Abstract
Flavonols are polyphenolic compounds with reported cardiovascular benefits and have been shown to exhibit antiplatelet properties in vitro. While some studies have shown inhibition of platelet aggregation following dietary supplementation with flavonol rich foods, few studies have assessed the ability of flavonols to inhibit platelet mediated thrombus generation in vivo. Furthermore, the duration of benefit and the influence of different dosing regimens remain unclear. In this study we investigate the ability of two structurally related flavonols; quercetin (Que) and 3′,4′-dihydroxyflavonol (DiOHF) to inhibit platelet aggregation, platelet granule exocytosis and vessel occlusion in a well characterized mouse model of platelet mediated arterial thrombosis. We investigated the effect of a single 6 mg/kg intravenous bolus and daily 6 mg/kg intraperitoneal doses over seven consecutive days. Carotid artery blood flow after injury was better maintained in mice treated with both Que and DiOHF when compared to the vehicle for both dosage regimens. This improved blood flow corresponded to inhibition of platelet aggregation and platelet dense granule exocytosis following chemical stimulation of PAR4. We therefore provide evidence of inhibition of platelet-mediated arterial thrombosis by flavonols in vivo, and demonstrate that this effect persists for at least 24 h after the last intraperitoneal dose. These data suggest a potential clinical role for flavonols as anti-platelet therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arterial thrombosis is one of the leading causes of death in the developed world [1]. It is well established that platelet-vessel wall interactions play an essential role in the formation of vascular thrombosis. Platelets adhere to thrombogenic substances exposed on the damaged endothelium surface such collagen via glycoprotein (GP) VI [2] leading to platelet activation, aggregation and ultimately thrombus formation.
There is increasing evidence that dietary flavonols exert cardiovascular benefits. Flavonols are phenolic substances widely found in fruits and vegetables [3, 4]. Epidemiological studies have indicated that consumption of a flavonol rich diet is associated with reduced deaths due to cardiovascular disease (CVD) [5]. The Rotterdam study showed a reduction of > 65 % in the occurrence of fatal myocardial infarction with flavonol intake > 33 mg/d [6].
Flavonols have been shown to exert both antioxidant and antiplatelet activity in vitro [4, 7, 8]. Rechner et al. [9] have shown that dietary polyphenolic compounds inhibit platelet aggregation, and Hubbard et al. [10] reported that following ingestion of the naturally occurring flavonol quercetin (Que), collagen-induced platelet aggregation was inhibited. Furthermore, previous studies have shown that ingestion of flavonol rich foods and beverages reduce platelet aggregation induced by different agonists [11–14]. A recent study by Jasuja, et al. has demonstrated the potential for quercetin-3-rutinoside to inhibit laser induced thrombosis in mouse cremaster arteries in vivo [15]. However, no studies have assessed the duration of this antiplatelet effect, or whether inhibition of thrombosis occurs with structurally related flavonols.
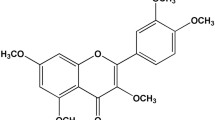
In addition to antiplatelet activity, flavonols exert well characterised antioxidant and vasorelaxant activity. Que has been shown to induce endothelium-independent vasodilation and to restore nitric oxide (NO) production and endothelial function in conditions of oxidative stress [16–18]. Que improves endothelium-dependant relaxation and increases cyclic AMP phosphodiesterases and protein C kinase (PKC) in rat aortae [17]. 3′,4′-Dihydroxyflavonol (DiOHF) is a synthetic flavonol with a structure suggested to improve antioxidant activity over natural flavonols [19]. It has been shown to be highly effective in restoring NO bioavailability [19]. DiOHF was also found to inhibit superoxide generation by blood vessels or in the presence of xanthine/xanthine oxidase and to reduce vascular contraction [20, 21]. It was also shown to reduce vascular damage due to ischaemia and reperfusion injury in animal models [22, 23]. However, no studies to date have assessed the antiplatelet potential of DiOHF.
The aim of this study was to examine the effect of Que and DiOHF on platelet function and platelet mediated thrombus generation in vivo. We sought to determine this both 30 min after a single intravenous (IV) dose, and 24 h after the last of seven daily intraperitoneal (IP) doses.
Materials and methods
Animals
All experimental procedures performed in this study were approved by the Animal Experimentation Ethics Committee of RMIT University and in accordance with the guidelines of the National Health and Medical Research Council of Australia for the care and use of animals for scientific purposes.
