Abstract
D-dimer is a product of cross linked fibrin degradation and is a measure of the amount of fibrin turnover. As such, D-dimer might be of utility in the prediction of both thrombotic and hemorrhagic events. Therefore, the aim of the present study was to evaluate whether elevated D-dimer levels on admission and at discharge could predict subsequent ischemic and hemorrhagic events in patients with acute myocardial infarction (AMI). D-dimer was measured on admission and at discharge in 461 out of a total of 3,602 patients in the HORIZONS-AMI trial, as part of the formal prespecified biomarker substudy. The predictive value for major adverse cardiovascular events (MACE) and non-CABG major bleeding after 3 year follow up was investigated by stratifying patients in groups of D-dimer level and comparing event rates using Kaplan–Meier and calculating hazard ratios using Cox proportional hazards models. D-dimer levels ≥ 0.71 μg/mL on admission were associated with an adjusted hazard ratio of 2.58 for MACE (p = 0.0014) and 4.61 for major bleeding (p = 0.0018). A discharge D-dimer level ≥ 1.26 μg/mL was associated with a higher risk for MACE by univariate analysis (HR 1.88, p = 0.037), but lost its significance after multivariate adjustment (HR 1.77, p = 0.070). High D-dimer levels on admission were associated with a higher risk of MACE and non-CABG major bleeding in STEMI patients undergoing pPCI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prognosis of initial survivors after acute myocardial infarction (AMI) is affected by the occurrence of recurrent ischemic events as well as major bleeding [1]. Plaque dislodgment and disruption of endothelium secondary to percutaneous coronary intervention (PCI), activation of the coagulation cascade and activation of platelets expose the patient to a high risk of recurrent ischemic events. Although antithrombotic therapy reduces this risk, it increases the risk for hemorrhagic complications [2, 3].
D-dimer is a product of the degradation of cross-linked fibrin and its serum levels reflect both fibrin formation and degradation. D-dimer concentrations were shown to remain elevated for several months after AMI treated with thrombolysis, illustrating the enhanced coagulable state post myocardial infarction patients are exposed to [4]. In addition, an enhanced coagulable state, caused by elevated blood fibrinogen levels, is associated with myocardial infarction, stroke and mortality [5–7]. Therefore, because of its potential to identify individuals with a hypercoagulable state, D-dimer might be a useful biomarker in predicting recurrent ischemic events.
Although an association between high levels of D-dimer and subsequent cardiovascular events has been well documented in healthy subjects and patients with unstable angina, there are no data regarding the prognostic value of D-dimer levels in a contemporary cohort of AMI patients treated with pPCI [8–15]. Moreover, in previous studies investigating the prognostic value of D-dimer, D-dimer was measured 2 weeks up to 2 months after the initial MI and no previous studies have investigated the prognostic value of D-dimer levels on admission or at discharge [9, 16].
In an exploratory analysis of the HORIZONS-AMI (harmonizing outcomes with revascularization and stents in AMI) biomarker study, we found an association between admission D-dimer levels and subsequent major bleeding. The aim of the present analysis was to further elaborate on D-dimer and the risk of bleeding and to investigate the relationship between admission D-dimer levels and the risk of subsequent cardiovascular events. In addition, we sought to investigate whether D-dimer levels at discharge could be of utility in the prediction for subsequent ischemic and hemorrhagic outcomes. Finally, we questioned if discharge D-dimer level and the change in D-dimer level during hospitalization were influenced by bivalirudin as compared to unfractionated heparin (UFH) plus a glycoprotein IIb/IIIa inhibitor (GPI).
Methods
The design and results of the HORIZONS-AMI trial have been previously published [17–20]. Briefly, between March 25, 2005 and May 7, 2007, a total of 3,602 STEMI patients were randomized open-label in a 1:1 ratio to treatment with bivalirudin alone (1,800 patients) or with UFH plus a GPI (1,802 patients). Consecutive patients ≥18 years of age who presented within 12 h after the onset of symptoms and who had ≥1 mm ST-segment elevation in two or more contiguous leads, new left bundle-branch block, or true posterior myocardial infarction were eligible for enrolment. Emergency coronary angiography with left ventriculography was performed after randomization, with subsequent triage to treatment with PCI, coronary artery bypass grafting (CABG), or medical management at physician discretion. After patency was restored in the infarct-related vessel, those patients assigned to PCI were randomized again, in a 3:1 ratio, to either paclitaxel-eluting stents (PES, TAXUS Express, Boston Scientific, Natick, Ma) or an uncoated, but otherwise identical bare metal stent (BMS) (Express, Boston Scientific, Natick, Ma). Follow-up angiography was performed routinely in a prespecified fraction of patients. Clinical follow-up was planned at 30 days, 6, and 12 months, and then yearly for 3 years total. An independent clinical events committee (Cardiovascular Research Foundation, NY, USA) that was masked to treatment assignment adjudicated all adverse ischemic and bleeding events from original source documents and procedural angiograms. The 3 year results of the HORIZONS-AMI trial have been published previously [17].
