Abstract
High central aortic pulse pressure (CPP) and thrombin-induced platelet–fibrin clot strength (TIP–FCS) have been associated with ischemic outcomes in patients with coronary artery disease in separate studies. But, the ischemic risk associated with these factors has never been analyzed in a single study and their interrelation is unknown. The primary aim of the study was to establish cut points for CPP and TIP–FCS measured at the time of catheterization associated with long term major adverse cardiovascular events. We enrolled 334 consecutive patients undergoing cardiac catheterization and assessed thrombogenicity by thrombelastography. Patients were followed up to 3 years. The primary endpoint was a composite of cardiovascular death, myocardial infarction, and ischemic stroke and the secondary endpoint was occurrence of the primary endpoint or recurrent ischemic events requiring hospitalization. Patients with primary and secondary endpoint occurrence had higher CPP (83 ± 20 vs. 60 ± 18 mmHg, p < 0.0001; 70 ± 21 vs. 59 ± 18 mmHg, p < 0.0001, respectively) and TIP–FCS (68.5 ± 5.8 vs. 65.5 ± 5.0 mm, p = 0.008; 67.4 ± 5.9 vs. 65.2 ± 4.8 mm, p = 0.001, respectively). CPP >60 mmHg and TIP–FCS >69 mm were both independent predictors of primary endpoint occurrence (p = 0.0001 and p = 0.02, respectively). ROC analysis for CPP and TIP–FCS showed a C-statistic of 0.81 (p < 0.0001) and 0.68 (p = 0.007) for the primary endpoint, respectively. Patients with CPP >60 mmHg had higher TIP–FCS (66.8 ± 5.1 vs. 64.8 ± 5.0 mm, p < 0.001) and primary and secondary endpoint occurrence (13 vs. 1.1%, p < 0.0001 and 31.8 vs. 14.4%, p = 0.0002, respectively). CPP >60 mmHg + TIP–FCS > 69 mm was associated with a markedly increased risk of primary endpoint occurrence [HR (95% CI) 5.4(2.3–12.5), p = 0.0001]. High CPP and thrombogenicity are interrelated; each are independently associated with increased cardiovascular risk; and simultaneous presence markedly enhances risk. The mechanistic link between CPP and thrombogenicity deserves further study.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Limited information is available on the relation between platelet function, hypercoagulability and hypertension in patients with coronary artery disease (CAD). Essential hypertension (EH) is associated with arterial thrombotic complications such as myocardial infarction (MI) and ischemic stroke. Platelet activation and aggregation play critical roles in ischemic event occurrences, including MI [1]. The observation of elevated platelet activation, an important characteristic of a prothrombotic state in EH patients, highlights the potential relation between peripheral blood pressure and thrombogenicity [2,3,4,5]. Nearly 10% of patients with CAD undergoing percutaneous coronary intervention (PCI) in the setting of acute coronary syndromes will experience recurrent ischemic events despite current standard dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 receptor inhibitor [6]. High thrombin-induced platelet–fibrin clot strength (TIP–FCS) measured by thrombelastography (TEG), also known as hypercoagulability, has been independently associated with post-PCI ischemic event occurrence [1, 7, 8]. The clinical significance of pulse pressure (PP), defined as the difference between systolic blood pressure and diastolic blood pressure, has been recently explored [9, 10]. Moreover, high aortic central pulse pressure (CPP) has been shown to be an independent predictor of CAD risk [11, 12], and is strongly associated with MI, cardiovascular (CV) mortality, and all-cause mortality in patients with CAD [13]. Despite the independent association of high CPP, and hypercoagulability in CAD patients, there has been no investigation exploring the association between platelet reactivity, hypercoagulability, and CPP in a single patient population. CPP more closely reflects the load placed on the left ventricle, and the coronary and cerebral vasculature. The primary aim of the study was to establish cut points for CPP and TIP–FCS measured at the time of catheterization associated with long term major adverse cardiovascular events (MACE). Secondly, we explored an interrelation between CPP and TIP–FCS along with their independent and shared association with MACE.