Administration of investigational agents
Mice (13 week old of both sexes) were treated with 6 mg/kg Que, 6 mg/kg DiOHF, or vehicle consisting of 0.5 % dimethylsulphoxide (DMSO) with 2.2 mM polyethylene glycol (PEG) in saline. DMSO and PEG were used to improve solubility of the flavonols in blood. The investigational agents were administered via an IP injection using a 26 gauge needle each day for 7 days, or a single IV bolus via the tail vein. Experimental procedures and blood collection were performed 24 h following the last IP treatment, or 30 min following the IV bolus treatment. 4.5 mg/kg IV eptifibatide (a GPIIb/IIIa antagonist) was used as a positive control.
Ferric chloride-induced carotid injury model
Ferric chloride-induced arterial injury was performed as previously published [24]. Briefly, mice were anaesthetised with ketamine and xylazine (200 : 10 mg/kg) by IP injection. A midline cervical incision was made and the carotid artery exposed. A laser Doppler flow probe (Moor Instruments, UK) was placed proximal to the carotid artery to measure baseline blood. After baseline blood flow was established, a 2 × 4 mm filter paper saturated with 20 % ferric chloride was applied to the carotid artery on the adventitial surface of the vessel for 4 min. Following the removal of the filter paper blood flow through the carotid artery was monitored for 30 min, or until 95 % vessel occlusion was reached. Area under the curve (AUC) and carotid artery blood flow at 15 and 30 min was recorded. At the end of each experiment and whilst under deep anaesthesia, the mouse was euthanized by cervical dislocation and the injured arterial segments were harvested to confirm the arterial injury by histological analysis.
Sample preparation for platelet aggregation and dense granule exocytosis
In separate mice, fresh whole blood was collected into tubes containing 100 μL of 3.2 % (w/v) sodium citrate via cardiac puncture. Platelet rich plasma (PRP) was obtained by centrifugation at 200xg for 15 min at room temperature with no brake. Platelet poor plasma (PPP) was obtained by centrifugation of the remaining blood at 1200xg for 15 min at room temperature. Platelet count was performed using a Beckman Coulter Ac.T 5 haematology analyser (Coulter Corporation, USA). Platelet count was normalised in all treatments groups to 100 x 109/L in Ringer citrate dextrose (RCD) buffer, pH 7.4 (108 mM NaCl, 38 mM KCl,1.7 mM NaHCO3, 21.2 mM sodium citrate, 27.8 mM glucose, and 1.1 mM MgCl2 · 6 H2O, with pH adjusted to 7.4).
Platelet aggregation
Platelet aggregation was measured by turbidimetric aggregometry using a Chrono-log 700 aggregometer (Chrono-log Corp, USA) [25]. In brief, PRP aggregation was performed in the presence of 100 μg/mL fibrinogen and 1 mM CaCl2 at 37 °C with constant stirring (200 rpm). Platelet aggregation baseline was set against murine PPP diluted 1 : 2 in RCD buffer. Platelet aggregation was stimulated using 250 μM of the protease-activated receptor 4 (PAR 4) agonist peptide (H–Ala–Tyr–Pro–Gly–Lys–Phe–NH2 (AYPGKF-NH2) (GL chemicals, China). The maximal aggregation amplitude over a 9 min period was recorded.
Assessment of granule exocytosis
Dense granule exocytosis was measured by flow cytometry using fluorescent quinacrine uptake and AYPGKF-NH2 induced release [26]. Mouse PRP was incubated with 100 μM quinacrine at 37 °C for 20 min in the dark. The platelets were then washed using RCD buffer by centrifugation at 500xg for 10 min with no brake at room temperature and resuspended in RCD buffer. Aliquots were incubated with 250 μM AYPGKF-NH2 at 37 °C for 5 min in the dark. The reaction was stopped by 1:12 dilution of RCD, and immediately analysed by flow cytometry as shown in Online Resource 1. Alpha granule exocytosis was measured in separate aliquots of PRP by incubating with anti-mouse CD62P-PE (BD Pharmingen, USA) and 250 μM AYPGKF-NH2 at 37 °C for 30 min. Flow cytometric analysis was performed using a FACSCanto II flow cytometer (BD Biosciences, USA). 10,000 individual platelets were analysed. Dense granule exocytosis was recorded as the percentage of decrease in quinacrine fluorescent intensity when compared to unstimulated platelets, while alpha granule exocytosis was recorded as CD62P mean fluorescent intensity (MFI).
Statistical analysis
All values are expressed as mean ± standard error of mean (SEM). Comparisons between test samples and control were performed using one-way ANOVA test with Dunnett’s test for post hoc comparisons. Comparison between single bolus and multiple dose vehicle regimens was made with an unpaired students t test.