Biomarkers substudy
A total of 502 patients within the angiographic follow-up cohort of the main trial who were randomized to receive PES were enrolled in the pre-specified biomarker substudy after appropriate additional written informed consent was obtained. The angiographic cohort consisted of the first 1,800 consecutive patients randomized to the stent arm in whom stent implantation was successful (diameter stenosis <10 % with TIMI-3 flow and no more than an NHLBI type A persistent dissection). A total of 26 biomarkers were measured at study enrolment, hospital discharge, 30 days and 1 year (a list of these 26 biomarkers is given in online resource 1). D-dimer levels were determined by Alere Inc., San Diego, CA, using Luminex immunoassay methods (Online resource 1). Detailed information regarding the immunoassays has been previously published [21].
Study endpoints and definitions
Primary endpoint of interest for the present study was the occurrence of a major adverse cardiovascular event (MACE). MACE was defined as the composite of all-cause death, recurrent MI, stroke, or target vessel revascularization for ischemia. The primary safety endpoint was non CABG related major bleeding. Major bleeding was defined as any bleeding that met the following criteria: intracranial bleeding, intraocular bleeding, retroperitoneal bleeding, access site hemorrhage requiring surgery or a radiologic or interventional procedure, hematoma ≥5 cm in diameter at the puncture site, reduction in hemoglobin concentration of ≥4 g/dL without an overt source of bleeding, reduction in hemoglobin concentration of ≥3 g/dL with an overt source of bleeding, reoperation for bleeding, or use of any blood product transfusion. Secondary endpoints included cardiac and non cardiac mortality, ischemic and hemorrhagic stroke and reinfarction. Endpoint definitions have been previously published in detail [20].
Anemia was defined using WHO criteria as a hematocrit value at initial presentation <39 % for men and <36 % for women [22]. Thrombocytopenia was defined as <150,000 platelets per cubic millimeter at baseline (notably, a baseline platelet count under 100,000/cc3 was an exclusion criterion). Creatinine clearance was calculated at baseline by the Cockcroft–Gault equation [23].
Statistical analysis
Categorical variables are presented as percentages and were compared with the Chi square test or the Fisher’s exact test. Continuous variables are presented as medians with interquartile ranges, and were compared using Mann–Whitney U test.
To compare patient and procedural characteristics in patients with different D-dimer levels and to evaluate the impact of D-dimer levels on outcome, patients were divided into three groups according to D-dimer values on admission and at discharge. To increase statistical power, cutoffs for low-, intermediate-, and high-D-dimer groups were determined according to thirds of D-dimer levels among those who suffered a MACE. For analyses investigating the relationship between discharge D-dimer and subsequent outcome, only patients who were alive at hospital discharge were included in the analysis and only events occurring after discharge were taken into consideration.
We used a stepwise logistic regression model to determine the determinants of a high D-dimer level (defined as values above the cut-off of the highest admission D-dimer group). Kaplan–Meier analyses were used to estimate the 3 year event rates and to plot time-to-event curves; comparisons between the three groups of D-dimer were tested for significance using the log-rank test. We calculated unadjusted hazard ratios for MACE and non-CABG major bleeding using Cox proportional hazard models. To determine univariate predictors of MACE, we performed univariate Cox regression analyses including all variables in Table 1. To determine predictors of MACE in our dataset, we developed a multivariable stepwise Cox regression model including the variables with the strongest association with MACE by univariate analysis (to avoid overfitting, up to eight variables were considered for inclusion). The adjusted hazard ratio of the highest D-dimer group was calculated by adjusting for these predictors of MACE. Similarly, we calculated adjusted HRs for discharge D-dimer groups using Cox regression models including the previously identified relevant predictors of MACE. Entry and exit criteria were set at p = 0.10. The adjusted hazard ratio for bleeding was calculated in a multivariate Cox model adjusting for a risk score for bleeding by Mehran et al. [24]. This bleeding risk score proposed by our group is an integer score derived from the following six baseline characteristics that were predictive of non-CABG major bleeding in the acute catheterization and urgent intervention triage strategy (ACUITY) trial and HORIZONS-AMI trial: age, gender, white blood cell count, serum creatinine (mg/dL), anemia, antithrombotic therapy (UFH + GPI vs bivalirudin) and presentation (STEMI vs NSTEMI) [24].