Methods
Study population
A total of 334 patients >18 years old undergoing elective cardiac catheterization for suspected severe CAD and with complete 3-years clinical follow-up data were included in this sub-analysis of Multi-Analyte, thrombogenic, and Genetic Markers of Atherosclerosis (MAGMA, NCT01276678) study. The MAGMA study is a prospective cohort study aimed to find a correlation between blood biomarkers and growth of the plaques, regardless of the presence of the classic risk factors for atherosclerosis. All patients provided written informed consent and study was approved by local Institutional Review Board. Patients were referred for elective cardiac catheterization for the following reasons: (1) a positive stress test with no angina; (2) a positive stress test with angina; and/or (3) a positive computerized tomography (CT) scan. Exclusion criteria included serious arrhythmias, significant valvular heart disease, LV ejection fraction <50%, concomitant anticoagulant therapy, pregnancy, infection, history of cancer, autoimmune or connective tissue disease, HIV, hepatitis C, or any abnormal laboratory value or physical finding that may interfere with the interpretation of the study results as per the investigator. CAD was defined angiographically as a luminal diameter stenosis ≥50%.
Cardiac catheterization
Central aortic pressure indices were measured in the ascending aorta using a 6F fluid-filled pigtail catheter (Boston Scientific Impulse 6F pigtail catheter, Tijuana, Mexico). Pressure tracings were recorded using a hemodynamic monitoring system (Philips Xper Information Management System, Melbourne, FL). The mean aortic pressure (MAP) was calculated as 1/3 systolic + 2/3 diastolic pressure. CPP was calculated as the difference between the peak systolic pressure and the pressure at end-diastole. Aortic pulsatility was defined as the ratio of CPP to mean aortic pressure (FPP). The ratio of CPP to diastolic pressure (pulsatility index, PI) was also applied for another index of aortic stiffness.
Blood sampling
Blood samples were obtained in the catheterization laboratory before coronary angiography. Blood was collected in a blood collection tube containing 3.8% trisodium citrate (Becton-Dickenson, Franklin Lakes, NJ) after discarding the first 2–3 mL of free-flowing blood. All assays were performed within 2 h of blood collection.
Thrombelastography
The Thrombelastography (TEG) haemostasis analyzer (TEG 5000 Thrombelastograph Hemostasis Analyzer system; Haemonetics, Braintree, MA) provides quantitative and qualitative measurement of the physical properties of a clot [14]. The citrated samples were assayed as per the manufacturer’s instructions to generate the TIP–FCS (recorded in mm) [1]. Briefly, 1 mL of citrated blood was transferred to a vial containing kaolin and mixed by inversion; 340 µL of the activated blood was immediately added to a sample cup in which 20 µL of 0.2 M calcium chloride had been previously added. TIP–FCS was determined by measuring the amplitude of the rotation of the pin, which increases proportionally with clot strength. Reaction time (R, min), a representative of the initiation phase of enzymatic clotting, is the time from the start of the sample run to the point of the first significant clot formation corresponding to an amplitude of 2 mm reading on the TEG tracing. K is a measure of the time to reach 20 mm clot strength from R. Coagulation index (CI) represents overall coagulation derived from R, K, TIP–FC, and angle of kaolin-activated whole blood tracings by the formula: CI = 0.3258 R − 01886 K + 0.1224 TIP–FCS + 0.0759 α − 707922 according to the manufacturer. Angle (α) is reflective of fibrinogen activity and is the degrees of the angle formed by the tangent line to TEG tracing measure at R [14].
Clinical outcomes and definitions
Patients were contacted by telephone at 12 and 36 months to record post-discharge MACE defined as CV death, MI, and ischemic stroke. Patient records including electronic source documents were obtained and reviewed by two physicians blinded to the study who adjudicated events. Cardiovascular death was defined as death secondary to any cardiovascular cause. MI was defined as a cardiac troponin > upper limits normal with ischemic symptoms and/or electrocardiographic abnormalities [15]. The primary composite endpoint was the occurrence of MACE. The secondary outcome included the composite of MACE and hospitalization for recurrent ischemia. Hypercoagulability was defined as TIP–FCS >69 mmHg [16]. Medical management of patients was done in accordance with the current clinical guidelines.
