hemorrhagic stroke and intracranial hemorrhage compared to VKA and less severe major bleeding events [18]. Although, the trials did confirm that major bleeding with a DOAC is less severe in nature than VKA therapy, the RE-LY, ROCKET, and ENGAGE-AF -TIMI trials did show that gastrointestinal bleeding is higher with dabigatran 150 mg, rivaroxaban, and edoxaban than VKA [15,16,17]. While the ARISTOTLE trial demonstrated that adverse events occurred in almost equal proportions of patients in the apixaban and warfarin arms [15].
When DOACs are not prescribed or utilized appropriately, the incidence of adverse events can increase. In fact, anticoagulant drugs–led by rivaroxaban–accounted for 21,996 reports of severe injuries in the US, including 3,018 reported deaths, per the Institute for Safe Medication Practices (ISMP) analysis of 2016 FDA adverse event data [13]. The majority of these injuries (n = 17,218) were from hemorrhages, making bleeding one of the most frequently reported serious adverse drug effects of all types [13]. Although data is limited on the clinical impact of adverse events associated with medication errors involving DOACs, studies have identified errors at the prescribing, dispensing and administration stages of the medication use process [19,20,21,22,23].
Inappropriate prescribing
DOACs are marketed for having fixed dosing as compared to warfarin, due to a wider therapeutic index and more predictable pharmacokinetic and pharmacodynamic properties [20]. When determining the appropriate dose for a patient, factors such as the indication, physiological properties and clinical parameters (age, weight, renal insufficiency, interacting drugs) of the patient should be taken into consideration [21]. The complexity of these DOAC specific parameters make their appropriate prescribing highly challenging [23].
Several studies have examined DOAC use and prescribing patterns in patients to assess compliance of patients’ prescriptions to guidelines and manufacturer recommendation [19,20,21,22,23,24,25]. According to a subgroup analysis regarding off label dosing of non-VKA oral anticoagulants of the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation phase III (ORBIT-AF III) trial, almost one in eight of US patients in the community received a DOAC dose inconsistent with labeling [19]. Two prospective studies conducted to evaluate the appropriateness of prescribing dabigatran and rivaroxaban in patients with non-valvular atrial fibrillation (NVAF) in the clinic setting found that the choice of drug and dosage were the most frequent inappropriate criterion among patients [24, 25]. The appropriateness of prescribing was assessed using the following criteria: indication, preferred choice, modalities and practicability of administration, drug–drug interactions (DDIs), drug-disease interactions, duplication, and duration from the Medication Appropriate Index (MAI) tool [26]. Some studies have identified rivaroxaban and apixaban most commonly associated with an inappropriate dose [19,20,21,22,23]. This could be potentially related to the dosing guidance specificity in patients with renal impairment or in the presence of drug interactions. Use of the Cockcroft–Gault method to calculate a patient’s renal function is mainly used, but certain patient populations are at risk for dosing discordance when advanced age, extremes in weight, or female gender are taken into consideration.
Although the clinical impact of adverse events associated with inappropriate prescribing has not been well established among various clinical studies, some have identified thrombotic and bleeding risks associated with suboptimal prescribing [19, 22, 23]. In a retrospective cohort analysis of 168 DOAC patients, three events of each, recurrent venous thromboembolism and major bleeding, were associated with inappropriately dosed DOACs [22]. While, Whitworth et al. found that the odds of bleeding doubled with each inappropriate use and adverse events [23]. Overall, an increase in cardiovascular hospitalizations can be associated with patients who are considered under dosed, while overdosing is associated with increased all-cause mortality as compared with recommended dosing [19].