Results
Of the 3,062 patients randomized in the HORIZONS-AMI trial, 502 were enrolled in the formal biomarker substudy (501 patients received a PES). Baseline characteristics and outcome of patients in the biomarker substudy versus those excluded for the biomarker substudy have been previously published [25]. 3 year clinical follow up was complete in 480 patients (96 %) and admission D-dimer was measured in 461 patients. Admission D-dimer was measured after a median of 9 min after start of the pPCI (IQR 4–33 min). In 15.3 % of patients D-dimer was measured before the start of the procedure, in 82.3 % of patients the measurement was after the start of PCI but within 60 min of the start of PCI. In the remaining 2.4 % of patients, D-dimer was measured after 1 h after the start of the procedure. Median D-dimer concentration was 0.32 μg/mL (IQR 0.21–0.55 μg/mL) on admission. Patients randomized to bivalirudin had a median D-dimer 0.30 μg/mL (IQR 0.19–0.51 μg/mL) on admission, as compared to 0.35 μg/mL (IQR 0.21–0.57 μg/mL) in the UFH + GPI group (p = 0.18). Discharge D-dimer was measured in 418 patients after a median duration of 5.0 days after hospital admission (IQR 3.0–6.7 days). The median increase in D-dimer during hospital admission was 0.31 μg/mL (IQR 0.10–0.69 μg/mL). At discharge the median D-dimer level was 0.73 μg/mL (IQR 0.44–1.23 μg/mL). There was no statistically significant difference in discharge D-dimer level or increase in D-dimer level for patients randomized to bivalirudin or heparin + GPI. The median discharge D-dimer level was 0.68 μg/mL (IQR 0.42–1.16 μg/mL) in the bivalirudin group and 0.79 μg/mL (IQR 0.48–1.32 μg/mL) in the UFH + GPI group (p = 0.11). The increase in D-dimer level during hospitalization was 0.28 (IQR 0.11–0.64 μg/mL) in the bivalirudin group versus 0.36 μg/mL (IQR 0.08–0.76 μg/mL) in the UFH + GPI group (p = 0.16).
Baseline characteristics according to the three groups of D-dimer at enrolment are given in Table 1. Patients with high D-dimer levels were typically female and of higher age. Coronary artery disease (CAD) tended to be more severely progressed in patients in the highest D-dimer group: they more often had a history of previous myocardial infarction, previous PCI and congestive heart failure as compared to patients in the lowest two groups of D-dimer. Also, they more often had a poor baseline left ventricular ejection fraction (LVEF < 40 %) and they more often presented with physical signs of heart failure (Killip class > 1). In addition, they more frequently had a history of peripheral artery disease, more often were anemic at presentation and had a lower creatinine clearance compared to patients in the lower groups of D-dimer. Conversely, patients in the lowest group of D-dimer level at admission more often had a family history of premature CAD. Finally, the total ischemic time tended to be longer with increasing D-dimer levels.
Predictors of high D-dimer
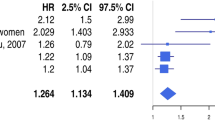
Table 2 displays the independent predictors of a high D-dimer level (≥0.71 μg/mL) on admission. Female patients had a twofold higher risk of having a high D-dimer level at presentation (OR 1.96, 95 % CI 1.05–3.70, p = 0.033). Per 10 years increase, age was associated with a 65 % increase in risk for a high D-dimer level (p = 0.0002). Those with a history of congestive heart failure or a poor LVEF had a markedly higher risk of having a high D-dimer (OR 2.72, p = 0.065 and OR 2.59, p = 0.027 respectively). Furthermore, patients with anemia on admission had a twofold higher risk of high D-dimer levels. Finally, the risk of a high D-dimer level increased with increasing total ischemic time (OR 1.11, 95 % CI 1.01–1.22, p = 0.027).