Statistical analysis
Continuous variables are presented as mean ± SD, and categorical variables are expressed as percentages. All continuous variables were normally distributed (assessed by the Kolmogorov–Smirnov test) and were compared using analysis of variance (ANOVA) method. A Chi square test was used to compare categorical variables. A multivariate Cox regression analysis was performed by including CVD risk factors, CPP, and hypercoagulability. ROC analysis was used to determine cut-point for CPP and TEG to predict primary and secondary endpoints. Using these cut-points, data was divided into patients with a high CPP and with low CPP and multivariate analysis used to determine significant factors between these two groups. A criterion of >60 mmHg was used to define a high CPP based on ROC analysis described in results. Quartile analysis was conducted for CPP with <50 mmHg as first quartile, 50–60 mmHg as second quartile, 61–74 mmHg as third quartile and >74 mmHg as fourth quartile. Hazards ratio was calculated using Cox regression model. Analyses were performed with MedCalc Software (version17, MedCalc Software, Ostend, Belgium). A two-tailed p value <0.05 was considered statistically significant.
Results
Clinical characteristics
The study cohort consisted of 334 patients and all patients underwent thrombogenicity testing. The baseline clinical characteristics are in Table 1. Patients with CPP >60 mmHg were older and more frequently women; and more often had hypertension and renal disease with a higher blood urea nitrogen and creatinine compared to patients with CPP ≤60 mmHg (Tables 1, 2). 242 (71.9%) were treated medically, 69 (20.6%) were treated with PCI and 25 (7.5%) were treated with coronary artery bypass grafting (CABG) (Table 2).
Receiver operator curve analysis and area under the curve (AUC)
ROC analysis was conducted and AUC calculated for prediction of primary and secondary endpoints with CPP and TIP–FCS (Fig. 1). The C-statistic for CPP was 0.81 (p < 0.0001) and 0.65 (p = 0.0001) for primary and secondary endpoints respectively (Fig. 1a, b). The C-statistic for TIP–FCS were 0.68 (p = 0.007) and 0.62 (p = 0.002) for primary and secondary endpoints, respectively (Fig. 1c, d). A cut-point of >60 mmHg for CPP and >69 mm for TIP–FCS were chosen based on this analysis.
Central aortic pressure measurements and cardiac catheterization data
Patients with CPP >60 mmHg had higher central aortic systolic blood pressure (SBP), MAP, CPP, PI, and FPP than patients with CPP ≤60 mmHg (p < 0.0001) (Table 3). Cardiac catheterization data showed a similar distribution of CAD severity amongst patients (Table 2).
Thrombogenicity in relation to CPP >60 mmHg
Patients with CPP >60 mmHg had higher TIP–FCS, CI, K and fibrinogen activity than patients with CPP ≤60 mmHg (p < 0.001, p = 0.04, p = 0.03 and p = 0.003, respectively) (Table 3). The relation of TIP–FCS to CPP quartiles is shown in Fig. 2. Patients in the third and fourth CPP quartiles had higher TIP–FCS compared to the lowest quartiles (p < 0.0001) (Fig. 2).
Relation between CPP and TIP–FCS to clinical outcomes
In total, there were 22 events in the primary endpoint (5 CV deaths, 13 MI and 4 ischemic stroke) and 75 events in the secondary endpoint (22 from the primary endpoint and 53 hospitalizations for recurrent ischemia with or without urgent revascularization).
Composite primary and secondary endpoints were higher in patients with CPP >60 mmHg (13 vs. 1.1%, p < 0.0001 and 31.8 vs. 14.4%, p = 0.0002, respectively) (Table 4).