Results
Effect of Que and DiOHF on FeCl3 induced arterial thrombosis in vivo
In order to assess the effect of Que and DiOHF on platelet mediated thrombosis in vivo, blood flow through the carotid artery of C57BL/6 mice was measured following FeCl3 injury. Vehicle treated mice had near complete vessel occlusion within the first 15 min following ferric chloride induced carotid artery damage with both the single IV dose (1.7 ± 1.7 % flow, Fig 1A) and multiple IP dose regimens (21.5 ± 9.2 % flow, Fig 1B, p = n.s. between regimens). As expected, the platelet GPIIb/IIIa receptor antagonist eptifibatide, administered at 4.5 mg/kg as a positive control maintained blood flow at near pre-injury levels (96.7 ± 3.3 % flow, Fig 1A, p < 0.05 vs. vehicle).
Arterial blood flow expressed as percentage of baseline. Blood flow was measured at 15 min (Panels A and B) and 30 min (Panels C and D) after arterial injury. Mice were treated with vehicle, 6 mg/kg quercetin, 6 mg/kg DiOHF or 4.5 mg/kg eptifibatide (positive control) either with a single IV bolus 30 min prior to arterial injury (Panels A and C, n = 5 for each treatment) or with daily IP doses over sequential days with the last dose 24 h prior to arterial injury (Panels B and D, n = 6 for each treatment). Data are mean ± SEM. One way ANOVA with Dunnett’s post-test. * p < 0.05 vs. vehicle control
Blood flow at 15 min was maintained at near pre-injury levels for mice treated with 6 mg/kg of Que for both the single IV (83.1 ± 17.0 % flow, Fig 1A, p < 0.05 vs. vehicle) and multiple IP regimen (100 ± 0 % flow, Fig 1B, p < 0.05 vs. vehicle). Likewise, blood flow at 15 min was well maintained in mice treated with 6 mg/kg DiOHF as either a single IV (100 ± 0 % flow, Fig 1A, p < 0.05 vs. vehicle) or with a multiple IP regimen (83.1 ± 17.0 % flow, Fig 1B, p < 0.05 vs. vehicle).
Blood flow remained completely absent for vehicle treated mice at 30 min following arterial injury for both the single IV (0 % flow, Fig 1C) and multiple IP regimens (0 % flow, Fig 1D, p = n.s. between regimens). Blood flow in mice treated with 6 mg/kg Que was lower at 30 min vs. 15 min for both the single IV (23.0 ± 4.7 % and 83.1 ± 17.0 % respectively, p < 0.05) and multiple IP regimens (52.0 ± 15.8 % and 100 ± 0 % respectively, p < 0.05), but remained significantly higher than the vehicle control (Fig. 1C, D, p < 0.05 vs. vehicle for each regimen). Similarly, mice treated with 6 mg/kg DiOHF had reduced blood flow at 30 min vs. 15 min for both the single IV dose (37.2 ± 16.1 % and 100 ± 0 % respectively, p < 0.05) and multiple IP dose regimens (27.5 ± 14.4 % and 83.1 ± 17.0 % respectively, p < 0.05). Nevertheless, mice treated with 6 mg/kg DiOHF as a single IV dose had improved blood flow at 30 min vs. vehicle control (37.2 ± 16.1 % vs. 0 %, Fig 1C, p < 0.05). However, while showing a similar trend, blood flow at 30 min was not significantly improved in mice treated with 6 mg/kg DiOHF as multiple IP doses (27.5 ± 14.4 % vs. 0 %, Fig 1D, p = n.s.).
Improved blood flow over the 30 min period follow arterial injury was also reflected in AUC. While greater overall blood flow was observed in mice treated with multiple IP vehicle vs. a single IV vehicle, this was not statistically significant (948 ± 156 vs. 476 ± 56 AU, p = 0.06). As expected, a single IV bolus of 4.5 mg/kg eptifibatide as a positive control significantly improved blood flow vs. vehicle over the 30 min period after injury (2471 ± 429 vs. 476 ± 56 AU, p < 0.05).
A single IV bolus of 6 mg/kg of Que significantly improved arterial blood flow over the 30 min following injury when compared to IV vehicle (2062 ± 296 vs. 476 ± 56 AU, Fig 2A, p < 0.05), and a similar improvement was seen with the multiple IP regimen (2705 ± 98 vs. 948 ± 156 AU, Fig 2B, p < 0.05). Similarly a single IV bolus of 6 mg/kg DiOHF significantly improved arterial blood flow over the 30 min following injury when compared to the IV vehicle (2472 ± 164 vs. 476 ± 56 AU, Fig 2A, p < 0.05), and a similar improvement was seen with the multiple IP regimen (2328 ± 289 vs. 948 ± 156 AU, Fig 2B, p < 0.05). Arterial injury was confirmed in all sections by histology.