D-dimer on admission and subsequent outcome
Table 3 shows clinical outcomes at 3 years according to the 3 groups of D-dimer concentration at baseline. Figure 1 displays the Kaplan–Meier curves for the three D-dimer groups at admission and subsequent outcome. The rates of MACE, all-cause mortality and stroke increased with increasing levels of D-dimer. By univariate analysis, compared with the lowest group, the hazard ratio for the highest group for 3 year MACE was 2.86 (95 % CI 1.69–4.83, p < 0.0001). The hazard ratio for the middle D-dimer group was 1.37 (95 % CI 0.82–2.32, p = 0.24). After adjustment for relevant predictors of MACE, the hazard ratio was 2.58 (95 % CI 1.44–4.63, p = 0.0014) for the highest group of admission D-dimer (≥0.71 μg/mL) relative to the lowest group. The hazard ratio for the middle group D-dimer was 1.41 (95 % CI 0.82–2.41, p = 0.21). Other predictors of 3 year MACE are given in online resource 2.
16.7 % of patients in the highest group D-dimer suffered a non-CABG major bleeding within 3 years follow up, as compared to 1.2 % in patients with a D-dimer level between 0.30 and 0.71 μg/mL and 2.8 % in the lowest group (p < 0.0001). By univariate analysis, patients in the highest D-dimer group had a sixfold higher risk of 3-year HORIZONS-AMI non-CABG major bleeding (HR 6.25, 95 % CI 2.40–16.26, p = 0.002) compared to patients in the lowest and middle group. After adjustment for the Mehran risk score for bleeding the hazard ratio for 3 year major bleeding was 4.61 (95 % CI 1.76–12.1, p = 0.0018). The hazard ratio for bleeding for this risk score was 1.09 per unit increment (95 % CI 1.03–1.15, p = 0.0021). Adjustment for other variables was not possible given the low number of events.
D-dimer at discharge and subsequent outcome and delta D-dimer and subsequent outcome
Table 4 shows 3 year clinical outcome according to groups of discharge D-dimer levels. At discharge, higher levels of D-dimer were associated with higher rates of subsequent MACE, death and stroke. By univariate analysis, D-dimer levels above 1.261 μg/mL were associated with a twofold higher risk of subsequent MACE compared to D-dimer levels below 0.61 μg/mL (HR 1.88, 95 % CI 1.04–3.39, p = 0.037). After adjustment for relevant predictors of MACE, the hazard ratio for the highest group lost its statistical significance but was materially unchanged: 1.77 (95 % CI 0.96–3.28, p = 0.070). Other predictors of MACE occurring after discharge up to 3 years are given in online resource 3.
Discussion
The primary finding of this study is that a serum D-dimer concentration ≥0.71 μg/mL measured on admission is associated with a higher risk of MACE and major bleeding occurring within 3 years follow-up. A high D-dimer level at discharge (≥1.26 μg/mL) was associated with a higher incidence of subsequent MACE, but after multivariable adjustment was no longer associated with a statistically significant higher risk of subsequent MACE.
Although the relationship between D-dimer and subsequent outcome has been previously reported in healthy subjects and patients with unstable angina, no such relationship has been previously investigated in a contemporary set of AMI patients treated with pPCI and dual antiplatelet therapy. Moreover, those studies investigating the role of D-dimer in the prediction of subsequent outcome in AMI patients treated with thrombolysis have yielded conflicting results. In a substudy of the ESTEEM trial, higher D-dimer levels were not associated with a higher risk for cardiovascular events in 518 patients 2 weeks after their initial MI [16]. By contrast, in a study conducted in 314 patients with acute anterior MI treated with thrombolysis, patients who suffered a recurrent MI or death within 90 days follow up, had a higher mean D-dimer level at 2 and 7 days after presentation, compared to those who had not [26]. Furthermore, in a study of 1,045 patients 2 months after an MI, a D-dimer level > 0.65 μg/mL was an independent predictor for cardiovascular death or MI (HR 2.43 after multivariable adjustment) [9]. In our study higher discharge D-dimer levels measured after a median of 5 days were associated with a twofold increase in risk for MACE. This confirms that higher D-dimer levels in post MI patients are able to predict ischemic cardiovascular events. A novel finding of our study was that admission D-dimer was a strong predictor of MACE occurring within 3 years follow up. In addition, we observed increasing rates of mortality, recurrent MI and stroke with increasing groups of D-dimer. Unfortunately, the small amount of events restricted us in our ability to adjust for confounders; therefore we were unable to determine if D-dimer was an independent predictor of the individual components of our composite outcome measure.