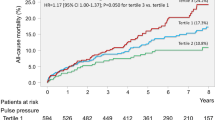
CPP and TIP–FCS were significantly greater in patients with composite primary and secondary endpoints compared to patients without event (Fig. 3). The composite primary and secondary endpoints were significantly higher in the fourth quartile of CPP compared to first quartile and increased in a step-wise progression across quartiles (p < 0.0001 and p = 0.001, respectively) (Fig. 4).
Comparison of primary and secondary clinical outcomes with, a CPP, b TIP–FCS. Primary outcome = the occurrence of cardiovascular death, myocardial infarction, and ischemic stroke. Secondary outcome = the primary outcome plus hospitalization for recurrent ischemia. CPP central pulse pressure, TIP–FCS thrombin induced platelet fibrin clot strength
Multivariate analysis and hazards ratio
Multivariate regression of significant CVD risk factors, CPP, and hypercoagulability was conducted for primary endpoint and only CPP >60 mmHg and TIP–FCS >69 mm retained statistical significance (p = 0.0001 and p = 0.02 respectively) (Table 5). The variables presenting an association with the outcome of interest with a p value <0.1 on univariate analysis were then entered in the multivariate analysis.
Hazards ratio (HR) was calculated for primary and secondary endpoints using CPP >60 mmHg and TIP–FCS >69 mm. HR in patients with CPP >60 mmHg for primary endpoint was 10.5 (95% CI 2.4–45.5, p = 0.001) and for secondary endpoint was 2.1 (95% CI 1.3–3.4, p = 0.002). HR for patients with TIP–FCS >69 mmHg for primary endpoint was 2.5 (95% CI 1.1–5.8, p = 0.03) and for secondary endpoint was 2.1 (95% CI 1.3–3.4, p = 0.001). When combined, i.e. patients with TIP–FCS >69 mm and CPP >60 mmHg, the HR for primary endpoint was 5.4 (95% CI 2.3–12.5, p = 0.0001) and for secondary endpoint was 2.8 (95% CI 1.7–4.7, p < 0.0001).
Discussion
To the best of our knowledge, this is the first study to assess the association between invasively determined CPP and thrombogenicity in CAD patients. Patients with high CPP and thrombogenicity are interrelated and independently associated with poorer cardiovascular outcomes. CPP >60 mmHg combined with TIP–FCS >69 mm was highly predictive of adverse outcomes.
Thrombelastography has been used to assess overall clotting kinetics and platelet–fibrin clot strength in whole blood in patients treated with PCI [1, 7, 8, 16]. In addition to the relation of hypercoagulability to long term ischemic event occurrences in patients treated with PCI, TIP–FCS has been correlated with fibrinogen, von Willebrand factor (vWF), plasminogen activator inhibitor-1(PAI-1), high-sensitivity C-reactive protein (hs-CRP), race, gender and CYP2C19 genotype [1, 8, 17, 18]. In this line, in the present study, CPP was associated with TIP–FCS.
Recent studies have demonstrated an association between platelet activation and blood pressure in hypertensive patients. In these studies, platelet activation was indicated by signal peptide–CUB–EGF domain—containing protein 1 (SCUBE1) and mean platelet volume (MPV) [19, 20]. Increased MPV values were observed in patients with prehypertension and hypertension compared with normotensive controls [3, 21, 22]. In addition, MPV was significantly correlated with hs-CRP levels; blood pressure and target organ damage in patients with EH [21,22,23,24]. Few studies have demonstrated the relation between hypercoagulability and complications of EH [25,26,27]. However, the present study is the first study to explore the relation of TIP–FCS measured by TEG to CPP in patients undergoing coronary angiography. A higher TIP–FCS was observed in patients with suspected CAD with a high CPP. The present study further supports the previous observation of an association between hypercoagulability and platelet activation in patients with EH [25,26,27]. TIP–FCS >69 mm, an indicator of hypercoagulability, was identified as a significant independent predictor of cardiovascular events 3-year follow-up in the current study which is similar to previous report [16]. Based on these observations, it is plausible that hypercoagulability is a critical underlying mechanism associated with clinical cardiovascular event occurrences in patients with CPP >60 mmHg.