Commonly used coagulation assays (e.g., activated partial thromboplastin time and prothrombin time) are available across all institutions and are easy to perform but lack the necessary sensitivity and specificity to be reliable for monitoring patients on DOACs [27]. The lack of routine monitoring, stable dosage regimen, and lesser interaction with drugs are some possible reasons why DOACs are less likely to be discontinued and are often seen as an advantage over warfarin [28]. However, situations exist when having a reliable method to assess the presence of an anticoagulant would be useful. The potential indications for coagulation testing may include emergency situations (e.g. trauma, urgent surgery, or acute bleed), overdose, acute thrombosis, medication adherence, and potential DDIs [29, 30]. Some studies have indicated that DOACs non-adherence rates have reached up to 50% if no special measures were taken [24, 31, 32].
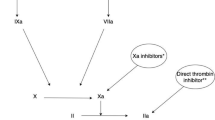
While DOACs have fewer DDIs than warfarin, the pharmacodynamics of DOACs can be enhanced by several drug classes, including other anticoagulants, antiplatelet, and nonsteroidal anti-inflammatory drugs (NSAIDs). The concomitant administration of these classes in addition to DOACs can cause an increased incidence of hemorrhage. A subgroup analysis examining concomitant use of rivaroxaban and NSAIDs found a 2.5-fold higher rate of major bleeding compared to those who were not taking NSAIDs [33]. Many of the drug interactions involving DOACs are dependent on varying degrees of P-glycoprotein (P-gp) and cytochrome P450 (3A4) pathways [34]. There is a substantial number of drugs that inhibit the P-gp system which as a result may enhance the absorption of DOACs [34]. These include antiretrovirals, certain macrolides, some psychotropic and, importantly, cardiovascular drugs such as amiodarone and verapamil, which are frequently used in conjunction with DOACs for rate or rhythm control in atrial fibrillation [34]. Alternatively, there are also drugs, P-gp or CYP 3A4 inducers, that will reduce levels of DOACS with variable magnitude such as certain anticonvulsants and herbal supplements for example St. John’s Wort [34]. Therapeutic management may require DOAC dosage adjustments or the avoidance of the concomitant use due to alterations in DOAC concentrations or increased risk of bleeding (Fig. 1).
Administration and dispensing
During the dispensing and administration stages of the medication use process, the focus is to ensure that the medication dispensed is concordant with the prescribed drug. Issues with dispensing and administration have been identified in the ambulatory and inpatient settings and may occur due to discontinuity of care across health sectors and interdisciplinary teams [35]. Errors that have been reported with DOACs at these stages include improper storage, administration of DOACs, and omission of dose. One of the recommendations to reduce medication errors and harm is to use the “five rights”: the right patient, the right drug, the right dose, the right route, and the right time [36].
Although studies have not identified clinical impact associated with errors of storage and administration of DOACs, it has been mentioned that some health care providers lack knowledge regarding the storage and administration of these medications. In the survey of health care providers, only half the respondents knew that dabigatran should not be crushed or exposed to moisture, and that rivaroxaban 20 mg should be taken with food [37].
The omission of an anticoagulant can pose a significant risk at which the patient can experience a thromboembolic event. Previously, various terms such as non-vitamin K or novel oral anticoagulants, often abbreviated as “NOAC” or target specific oral anticoagulants (TSOAC) were used to describe direct oral anticoagulants. However, the ISMP identified “NOAC” as an error-prone abbreviation due to being misconstrued as “no anticoagulation. There is at least one reported account where the term “NOAC” written in the medical record was interpreted as meaning “no anticoagulation” potentially resulting in the patient not receiving the critical therapy that was intended [38]. In addition, lapses with “hold orders” in the inpatient setting can also lead to omission of therapy, when the medication is not re-initiated when appropriate. An example could be described as “holding” two to three doses of a DOAC for a procedure.
A descriptive Danish study conducted by Henrisken et al. showed potentially fatal and serious events were most frequently associated with sector change (admission to or discharge from hospital or undergoing surgery) and resulted from insufficient or excess dosing [35]. Although the study did not highlight differences between anticoagulant drug classes, it did show anticoagulants are associated with serious and potentially fatal adverse events to patients during a sector change [35].