Several mechanisms might underlie the predictive value of D-dimer for MACE in AMI patients. First, D-dimer levels may be a measure of atherosclerotic burden and corresponding thrombotic activity. The degree of atherosclerotic disease was recently shown to be associated with platelet reactivity [27]. Moreover, atherosclerosis is thought to cause a hypercoagulable state by increasing IL-6 induced tissue factor expression on circulating monocytes and microparticles. This in turn results in increased fibrin formation and thus, after proteolysis of fibrin, higher levels of circulating D-dimer [28, 29]. Furthermore, tissue factor expression on macrophages and smooth muscle cells within the plaque results in activation of the coagulation cascade resulting in fibrin formation. Consequently, D-dimer is generated by proteolysis in atheromatous plaques [29]. It is therefore not surprising that circulating D-dimer levels have been shown in clinical studies to be a reflection of the severity of peripheral and coronary atherosclerotic disease [30, 31]. Second, D-dimer may in turn aggravate atherosclerosis, by stimulating smooth muscle cell proliferation, and by serving as a chemotactic agent for monocytes [32, 33]. D-dimer also increases the synthesis of fibrinogen by stimulating the release of hepatocyte-stimulating factor from leukocytes, which in turn stimulates fibrinogen synthesis in the liver [34].
D-dimer and the risk of bleeding
No previous studies have shown an increased risk of bleeding in AMI patients with high D-dimer levels. Interestingly, D-dimer has recently been shown to be an independent predictor of major hemorrhagic events in 269 atrial fibrillation patients treated with warfarin. After approximately 2 years follow up, a D-dimer level > 0.5 μg/mL was associated with a strong increase in risk for hemorrhagic complications (HR 29.3; 95 % CI: 3.65–235, p < 0.01) after adjustment of baseline PT-INR [35]. In 142 AMI patients treated with thrombolysis, a trend towards higher D-dimer levels was found in patients who suffered a hemorrhagic complication (13.9 vs 9.4 μg/mL, p = 0.07) [36]. In 461 AMI patients treated with pPCI, we observed a marked increase in risk for 3-year non CABG major bleeding in patients with D-dimer ≥ 0.71 μg/mL. A possible explanation for the association between D-dimer levels and the risk of bleeding could be that patients with high D-dimer levels are at high risk of recurrent ischemic events and these patients are more likely to require invasive procedures and aggressive antithrombotic treatment and are therefore exposed to a higher likelihood of bleeding. Moreover, D-dimer may be elevated in patients with risk factors associated with bleeding. Since there were only 22 hemorrhagic events, we had limited ability to control for these confounding variables.
Predictors of high D-dimer levels
We found a longer total ischemic time (time from symptom onset to balloon inflation) to be associated with high D-dimer levels. Longer time from symptom onset to balloon reflects a longer period of time from initiation of the coagulation cascade and first fibrin deposition to hospital admission and corresponding initiation of antithrombotic therapy. It therefore seems plausible that longer ischemic time results in higher fibrin turnover and hence higher D-dimer levels.
Patients with a history of congestive heart failure and reduced LVEF had a higher risk of high D-dimer levels. This suggests that patients with heart failure have a higher rate of fibrin turnover. Indeed, patients with heart failure have been shown to be exposed to a persistent procoagulant state with resultant further disease progression [37].
Limitations
A number of potential limitations must be addressed. The present study follows a recent study, in which we explored the relationship between 26 biomarkers and the risk of subsequent bleeding. Given the multiple comparisons made for the 26 biomarkers as part of the HORIZONS-AMI biomarker substudy, the possibility of finding spurious associations was increased.
Because of the small amount of events (bleeding events in particular), we had limited ability to control for large differences in confounding variables. However, in an attempt to adjust for a broader set of variables, we adjusted for a risk score for bleeding. This notwithstanding, we cannot exclude that the associations found between D-dimer and bleeding is explained by residual confounding.
The association we found between high D-dimer levels and subsequent MACE might not be specific for D-dimer, but also be observed for other markers associated with coagulation that are closely related to D-dimer, such as lipoprotein(a). Unfortunately these were not measured in the HORIZONS-AMI biomarker substudy. Therefore, we were unable to determine if D-dimer has incremental value in the prediction of future cardiovascular events in post STEMI patients, on top of markers such as fibrinogen or lipoprotein (a). As 99.9 % of patients (501/502) in the biomarker substudy of the HORIZONS-AMI were treated with PES, these results cannot directly be extrapolated to AMI patients treated with BMS or other DES such as everolimus-eluting stents. Also, all patients underwent primary PCI for STEMI, and these results may therefore not be applicable to ACS patients. Finally, due to the retrospective nature of our analysis, causality between D-dimer and subsequent outcome cannot be established and our analyses should be considered hypothesis generating.