CPP has been shown to be independently associated with the occurrence and extent of CAD in patients undergoing coronary angiography [12, 28, 29]. Furthermore, CPP is an independent and a stronger predictor of cardiovascular outcome and all-cause mortality than brachial pulse pressures, PP-24 h, SBP-24 h and central SBP [9, 30,31,32]. In this line, in the current study patients with the higher CPP quartile had significantly greater values for TIP–FCS, compared with the lowest CPP quartile. The TIP–FCS in patients with CPP >60 mmHg was markedly higher than in patients with CPP ≤60 mmHg. Furthermore, total events, all-cause death, CV death, MI and composite event occurrences in patients with CPP >60 mmHg, were significantly higher than in patients with CPP ≤60 mmHg. Cox regression analysis showed that CPP >60 mmHg combined with TIP–FCS >69 mm was a strong predictive factor for cardiovascular events. This is inconsistent with a previous report of relation of cutoff of CPP value (≥50 mmHg) to adverse cardiovascular outcome [30]. The latter observation may be attributed to a difference in method of measuring used to assess CPP.
Although the exact mechanism for the relation between hypercoagulability and CPP remains unknown, it can be speculated that increased shear stress caused by higher CPP might induce platelet activation, release of procoagulant factors and finally resulting in hypercoagulability state. High flow and pressure gradient also promote the accumulation of thrombin and fibrin, which could further promote formation/growth of a clot. Finally, with growth of a clot, shear stresses can become sufficiently extreme in diseased arteries to drive von-Willebrand factor self-association into massive fibers, potentially causing the final burst of clot growth towards full thrombotic occlusion [33]. Additionally, hypercoagulability characterized by elevated platelet reactivity, fibrinogen, vWF, PAI-1 [7, 17], specific inflammation markers including C-reactive protein (CRP), interleukin-6 (IL-6), interleukin-8 (IL-8) and monocyte chemoattractant (MCP)-1, have been linked to ischemic event occurrences in CAD patients [7, 17]. Furthermore, activated platelets release soluble CD40 ligand (sCD40L), SCUBE1, and vasoactive agents, such as platelet-derived growth factors (PDGF), vascular endothelial growth factors (VEGF), thereby accelerating thrombotic event occurrences [19, 34]. Further larger-scale randomized controlled trials studies are needed to validate our findings.
Limitations
The present study has the following limitations. This is a hypothesis generating observational study and limited by a small sample size to evaluate clinical event occurrences. Secondly, the study patients were on different vasoactive medications which affect aortic blood pressure. However, in routine clinical practice, patients undergoing coronary angiography are usually being administered standard cardiovascular medications such as nitrates, beta-blockers, calcium channel blocker, etc. Thus, our results might be representative of a real world scenario.
Conclusions
High CPP and thrombogenicity are interrelated; each are independently associated with increased cardiovascular risk; and simultaneous presence markedly enhances risk. The mechanistic link between CPP and thrombogenicity deserves further study.