Periprocedural management of DOACs
In 2017, the American College of Cardiology released a consensus statement regarding the periprocedural management of DOAC’s for patients with non-valvular atrial fibrillation to provide practical guidance in the bridging or interruption of therapy [39]. Clinical considerations for periprocedural management regarding interruption of therapy are dependent on predisposing factors, procedural bleed risk, and kidney function [39]. The exact duration for which a DOAC should be withheld is also dependent on the specified agent.
Given the pharmacokinetic profiles of DOACs and their short-half lives, reinitiating a DOAC will render the patient therapeutically anticoagulated within hours after the first full dose and bridge therapy is generally not required [39]. Prior to reinitiating DOAC therapy, practitioners should ensure complete homeostasis [39]. Patient related-factors and risk of procedural site bleed could potentiate the likelihood of bleeding complications. Resuming DOAC therapy may be a cumbersome task for practitioners, as they are tasked with minimizing preoperative thrombotic risks with temporary interruption of anticoagulation therapy and postoperative bleeding risks associated with resumption of therapy. Following procedures with low post procedural bleed risk, DOAC therapy could be resumed sooner than with high post procedural bleed risks [39]. In the setting of neuraxial anesthesia, the American Society of Regional Anesthesia and Pain Management does recommend discontinuing DOAC’s prior to neuraxial procedures due to increased risk of spinal or epidural hematoma [40]. All DOAC’s carry a black box warning regarding their use in the setting of neuraxial anesthesia and reinitiating DOAC therapy is dependent on the specific agent and could vary from at least 24–72 h after the procedure [40]. Clinical considerations for procedural management regarding the interruption and reinstitution of DOAC therapy is listed in Table 2.
Use of reversal agents
In the event of a life-threatening or uncontrolled bleeding event, there are currently two reversal agents, coagulation factor Xa, recombinant, inactivated-zhzo and idarucizumab marketed for specific DOACs. Coagulation factor Xa, recombinant, inactivated-zhzo was recently approved for the reversal of apixaban and rivaroxaban [41]. It is not indicated for the treatment of bleeding related to any factor Xa inhibitors other than apixaban or rivaroxaban. Dosing of coagulation factor Xa, recombinant, inactivated-zhzo is based upon the dose of rivaroxaban and apixaban and the time since last administration. Treatment consists of either a single 400- or 800-mg intravenous bolus dose, followed by an infusion at a rate of 4 or 8 mg/min for up to 120 min [41]. Dosing and administration recommendations with the use of factor Xa, recombinant, inactivated-zhzho are included in algorithm (Fig. 2).
However, practitioners should be cautioned with the use of coagulation factor Xa, recombinant, inactivated-zhzo due to reports of arterial and venous thromboembolic events and sudden deaths observed within 30 days of use according to early reports from the Andexanet Alfa for acute major bleeding associated with factor Xa inhibitors (ANNEXA-4) study [42].
Idarucizumab is a humanized monoclonal antibody fragment that binds directly to dabigatran, neutralizing its activity [43]. Idarucizumab is indicated in patients treated with dabigatran when the reversal of the anticoagulants effects of dabigatran is needed. The recommended dose of Idarucizumab is 5 g provided as 2 separate vials each containing 2.5 g [43]. Idarucizumab is administered intravenously as a bolus injection or as two consecutive infusions of 2.5 g over 5 min to 10 min each (administered no more than 15 min apart) [43].
With reversing any anticoagulation agent, there is a risk of exposing patients to thrombotic risks of their underlying disease. The clinical benefit should be assessed prior to initiation of any reversal agents to mitigate any risks that may be associated with reversal therapy.