Arterial blood flow area under the curve (AUC) over 30 min for mice treated with vehicle, 6 mg/kg quercetin, 6 mg/kg DiOHF or 4.5 mg/kg eptifibatide (positive control) following ferric chloride induced arterial injury. (Panel A) Mice treated by intravenous injection 30 min prior to arterial injury (n = 5 for each treatment). (Panel B) Mice treated by intraperitoneal injection once per day for seven consecutive days, with the last dose 24 h prior to arterial injury (n = 6 for each treatment). Data are mean ± SEM. One way ANOVA with Dunnett’s post-test. * p < 0.05 vs. vehicle control
Effect of Que and DiOHF on PAR-4 mediated platelet aggregation and granule exocytosis
In order to assess the effect of Que and DiOHF on platelet function, blood platelets from C57BL/6 mice treated with 6 mg/kg of each agent or vehicle control as a single IV bolus or multiple IP doses were assessed for PAR-4 induced platelet aggregometry and granule exocytosis, as shown in Online Resource 2.
Stimulation with 250 μM AYPGKF-NH2 induced 57.6 ± 6.1 % platelet aggregation in mice treated with a single IV vehicle (Fig 3A) and 73.4 ± 4.6 % aggregation in mice treated with multiple IP doses of vehicle (Fig 3B, p = 0.06 between regimens). As expected, a single IV bolus of 4.5 mg/kg eptifibatide significantly inhibited 250 μM AYPGKF-NH2 induced platelet aggregation vs. vehicle (31.0 ± 2.1 vs. 57.6 ± 6.1 %, Fig 3B, p < 0.05).
PRP platelet aggregation stimulated with 250 μM of the PAR 4 agonist AYPGKF-NH2. PRP was derived from mice treated with vehicle, 6 mg/kg quercetin, 6 mg/kg DiOHF or 4.5 mg/kg eptifibatide (positive control) either as a single IV bolus 30 min prior to blood collection (Panel A, n = 8 for each treatment) or as multiple IP injections over seven consecutive days with the final dose 24 h prior to blood collection (Panel B, n = 8 for each treatment). Data are mean ± SEM. One way ANOVA with Dunnett’s post-test. * p < 0.05 vs. vehicle control
Que significantly inhibited 250 μM AYPGKF-NH2 induced aggregation vs. vehicle control when administered as a single IV (47.0 ± 4.0 vs. 57.6 ± 6.1 % respectively, Fig 3A, p < 0.05) and multiple IP doses (50.4 ± 6.6 vs. 73.4 ± 4.6 % respectively, Fig 3B, p < 0.05). Similarly DiOHF significantly inhibited 250 μM AYPGKF-NH2 induced aggregation vs. vehicle control when administered as a single IV (46.3 ± 7.0 vs. 57.6 ± 6.1 % respectively, Fig 3A, p < 0.05) and multiple IP doses (49.9 ± 6.5 vs. 73.4 ± 4.6 % respectively, Fig 3B, p < 0.05).
Dense granule exocytosis was measured by fluorescent quinacrine release. Stimulation with 250 μM AYPGKF-NH2 induced 55.0 ± 4.1 % release of fluorescent quinacrine with a single IV vehicle (Fig 4A) and 61.9 ± 3.6 % release with multiple IP vehicle (Fig 4B, p = n.s. between regimens). As expected, treatment with a single IV bolus of 4.5 mg/kg eptifibatide did not affect dense granule exocytosis (52.3 ± 4.0 vs. 55.0 ± 4.1 %, Fig 4A, p = n.s.).
AYPGKF-NH2 induced platelet dense granule exocytosis, as measured by release of fluorescent quinacrine by flow cytometry. PRP was derived from mice treated with vehicle, 6 mg/kg quercetin, 6 mg/kg DiOHF or 4.5 mg/kg epitifibatide (positive control) either as a single IV bolus 30 min prior to blood collection (Panel A, n = 6 for each treatment) or as multiple IP injections over seven consecutive days with the final dose 24 h prior to blood collection (Panel B, n = 6 for each treatment). Data are mean ± SEM. One way ANOVA with Dunnett’s post-test. * p < 0.05 vs. vehicle control
Que significantly inhibited 250 μM AYPGKF-NH2 induced release of fluorescent quinacrine vs. vehicle control when administered as a single IV (32.0 ± 11.0 vs. 55.0 ± 4.1 %, Fig 4A, p < 0.05) and multiple IP doses (38.2 ± 7.8 vs. 61.9 ± 3.6 %, Fig 4B, p < 0.05). Similarly DiOHF significantly inhibited 250 μM AYPGKF-NH2 induced release of fluorescent quinacrine vs. vehicle control when administered as a single IV (29.3 ± 12.5 vs. 55.0 ± 4.1 %, Fig 4A, p < 0.05) and multiple IP doses (34.7 ± 6.7 vs. 61.9 ± 3.6 %, Fig 4B, p < 0.05).