References
Gurbel PA, Bliden KP, Guyer K, Cho PW, Zaman KA, Kreutz RP, Bassi AK, Tantry US (2005) Platelet reactivity in patients and recurrent events post-stenting: results of the PREPARE POST-STENTING study. J Am Coll Cardiol 46:1820–1826
Gkaliagkousi E, Corrigall V, Becker S, de Winter P, Shah A, Zamboulis C, Ritter J, Ferro A (2009) Decreased platelet nitric oxide contributes to increased circulating monocyte-platelet aggregates in hypertension. Eur Heart J 30:3048–3054
Erdogan D, Icli A, Aksoy F, Akcay S, Ozaydin M, Ersoy I, Varol E, Dogan A (2013) Relationships of different blood pressure categories to indices of inflammation and platelet activity in sustained hypertensive patients with uncontrolled office blood pressure. Chronobiol Int 30:973–980
Varol E, Akcay S, Icli A, Yucel H, Ozkan E, Erdogan D, Ozaydin M (2010) Mean platelet volume in patients with prehypertension and hypertension. Clin Hemorheol Microcirc 45:67–72
Gkaliagkousi E, Passacquale G, Douma S, Zamboulis C, Ferro A (2010) Platelet activation in essential hypertension: implications for antiplatelet treatment. Am J Hypertens 23:229–236
Gurbel PA, Tantry US (2010) Combination antithrombotic therapies. Circulation 121:569–583
Gurbel PA, Bliden KP, Kreutz RP, Dichiara J, Antonino MJ, Tantry US (2009) The link between heightened thrombogenicity and inflammation: pre-procedure characterization of the patient at high risk for recurrent events after stenting. Platelets 20:97–104
Jeong YH, Bliden KP, Shuldiner AR, Tantry US, Gurbel PA (2014) Thrombin-induced platelet-fibrin clot strength: relation to high on-clopidogrel platelet reactivity, genotype, and post-percutaneous coronary intervention outcomes. Thromb Haemost 111:713–724
Huang CM, Wang KL, Cheng HM, Chuang SY, Sung SH, Yu WC, Ting CT, Lakatta EG, Yin FC, Chou P, Chen CH (2011) Central versus ambulatory blood pressure in the prediction of all-cause and cardiovascular mortalities. J Hypertens 29:454–459
Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C (2010) Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J 31:1865–1871
Franklin SS, Khan SA, Wong ND, Larson MG, Levy D (1999) Is pulse pressure useful in predicting risk for coronary heart disease? The Framingham heart study. Circulation 100:354–360
Philippe F, Chemaly E, Blacher J, Mourad JJ, Dibie A, Larrazet F, Laborde F, Safar ME (2002) Aortic pulse pressure and extent of coronary artery disease in percutaneous transluminal coronary angioplasty candidates. Am J Hypertens 15:672–677
Lin MJ, Chen CY, Lin HD, Lin CS, Wu HP (2016) Prognostic significance of central pulse pressure for mortality in patients with coronary artery disease receiving repeated percutaneous coronary intervention. Medicine 95:e3218.
Mahla E, Suarez TA, Bliden KP, Rehak P, Metzler H, Sequeira AJ, Cho P, Sell J, Fan J, Antonino MJ, Tantry US, Gurbel PA (2012) Platelet function measurement-based strategy to reduce bleeding and waiting time in clopidogrel-treated patients undergoing coronary artery bypass graft surgery: the timing based on platelet function strategy to reduce clopidogrel-associated bleeding related to CABG (TARGET-CABG) study. Circ Cardiovasc Interv 5:261–269
Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60:1581–1598
Gurbel PA, Bliden KP, Navickas IA, Mahla E, Dichiara J, Suarez TA et al (2010) Adenosine diphosphate-induced platelet-fibrin clot strength: a new thrombelastographic indicator of long-term poststenting ischemic events. Am Heart J 160:346–354
Tantry US, Bliden KP, Suarez TA, Kreutz RP, Dichiara J, Gurbel PA (2010) Hypercoagulability, platelet function, inflammation and coronary artery disease acuity: results of the Thrombotic Risk Progression (TRIP) study. Platelets 21:360–367
Lev EI, Bliden KP, Jeong YH, Pandya S, Kang K, Franzese C et al (2014) Influence of race and sex on thrombogenicity in a large cohort of coronary artery disease patients. J Am Heart Assoc 3:e001167
Ozkan G, Ulusoy S, Mentese A, Karahan SC, Cansiz M (2013) New marker of platelet activation, SCUBE1, is elevated in hypertensive patients. Am J Hypertens 26:748–753