Risk reduction strategies
Opportunities for lowering risks of medications errors exist for healthcare providers to optimize patient care and outcomes. The anti-thrombotic self-assessment tool and 10 key system elements of medication use provided by the ISMP could evaluate current systems related to the use of anticoagulant agents, proactively identify potential improvement areas and opportunities for reducing patient harm [44]. Prior to initiating a DOAC agent, pertinent patient demographic information (age, actual body weight) and clinical information such as clinical indication and most recent laboratory data (renal and hepatic function) can assist with selecting the appropriate medication and prevent complications in therapy. Dosage adjustments may be necessary for patients dependent upon renal function, age, or weight. Patients that are not candidates for DOAC therapy include those with several renal dysfunction, mechanical valve prosthesis, and women who are pregnant [45]. A standardized protocol in which computer order entry system alerts healthcare practitioners of duplicate class orders for anticoagulants and highlights potentially dangerous DDIs could mitigate the risks of administration and dispensing errors. In addition, the computer order entry system should be directly interfaced with the laboratory system in which abnormal values automatically notify practitioners for a potential need for modification of therapy [44, 46].
Medication reconciliation should be performed for all patients at times of sector transfer, which includes hospital admission/discharge and peri-operative management. Serious incidents and sometimes fatality may occur when a patient is transitioning care, in which insufficient or excess anticoagulation is administered. Upon patient discharge, verify that the patient will be able to obtain the medication prescribed.
Warfarin is a practical option for patients who may be underinsured or financially unstable, if their level of anticoagulation can be monitored properly. In patients with mechanical heart valves, warfarin provides excellent protection against thromboembolic complications [47]. Dabigatran was compared to warfarin in patients with mechanical heart valves in a Phase II Study (RE-ALIGN) [47]. The study was discontinued early due to excess thromboembolic and bleeding events in the patients randomized to the dabigatran group [47].
Conclusion
As DOACs continue to be integrated in to clinical practice, it is important to utilize and manage these agents appropriately. Results from studies describe improper DOAC utilization and potentially inappropriate prescribing, which may advertently lead to adverse events. Varying indications and complexities in dosing regimens, renal function, and drug–drug interactions can contribute to the inappropriate prescribing of DOACs. In addition, patient-related factors such as advanced age, comorbid cardiovascular conditions, and compliance could play an integral role in risk stratification to identify patients at an increased risk of bleeding events. Due to the differences among the oral anticoagulants, it is important for healthcare providers to utilize these agents appropriately to achieve the safest and most efficacious use to optimize patient outcomes. Possible interventions to reduce medication errors involve strengthening the education of healthcare providers, establishing DOAC management protocols, and implementing anticoagulant services for all patients when they are discharged from the hospital and transition care to the outpatient setting.
References
Barnes GD, Lucas E, Alexander GC, Goldberger ZD (2015) National trends in ambulatory oral anticoagulant use. Am J Med 128(12):1300–135.e2. https://doi.org/10.1016/j.amjmed.2015.05.044
Bristol-Myers Squibb Company (2016) Eliquis®: highlights of prescribing information. https://packageinserts.bms.com/pi/pi_eliquis.pdf. Accessed 3 Oct 2017