Alpha granule exocytosis was measured by platelet surface P-selectin (CD62P) expression. Stimulation with 250 μM AYPGKF-NH2 induced CD62P expression vs. no agonist control (3257 ± 297 vs. 75 ± 14 MFI, p < 0.05) in vehicle treated mice.
Que had no effect on 250 μM AYPGKF-NH2 induced CD62P expression vs. vehicle control when administered as multiple IP doses (2671 ± 463 vs. 3257 ± 297 MFI, Fig 5, p = n.s.). DiOHF also had no effect on 250 μM AYPGKF-NH2 induced CD62P expression vs. vehicle control when administered as multiple IP doses (3306 ± 694 MFI vs. 3257 ± 297 MFI, Fig 5, p = n.s.). Single IV doses were not examined for CD62P expression.
AYPGKF-NH2 induced platelet alpha granule exocytosis, as measured by P-selectin expression by flow cytometry. PRP was derived from blood collected from mice treated with 6 mg/kg Que (n = 6), 6 mg/kg DiOHF (n = 6) or vehicle (n = 6) as multiple IP injections over 7 consecutive days with the final dose 24 h prior to blood collection. Data are mean ± SEM. One way ANOVA with Dunnett’s post-test. * p < 0.05 vs. vehicle control
Discussion
We demonstrate that the naturally occurring flavonol Que and the synthetic flavonol DiOHF improve carotid artery blood flow for up to 30 min following injury in a well-established mouse model of acute platelet mediated arterial thrombosis. These improvements in arterial flow correspond to inhibition of platelet aggregation and dense granule exocytosis. Improvements in both blood flow and inhibition of platelet aggregation and exocytosis were demonstrated with both a single IV bolus just prior to, or with multiple doses over days leading to the arterial injury. These results corroborate those of a very recent study showing the antithrombotic effects of a related dietary flavonol, quercetin-3-rutinoside in laser-induced injury to mouse cremaster arteries [15].
At 15 min after arterial injury, blood flow in the vehicle control had reduced to close to zero, while blood flow for 6 mg/kg Que and DiOHF were maintained with both dosage regimens. However, at 30 min after injury, while still significantly improved over the vehicle control, blood flow in the mice treated with Que and DiOHF had fallen below 50 % of pre-injury levels. This suggests mice treated with flavonols maintained the ability to form a thrombus, but that thrombus formation was reduced and occurred at a reduced rate.
Platelet aggregation is one of the final steps in the thrombus formation, and is preceded by adhesion to the site of injury, intracellular signalling, shape change and a platelet release reaction in which dense and alpha granules release mediators necessary for amplification of platelet activation and propagation of the platelet rich thrombus [27, 28]. This study demonstrates inhibition of dense granule exocytosis with 6 mg/kg of Que or DiOHF either by single IV or multiple IP injections. Dense granule exocytosis is necessary for in situ delivery of ADP to the growing thrombus, which is critical to thrombus propagation. Inhibition of dense granule exocytosis limits release of ADP and is consistent with a delay in thrombus generation. In contrast to eptifibatide, inhibition of thrombus growth and platelet aggregation with Que and DiOHF corresponded to inhibition of dense granule exocytosis. Thus inhibition of ADP release from dense granules with DiOHF and Que may contribute to the impaired thrombus generation observed in this study.
The model used is a well characterised model of platelet-mediated arterial thrombosis and the current study demonstrates direct antiplatelet potential of flavonols. However, the ability of flavonols to improve vascular relaxation and function is likely to also contribute to the observed improvements in blood flow. In this model ferric chloride injures the carotid artery by a redox-active mechanism requiring erythrocyte hemolysis [29]. Ferric chloride denudes the endothelium exposing the thrombogenic subendothelial matrix and subendothelial collagen. Circulating platelets recognise collagen and begin binding to it via the GPVI and GPIb-IX-V complex receptor on the platelet surface, initiating platelet activation, and subsequent thrombus formation at the site of endothelial injury [24]. Propagation of the growing thrombus beyond the site of injury is limited by expression of mediators such as NO and prostacyclin by the surrounding healthy endothelium. We [22, 30] and others [16–18] have previously demonstrated the capacity of flavonols to improve endothelial NO bioavailability in conditions of oxidative stress. While no studies have assessed Que and DiOHF on prostacyclin production, structurally related flavonoids found in several foods been shown to double the production of 6-keto-prostaglandin F1α (a stable metabolite of prostacyclin) in endothelial cells [31, 32]. This preservation of endothelial function and increased expression of key anti-platelet mediators, combined with well characterized enhancement of endothelium-dependant relaxation [11, 22, 30, 33] in addition to the direct anti-platelet activity we have demonstrated, may contribute to the improved blood flow following injury observed in this study.