El Haouari M, Rosado JA (2009) Platelet function in hypertension. Blood Cells Mol Dis 42:38–43
Coban E, Yazicioglu G, Berkant Avci A, Akcit F (2005) The mean platelet volume in patients with essential and white coat hypertension. Platelets 16:435–438
Nadar SK, Blann AD, Kamath S, Beevers DG, Lip GY (2004) Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). J Am Coll Cardiol 44:415–422
Inanc T, Kaya MG, Yarlioglues M, Ardic I, Ozdogru I, Dogan A et al (2010) The mean platelet volume in patients with non-dipper hypertension compared to dippers and normotensives. Blood Press 19:81–85
Yarlioglues M, Kaya MG, Ardic I, Dogdu O, Kasapkara HA, Gunturk E et al (2011) Relationship between mean platelet volume levels and subclinical target organ damage in newly diagnosed hypertensive patients. Blood Press 20:92–97
Lee AJ (1997) The role of rheological and haemostatic factors in hypertension. J Hum Hypertens 11:767–776
Khaleghi M, Singletary LA, Kondragunta V, Bailey KR, Turner ST, Mosley TH Jr et al (2009) Haemostatic markers are associated with measures of vascular disease in adults with hypertension. J Hum Hypertens 23:530–537
Yang P, Liu YF, Yang L, Wei Q, Zeng H (2010) Mechanism and clinical significance of the prothrombotic state in patients with essential hypertension. Clin Cardiol 33:E81–E86
Jankowski P, Kawecka-Jaszcz K, Czarnecka D, Brzozowska-Kiszka M, Styczkiewicz K, Styczkiewicz M et al (2004) Ascending aortic, but not brachial blood pressure-derived indices are related to coronary atherosclerosis. Atherosclerosis 176:151–155
Nishijima T, Nakayama Y, Tsumura K, Yamashita N, Yoshimaru K, Ueda H et al (2001) Pulsatility of ascending aortic blood pressure waveform is associated with an increased risk of coronary heart disease. Am J Hypertens 14:469–473
Roman MJ, Devereux RB, Kizer JR, Okin PM, Lee ET, Wang W et al (2009) High central pulse pressure is independently associated with adverse cardiovascular outcome the strong heart study. J Am Coll Cardiol 54:1730–1734
Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T et al (2007) Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension 50:197–203
Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarc’h PM et al (2002) Central pulse pressure and mortality in end-stage renal disease. Hypertension 39:735–738
Diamond SL (2016) Flow and delta-P dictate where thrombin, fibrin, and von Willebrand factor will be found. Thromb Res 141(Suppl 2):S22–S24
Duhamel TA, Xu YJ, Arneja AS, Dhalla NS (2007) Targeting platelets for prevention and treatment of cardiovascular disease. Expert Opin Ther Targets 11:1523–1533
Acknowledgements
This study was supported by Platelet and Thrombosis Research, LLC and Haemonetics. VAP© testing was provided by Atherotech® Inc. Urinary 11-dehydrothromboxane B2 and AtherOx testing were provided by Corgenix Medical Corp. We wish to acknowledge Tania Gesheff, RN, MSN, Kiran Kalra, MBBS, and Cescelle Barbour RN, MSN for their contributions to this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Dr. Gurbel reports serving as a consultant for Bayer, Merck, and Haemonetics; receiving grants from the National Institutes of Health, Harvard Clinical Research Institute, Bayer, Haemonetics, Duke Clinical Research Institute, Amgen, Medicure Inc.; receiving honoraria and payment for lectures, consultations, including service on speakers’ bureaus from Bayer, Janssen, Merck and Boehringer Ingleheim; Dr. Gurbel is holding stock or stock options in Merck, Medtronic, and Pfizer; and holding patents in the area of personalized antiplatelet therapy and interventional cardiology. Other authors report no disclosures.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Gailing Chen and Kevin P. Bliden have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, G., Bliden, K.P., Chaudhary, R. et al. Central aortic pulse pressure, thrombogenicity and cardiovascular risk. J Thromb Thrombolysis 44, 223–233 (2017). https://doi.org/10.1007/s11239-017-1524-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-017-1524-y