Conclusions
D-dimer at presentation is associated with MACE and non CABG major bleeding in patients with AMI treated with primary PCI and PES. This implies a significant relation between the amount of fibrin formation and degradation and subsequent risk for ischemic and hemorrhagic outcome after AMI. A larger trial is warranted to definitively address the prognostic value of D-dimer for subsequent MACE and bleeding after acute MI.
Abbreviations
- PCI:
-
Percutaneous coronary intervention
- AMI:
-
Acute myocardial infarction
- CABG:
-
Coronary artery bypass grafting
- UFH:
-
Unfractionated heparin
- GPI:
-
Glycoprotein IIb/IIIa inhibitor
- HORIZONS-AMI:
-
Harmonizing outcomes with revascularization and stents in acute myocardial infarction trial
- TIMI:
-
Thrombolysis in myocardial infarction
- MACE:
-
Major adverse cardiovascular events
- PES:
-
Paclitaxel-eluting stent
- BMS:
-
Bare metal stent
References
Pocock SJ, Mehran R, Clayton TC, Nikolsky E, Parise H, Fahy M, Lansky AJ, Bertrand ME, Lincoff AM, Moses JW, Ohman EM, White HD, Stone GW (2010) Prognostic modeling of individual patient risk and mortality impact of ischemic and hemorrhagic complications. Circulation 121(1):43–51. doi:10.1161/circulationaha.109.878017
Antithrombotic Trialists’ Collaboration (2002) Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324(7329):71–86
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357(20):2001–2015. doi:10.1056/NEJMoa0706482
Merlini PA, Bauer KA, Oltrona L, Ardissino D, Cattaneo M, Belli C, Mannucci PM, Rosenberg RD (1994) Persistent activation of coagulation mechanism in unstable angina and myocardial infarction. Circulation 90(1):61–68
Heinrich J, Balleisen L, Schulte H, Assmann G, van de Loo J (1994) Fibrinogen and factor VII in the prediction of coronary risk. Results from the PROCAM study in healthy men. Arterioscler Thromb 14(1):54–59
Meade TW, Mellows S, Brozovic M, Miller GJ, Chakrabarti RR, North WR, Haines AP, Stirling Y, Imeson JD, Thompson SG (1986) Haemostatic function and ischaemic heart disease: principal results of the Northwick Park Heart Study. Lancet 2(8506):533–537
Wilhelmsen L, Svardsudd K, Korsan-Bengtsen K, Larsson B, Welin L, Tibblin G (1984) Fibrinogen as a risk factor for stroke and myocardial infarction. N Engl J Med 311(8):501–505. doi:10.1056/nejm198408233110804
Oldgren J, Linder R, Grip L, Siegbahn A, Wallentin L (2001) Coagulation activity and clinical outcome in unstable coronary artery disease. Arterioscler Thromb Vasc Biol 21(6):1059–1064. doi:10.1161/01.atv.21.6.1059
Moss AJ, Goldstein RE, Marder VJ, Sparks CE, Oakes D, Greenberg H, Weiss HJ, Zareba W, Brown MW, Liang C-S, Lichstein E, Little WC, Gillespie JA, Van Voorhees L, Krone RJ, Bodenheimer MM, Hochman J, Dwyer EM, Arora R, Marcus FI, Watelet LFM, Case RB (1999) Thrombogenic factors and recurrent coronary events. Circulation 99(19):2517–2522. doi:10.1161/01.cir.99.19.2517
Ridker PM, Hennekens CH, Cerskus A, Stampfer MJ (1994) Plasma concentration of cross-linked fibrin degradation product (D-dimer) and the risk of future myocardial infarction among apparently healthy men. Circulation 90(5):2236–2240. doi:10.1161/01.cir.90.5.2236
Lowe GD, Yarnell JW, Sweetnam PM, Rumley A, Thomas HF, Elwood PC (1998) Fibrin D-dimer, tissue plasminogen activator, plasminogen activator inhibitor, and the risk of major ischaemic heart disease in the Caerphilly Study. Thromb Haemost 79(1):129–133
Cushman M, Lemaitre RN, Kuller LH, Psaty BM, Macy EM, Sharrett AR, Tracy RP (1999) Fibrinolytic Activation markers predict myocardial infarction in the elderly. Arterioscler Thromb Vasc Biol 19(3):493–498. doi:10.1161/01.atv.19.3.493