Another potential mechanism by which Que and DiOHF might reduce thrombus formation in the FeCl3 induced injury model is through the potential for antioxidant activity ameliorating the oxidative damage produced by ferric chloride, rather than by direct inhibition of platelet function. However, this possibility is considered unlikely as arterial injury was confirmed by examination of histological segments irrespective of treatment, and is corroborated by data from Jasuja et al. [15] where a laser induced injury was used.
An unexpected finding of this study was that at a dosage capable of achieving inhibition of dense granule exocytosis, no inhibition of alpha granule exocytosis was observed. Platelet alpha granule secretion occurs more readily than dense granule secretion, however the mechanisms leading to membrane fusion and exocytosis of the two granule types have generally been assumed to be similar [34, 35]. Studies have shown that aspirin at certain concentrations is capable of inhibiting ADP induced serotonin release (a dense granule component) whilst P-selectin expression is unaffected [35], suggesting the potential for selective inhibition of exocytosis of the different granule types. The results obtained in the current study suggest a similar mechanism of selective inhibition of dense granule exocytosis whilst alpha granule exocytosis is maintained. However, recent studies have suggested that α-granules are heterogeneous in composition [34, 36]. While all α-granules contain P-selectin, subtypes have been identified with differential expression of pro- and anti-angiogenic factors [36] and vWF [37]. While our results demonstrate that overall α-granule exocytosis, as measured by P-selectin expression is not inhibited by Que or DiOHF, it remains possible that subtypes of α-granules may be inhibited, and further studies are warranted to elucidate this. Further investigation of the different concentrations of the flavonols and exocytosis induced by different chemical agonists is also warranted before conclusions can be drawn from this interesting observation.
While not significantly different, there was lower AYPGKF-NH2 induced aggregation in mice treated with IV vehicle 30 min prior to blood collection compared to those treated with IP vehicle over 7 days. The C57BL/6 mice in the IP groups were 2 weeks older than IV treated mice at the time of testing, and were sourced from a different supplier than the IV group. These variables may have contributed to the difference in vehicle treated aggregation. It is unlikely that the vehicle consisting of 0.5 % DMSO and 2.2 mM PEG in inhibited platelet function when administered by IV 30 min prior to blood collection as no effect of vehicle when added to platelets in vitro at the dose used was observed.
In this study IV treatment was used to assess the acute effect, while the multiple IP treatments were used to investigate the longer lasting effects of Que and DiOHF treatment. IV injection of 6 mg/kg will result in approximately 200 μM of Que and 270 μM of DiOHF in the plasma. This is much higher than concentrations associated with dietary intake of Que (20–30 nM [38]). IP treatments of the same dose are absorbed through the peritoneal circulation, while some of it is metabolised by the liver. It has been suggested that IP administered Que reaches the liver unchanged [39] where it is conjugated [40]. Therefore, the plasma flavonol concentration following IP administration would be less than that of IV treatment. However, a characteristic feature of the bioavailability of quercetin is the elimination of this flavonol and its metabolites is quite slow, with reported half-lives ranging from 11–28 h [41]. This could favour accumulation in the plasma with multiple IP doses. The pharmacodynamics of DiOHF are not yet established. We have shown that seven consecutive IP injections of 6 mg/kg of Que or DiOHF significantly reduce thrombus formation in the carotid artery. This corresponds to doses previously shown to significantly reduce oxidative damage produced by oxygen free radicals and reverse endothelial dysfunction by restoring endothelium dependant relaxation and increased nitric oxide bioavailability [11, 22, 30, 33]. Both single IV and multiple IP treatments with Que and DiOHF resulted in incomplete, yet significant inhibition of platelet aggregation and dense granule exocytosis accompanied with a significant reduction in thrombus formation.
Conclusion
We provide evidence that the naturally occurring flavonol Que and the synthetic flavonol DiOHF reduce thrombus formation and improve carotid artery blood flow in a well-established mouse model of acute platelet mediated arterial thrombosis. These improvements occur with both IV administration just prior to, or with multiple IP doses over 7 days with the last dose 24 h before arterial injury. The improvements in arterial flow correspond to inhibition of platelet aggregation and dense granule exocytosis.