Boehringer Ingelheim Pharmaceuticals, Inc (2015) [revised 2018]. Pradaxa®: highlights in prescribing information. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Pradaxa/Pradaxa.pdf. Accessed 3 Oct 2017
Daiichi Sankyo, Co. (2015) Savaysa®: highlights of prescribing information. http://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. Accessed 3 Oct 2017
Janssen Ortho, LLC (2017) Xarelto®: highlights in prescribing information. http://dsi.com/prescribing-information-portlet/getPIContent?productName=Savaysa&inline=true. Accessed 3 Oct 2017
Bristol-Myers Squibb Company (2017) Warfarin®: highlights of prescribing information. https://packageinserts.bms.com/pi/pi_coumadin.pdf. Accessed 3 Oct 2017
Tomaselli GF, Mahaffey KW, Cuker A, Dobesh PP, Doherty JU, Eikelboom JW, Florido R, Hucker W, Mehran R, Messé SR, Pollack CV Jr, Rodriguez F, Sarode R, Siegal D, Wiggins BS (2017) 2017 ACC expert consensus decision pathway on management of bleeding in patients on oral anticoagulation. J Am Coll Cardiol 70(24):3042–3067
Huisman VTEI, Buller HR, Decousus H et al (2017) The changing landscape of stroke prevention in AF: findings from the GLORIA-AF registry phase 2. J AM Coll Cardiol 69(7):777–785
Kearon C, Akl EA, Ornelas J et al (2016) Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 149(2):315–352
Kirchhof P, Benussi S, Kotecha D et al (2016) 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J 37(38):2893–2962
January CT, Wann LS, Alpert JS et al (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130(23):e199–e267
Shoeb M, Fang MC (2013) Assessing bleeding risk in patients taking anticoagulants. J Thromb Thrombolysis 35(3):312–319
Moore T, Cohen M, Furberg C. Quarter W (2016) Quarter 4. 2017. https://www.ismp.org/sites/default/files/attachments/2018-01/2016Q4_1.pdf
Hellenbart EL, Faulkenberg KD, Finks SW (2017) Evaluation of bleeding in patients receiving direct oral anticoagulants. Vasc Health Risk Manag 13:325–342
Granger CB, Alexander JH, McMurray JJ et al (2011) Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med 365:981–992
Patel MR, Mahaffey KW, Garg J et al (2011) Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 365:883–891
Giugliano RP, Ruff CT, Braunwald E et al (2013) Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med 369:2093–2104
Ruff CT, Guigliano RP, Braunwald E et al (2014) Comparisons of the efficacy of safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomized trials. Lancet 383(9921):955–962
Steinberg B, Shrader P, Thomas L et al (2016) Off-label dosing of non-vitamin K antagonist oral anticoagulants and adverse outcomes: the ORBIT-AF II Registry. J Am Coll Cardiol 68(24):2597–2604
Howard M, Lipshutz A, Roess B et al (2017) Identification of risk factors for inappropriate and suboptimal initiation of direct oral anticoagulants. J Thromb Thrombolysis 43:149–156
Viprey M, Jeannin R, Piriou V, Chevalier P, Michel C, Aulagner G, Berthiller J, Armoiry X (2017) Prevalence of drug-related problems associated with direct oral anticoagulants in hospitalized patients: a multicenter, cross-sectional study. J Clin Pharm Ther 42:58–63
Tran E, Duckett A, Fisher S et al (2017) Appropriateness of direct oral anticoagulant dosing for venous thromboembolism treatment. J Thromb Thrombolysis 43:505–513
Whitworth MM, Haase KK, Fike DS, Bharadwaj RM, Young RB, MacLaughlin EJ (2017) Utilization and prescribing patterns of direct oral anticoagulants. Int J Gen Med 10:87–94
Larock AS, Mullier F, Sennesael AL et al (2014) Appropriateness of prescribing dabigatran etexilate and rivaroxaban in patients with nonvalvular atrial fibrillation: a prospective study. Ann Pharmacother 48:1258–1268
Basaran O, Filiz Basaran N, Cekic EG et al (2015) Prescription Patterns of oral anticoagulants in nonvalvular atrial fibrillation (PROPER study). Clin Appl Throm Hemost 23(4):384–391
Hanlon JT, Schmader KE, Samsa GP, Weinberger M, Uttech KM, Lewis IK et al (1992) A method for assessing drug therapy appropriateness. J Clin Epidemiol 45:1045–1045