Fowkes FGR, Housley E, Rattray A, Lowe GDO, Rumley A, Elton RA, MacGregor IR, Dawes J (1993) Cross-linked fibrin degradation products, progression of peripheral arterial disease, and risk of coronary heart disease. Lancet 342(8863):84–86. doi:10.1016/0140-6736(93)91288-w
Menown IBA, Mathew TP, Gracey HM, Nesbitt GS, Murray P, Young IS, Adgey AAJ (2003) Prediction of recurrent events by D-dimer and inflammatory markers in patients with normal cardiac troponin I (PREDICT) study. Am Heart J 145(6):986–992. doi:10.1016/s0002-8703(03)00169-8
Linder R, Oldgren J, Egberg N, Grip L, Larson G, Siegbahn A, Wallentin L (1999) The effect of a low molecular mass thrombin inhibitor, inogatran, and heparin on thrombin generation and fibrin turnover in patients with unstable coronary artery disease. Eur Heart J 20(7):506–518. doi:10.1053/euhj.1998.1336
Christersson C, Oldgren J, Bylock A, Siegbahn A, Wallentin L (2007) Early decrease in coagulation activity after myocardial infarction is associated with lower risk of new ischaemic events: observations from the ESTEEM Trial. Eur Heart J 28(6):692–698
Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Fahy M, Parise H, Mehran R (2011) Heparin plus a glycoprotein IIb/IIIa inhibitor versus bivalirudin monotherapy and paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction (HORIZONS-AMI): final 3-year results from a multicentre, randomised controlled trial. Lancet 377(9784):2193–2204. doi:10.1016/s0140-6736(11)60764-2
Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R (2008) Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med 358(21):2218–2230. doi:10.1056/NEJMoa0708191
Stone GW, Lansky AJ, Pocock SJ, Gersh BJ, Dangas G, Wong SC, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Mockel M, Ochala A, Kellock A, Parise H, Mehran R (2009) Paclitaxel-eluting stents versus bare-metal stents in acute myocardial infarction. N Engl J Med 360(19):1946–1959. doi:10.1056/NEJMoa0810116
Mehran R, Brodie B, Cox DA, Grines CL, Rutherford B, Bhatt DL, Dangas G, Feit F, Ohman EM, Parise H, Fahy M, Lansky AJ, Stone GW (2008) The harmonizing outcomes with revascularization and stents in acute myocardial infarction (HORIZONS-AMI) trial: study design and rationale. Am Heart J 156(1):44–56. doi:10.1016/j.ahj.2008.02.008
Claessen BE, Stone GW, Mehran R, Witzenbichler B, Brodie BR, Wohrle J, Witkowski A, Guagliumi G, Zmudka K, Henriques JP, Tijssen JG, Sanidas EA, Chantziara V, Hakim D, Leon S, Xu K, Dangas GD (2012) Relationship between biomarkers and subsequent clinical and angiographic restenosis after paclitaxel-eluting stents for treatment of STEMI: a HORIZONS-AMI substudy. J Thromb Thrombolysis 34(2):165–179. doi:10.1007/s11239-012-0706-x
Nutritional anaemias. Report of a WHO scientific group (1968). World Health Organization technical report series 405:5–37
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW (2010) A Risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 55(23):2556–2566. doi:10.1016/j.jacc.2009.09.076
Kikkert W, Claessen B, Stone G, Mehran R, Witzenbichler B, Brodie B, Wöhrle J, Witkowski A, Guagliumi G, Zmudka K, Henriques JS, Tijssen JP, Sanidas E, Chantziara V, Xu K, Dangas G (2013) Relationship between biomarkers and subsequent bleeding risk in ST-segment elevation myocardial infarction patients treated with paclitaxel-eluting stents: a HORIZONS-AMI substudy. J Thromb Thrombolysis 35(2):200–208. doi:10.1007/s11239-012-0837-0
Dempfle CE, Kontny F, Abildgaard U (1999) Predictive value of coagulation markers concerning clinical outcome 90 days after anterior myocardial infarction. Thromb Haemost 81(5):701–704