References
Michelson AD (2010) Antiplatelet therapies for the treatment of cardiovascular disease. Nat Rev Drug Discov 9(2):154–169
Willoughby S, Holmes A, Loscalzo J (2002) Platelets and cardiovascular disease. Eur J Cardiovasc Nurs 1(4):273–288
Anjaneyulu M, Chopra K (2004) Quercetin, an anti-oxidant bioflavonoid, attenuates diabetic nephropathy in rats. Clin Exp Pharmacol Physiol 31(4):244–248
Engler MB, Engler MM (2006) The emerging role of flavonoid-rich cocoa and chocolate in cardiovascular health and disease. Nutr Rev 64(3):109–118
McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT (2012) Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr 95(2):454–464
Geleijnse JM, Launer LJ, Van der Kuip DA, Hofman A, Witteman JC (2002) Inverse association of tea and flavonoid intakes with incident myocardial infarction: the Rotterdam Study. Am J Clin Nutr 75(5):880–886
Beretz A, Cazenave JP, Anton R (1982) Inhibition of aggregation and secretion of human platelets by quercetin and other flavonoids: structure-activity relationships. Agents Actions 12(3):382–387
Pietta PG (2000) Flavonoids as antioxidants. J Nat Prod 63(7):1035–1042
Rechner AR, Kroner C (2005) Anthocyanins and colonic metabolites of dietary polyphenols inhibit platelet function. Thromb Res 116(4):327–334
Hubbard GP, Wolffram S, Lovegrove JA, Gibbins JM (2004) Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J Thromb Haemost 2(12):2138–2145
Chong MF, Macdonald R, Lovegrove JA (2010) Fruit polyphenols and CVD risk: a review of human intervention studies. Br J Nutr 104(Suppl 3):S28–S39
Vitseva O, Varghese S, Chakrabarti S, Folts JD, Freedman JE (2005) Grape seed and skin extracts inhibit platelet function and release of reactive oxygen intermediates. J Cardiovasc Pharmacol 46(4):445–451
Freedman JE, Parker C 3rd, Li L, Perlman JA, Frei B, Ivanov V, Deak LR, Iafrati MD, Folts JD (2001) Select flavonoids and whole juice from purple grapes inhibit platelet function and enhance nitric oxide release. Circulation 103(23):2792–2798
Briggs WH, Folts JD, Osman HE, Goldman IL (2001) Administration of raw onion inhibits platelet-mediated thrombosis in dogs. J Nutr 131(10):2619–2622
Jasuja R, Passam FH, Kennedy DR, Kim SH, van Hessem L, Lin L, Bowley SR, Joshi SS, Dilks JR, Furie B, Furie BC, Flaumenhaft R (2012) Protein disulfide isomerase inhibitors constitute a new class of antithrombotic agents. J Clin Invest 122(6):2104–2113
Perez-Vizcaino F, Duarte J (2010) Flavonols and cardiovascular disease. Mol Aspects Med 31(6):478–494
Duarte J, Perez-Vizcaino F, Zarzuelo A, Jimenez J, Tamargo J (1993) Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol 239(1–3):1–7
Perez-Vizcaino F, Ibarra M, Cogolludo AL, Duarte J, Zaragoza-Arnaez F, Moreno L, Lopez–Lopez G, Tamargo J (2002) Endothelium-independent vasodilator effects of the flavonoid quercetin and its methylated metabolites in rat conductance and resistance arteries. J Pharmacol Exp Ther 302(1):66–72
Woodman OL, Meeker WF, Boujaoude M (2005) Vasorelaxant and antioxidant activity of flavonols and flavones: structure-activity relationships. J Cardiovasc Pharmacol 46(3):302–309
Woodman OL, Chan E (2004) Vascular and anti-oxidant actions of flavonols and flavones. Clin Exp Pharmacol Physiol 31(11):786–790
Kim HY, Seok YM, Woodman OL, Williams SJ, Kim IK (2011) 3′,4′-Dihydroxyflavonol reduces vascular contraction through Ca(2 +) desensitization in permeabilized rat mesenteric artery. Naunyn Schmiedebergs Arch Pharmacol 385(2):191–202
Chan EC, Drummond GR, Woodman OL (2003) 3′,4′-dihydroxyflavonol enhances nitric oxide bioavailability and improves vascular function after ischemia and reperfusion injury in the rat. J Cardiovasc Pharmacol 42(6):727–735
Wang S, Thomas CJ, Dusting GJ, Woodman OL, May CN (2009) 3′,4′-Dihydroxyflavonol improves post-ischaemic coronary endothelial function following 7 days reperfusion in sheep. Eur J Pharmacol 624(1–3):31–37