Conway S, Hwang A, Ponte C, Gums J (2017) Laboratory and clinical monitoring of direct oral anticoagulants: what clinicians need to know. Pharmacotherapy 37(2):236–248
Garkina SV, Vavilova TV, Lebedev DS, Mikhaylov EN (2016) Compliance and adherence to oral anticoagulation therapy in elderly patients with atrial fibrillation in the era of direct oral anticoagulants. J Geriatr Cardiol 13(9):807–810
Favaloro EJ, Lipppi G, Koutts J (2011) Laboratory testing of anticoagulants: the present and the future. Pathology 43:682–692
Blann AD, Lip GYH (2014) Laboratory monitoring of the non-vitamin K oral anticoagulants. J Am Coll Cardiol 64:1140–1142
Ten Cate H (2013) New oral anticoagulants: discussion on monitoring and adherence should start now! Thromb J 11:8
Rodriguez RA, Carrier M, Wells PS (2013) Non-adherence to new oral anticoagulants: a reason for concern during long-term anticoagulation? J Thromb Haemost 11:390–394
Davidson BL, Verheijen S, Lensing AW, Gebel M, Brighton TA, Lyons RM, Rehm J, Prins MH (2014) Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 174(6):947–953
Fitzgerald JL, Howes LG (2016) Drug interactions of direct acting oral anticoagulants. Drug Saf 39:841–845
Henrisksen JN, Nielsen LP, Hellebeck A, Poulsen BK (2017) Medication errors involving anticoagulants: data from the Danish patient safety database. Pharmacol Res Perspect 5(3):e00307
The five rights: destination without A, Map A. Institute For Safe Medication Practices. http://www.ismp.org/resources/five-rights-destination-without-map. (2007). Accessed 8 Aug 2018
Piran S, Schulman S, Panju M et al (2018) Oral anticoagulant dosing, administration, and storage: a cross-sectional survey of Canadian health care providers. J Thromb Thrombolysis 45:180–185
Institute for Safe Medication Practices. Medication Safety Alert. Volume 18, Issue 25. 12 December 2013
Doherty JU, Gluckman TJ, Hucker WJ et al (2017) 2017 ACC Expert Consensus Decision Pathway for Periprocedural Management of Anticoagulation in patients with Nonvalvular Atrial Fibrillation. J Am Coll Cardiol 69(7):871–898
Horlocker T, Vandermeuelen E, Kopp S et al (2018) Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy. Reg Anesth Pain Med 43(5):566
Portola Pharmaceuticals, Inc, (2018) Andexxa® (coagulation factor Xa (recombinant), inactivated-zhzo) highlights in prescribing information. https://www.andexxa.com/wp-content/uploads/Andexxa%20USPI-v1.4-may2018.pdf. Accessed 12 May 2018
Connolly SJ, Milling TJ, Eikelboom JW et al (2016) Andexanet alfa for acute major bleeding associated with factor Xa inhibitors. N Engl J Med 375(12):1131–1141
Boehringer Ingelheim Pharmaceuticals, Inc, (2015) Praxbind®: highlights in prescribing information. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Praxbind/Praxbind.pdf. Accessed 3 Oct 2017
Institute for Safe Medication Practices (2017) Medication Safety Self-Assessment® for Antithrombotic Therapy. https://www.ismp.org/assessments/antithrombotic-therapy. Accessed 1 Oct 2017
Burnett AE, Mahan CE, Vazquez SR, Oertel LB, Garcia DA, Ansell J (2016) Guidance for the practical management of the direct oral anticoagulants (DOACs) in VTE treatment. J Thromb Thrombolysis 41:206–232
Kuperman GJ, Bobb A, Payne TH et al (2007) Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc 14(1):29–40
Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, Blatchford J, Devenny K, Friedman J, Guiver K, Harper R, Khder Y, Lobmeyer MT, Maas H, Voigt JU, Simoons ML, Van de Werf F, RE-ALIGN Investigators (2013) Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med 369(13):1206–1214
Funding
This study was performed without financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants
This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Barr, D., Epps, Q.J. Direct oral anticoagulants: a review of common medication errors. J Thromb Thrombolysis 47, 146–154 (2019). https://doi.org/10.1007/s11239-018-1752-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1752-9