Chirumamilla AP, Maehara A, Mintz GS, Mehran R, Kanwal S, Weisz G, Hassanin A, Hakim D, Guo N, Baber U, Pyo R, Moses JW, Fahy M, Kovacic JC, Dangas GD (2012) High platelet reactivity on clopidogrel therapy correlates with increased coronary atherosclerosis and calcification: a volumetric intravascular ultrasound study. JACC Cardiovasc Imaging 5(5):540–549. doi:10.1016/j.jcmg.2011.12.019
Borissoff JI, Heeneman S, Kilinc E, Kassak P, Van Oerle R, Winckers K, Govers-Riemslag JW, Hamulyak K, Hackeng TM, Daemen MJ, ten Cate H, Spronk HM (2010) Early atherosclerosis exhibits an enhanced procoagulant state. Circulation 122(8):821–830. doi:10.1161/circulationaha.109.907121
Spronk HM, van der Voort D, Ten Cate H (2004) Blood coagulation and the risk of atherothrombosis: a complex relationship. Thromb J 2(1):12. doi:10.1186/1477-9560-2-12
Lassila R, Peltonen S, Lepäntalo M, Saarinen O, Kauhanen P, Manninen V (1993) Severity of peripheral atherosclerosis is associated with fibrinogen and degradation of cross-linked fibrin. Arterioscler Thromb Vasc Biol 13(12):1738–1742. doi:10.1161/01.atv.13.12.1738
Folsom AR, Aleksic N, Park E, Salomaa V, Juneja H, Wu KK (2001) Prospective study of fibrinolytic factors and incident coronary heart disease. Arterioscler Thromb Vasc Biol 21(4):611–617. doi:10.1161/01.atv.21.4.611
Smith EB, Keen GA, Grant A, Stirk C (1990) Fate of fibrinogen in human arterial intima. Arterioscler Thromb Vasc Biol 10(2):263–275. doi:10.1161/01.atv.10.2.263
Richardson DL, Pepper DS, Kay AB (1976) Chemotaxis for human monocytes by fibrinogen-derived peptides. Br J Haematol 32(4):507–513
Ritchie DG, Levy BA, Adams MA, Fuller GM (1982) Regulation of fibrinogen synthesis by plasmin-derived fragments of fibrinogen and fibrin: an indirect feedback pathway. Proc Natl Acad Sci USA 79(5):1530–1534
Sadanaga T, Sadanaga M, Ogawa S (2010) Evidence that D-dimer levels predict subsequent thromboembolic and cardiovascular events in patients with atrial fibrillation during oral anticoagulant therapy. J Am Coll Cardiol 55(20):2225–2231
Meierhenrich R, Carlsson J, Seifried E, Pfarr E, Smolarz A, Neuhaus K-L, Tebbe U (1998) Effect of reteplase on hemostasis variables: analysis of fibrin specificity, relation to bleeding complications and coronary patency. Int J Cardiol 65(1):57–63. doi:10.1016/s0167-5273(98)00100-4
Shantsila E, Lip GY (2009) The endothelium and thrombotic risk in heart failure. Thromb Haemost 102(2):185–187. doi:10.1160/th09-06-0344
Acknowledgments
The HORIZONS-AMI trial was supported by the Cardiovascular Research Foundation, with grant support from Boston Scientific and the Medicines Company.
Conflict of interest
The HORIZONS-AMI trial was supported by the Cardiovascular Research Foundation, with grant support from Boston Scientific and the Medicines Company. Dr. Stone has served as a consultant to the Medicines Company and Boston Scientific. Dr. Dangas and Dr. Mehran have received speaker grants from Sanofi Aventis, Bristol-Meiers Squibb, The Medicines Co, Eli Lilly, Daiichi Sankyo, and honoraria from Astra Zeneca, Johnson & Johnson, and Abbott Vascular. Dr. Witzenbichler has received lecture honoraria from Boston Scientific and The Medicines Company. Dr. Witkowski has received honoraria from Medtronic, Abbott Vascular and Eli Lilly. Dr. Guagliumi has served as a consultant to Boston Scientific and St. Jude Medical and is receiving grant support from Abbott Vascular, Medtronic, Boston Scientific and St. Jude Medical. The other authors report no conflicts.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kikkert, W.J., Claessen, B.E., Stone, G.W. et al. D-dimer levels predict ischemic and hemorrhagic outcomes after acute myocardial infarction: a HORIZONS-AMI biomarker substudy. J Thromb Thrombolysis 37, 155–164 (2014). https://doi.org/10.1007/s11239-013-0953-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-013-0953-5