Orlowski E, Chand R, Yip J, Wong C, Goschnick MW, Wright MD, Ashman LK, Jackson DE (2009) A platelet tetraspanin superfamily member, CD151, is required for regulation of thrombus growth and stability in vivo. J Thromb Haemost 7(12):2074–2084
Goschnick MW, Lau LM, Wee JL, Liu YS, Hogarth PM, Robb LM, Hickey MJ, Wright MD, Jackson DE (2006) Impaired “outside-in” integrin alphaIIbbeta3 signaling and thrombus stability in TSSC6-deficient mice. Blood 108(6):1911–1918
Linden MD, Frelinger AL 3rd, Barnard MR, Przyklenk K, Furman MI, Michelson AD (2004) Application of flow cytometry to platelet disorders. Semin Thromb Hemost 30(5):501–511
Kinlough-Rathbone RL, Packham MA, Reimers HJ, Cazenave JP, Mustard JF (1977) Mechanisms of platelet shape change, aggregation, and release induced by collagen, thrombin, or A23,187. J Lab Clin Med 90(4):707–719
Jackson SP (2007) The growing complexity of platelet aggregation. Blood 109(12):5087–5095
Woollard KJ, Sturgeon S, Chin-Dusting JP, Salem HH, Jackson SP (2009) Erythrocyte hemolysis and hemoglobin oxidation promote ferric chloride-induced vascular injury. J Biol Chem 284(19):13110–13118
Leo CH, Hart JL, Woodman OL (2011) 3′,4′-Dihydroxyflavonol reduces superoxide and improves nitric oxide function in diabetic rat mesenteric arteries. PLoS ONE 6(6):e20813
Schramm DD, Wang JF, Holt RR, Ensunsa JL, Gonsalves JL, Lazarus SA, Schmitz HH, German JB, Keen CL (2001) Chocolate procyanidins decrease the leukotriene-prostacyclin ratio in humans and human aortic endothelial cells. Am J Clin Nutr 73(1):36–40
Polagruto JA, Schramm DD, Wang-Polagruto JF, Lee L, Keen CL (2003) Effects of flavonoid-rich beverages on prostacyclin synthesis in humans and human aortic endothelial cells: association with ex vivo platelet function. J Med Food 6(4):301–308
Ishizawa K, Izawa-Ishizawa Y, Ohnishi S, Motobayashi Y, Kawazoe K, Hamano S, Tsuchiya K, Tomita S, Minakuchi K, Tamaki T (2009) Quercetin glucuronide inhibits cell migration and proliferation by platelet-derived growth factor in vascular smooth muscle cells. J Pharmacol Sci 109(2):257–264
Ren Q, Ye S, Whiteheart SW (2008) The platelet release reaction: just when you thought platelet secretion was simple. Curr Opin Hematol 15(5):537–541
Rinder CS, Student LA, Bonan JL, Rinder HM, Smith BR (1993) Aspirin does not inhibit adenosine diphosphate-induced platelet alpha-granule release. Blood 82(2):505–512
Italiano JE Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, Ryeom S, Folkman J, Klement GL (2008) Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood 111(3):1227–1233
van Nispen tot Pannerden H, de Haas F, Geerts W, Posthuma G, van Dijk S, Heijnen HF (2010) The platelet interior revisited: electron tomography reveals tubular alpha-granule subtypes. Blood 116 (7):1147-1156
Radtke J, Linseisen J, Wolfram G (2002) Fasting plasma concentrations of selected flavonoids as markers of their ordinary dietary intake. Eur J Nutr 41(5):203–209
Siess MH, Vernevaut MF (1982) The influence of food flavonoids on the activity of some hepatic microsomal monooxygenases in rats. Food Chem Toxicol 20(6):883–886
Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC (2007) A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol 45(11):2179–2205
Manach C, Williamson G, Morand C, Scalbert A, Remesy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81(1 suppl):230S–242S
Acknowledgments
The authors wish to gratefully acknowledge Dr Eunice Yang and Mr Musaed Alshahrani for their help with the animal model. Flow cytometry was performed at the RMIT University Flow Cytometry Core Facility.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mosawy, S., Jackson, D.E., Woodman, O.L. et al. Treatment with quercetin and 3′,4′-dihydroxyflavonol inhibits platelet function and reduces thrombus formation in vivo. J Thromb Thrombolysis 36, 50–57 (2013). https://doi.org/10.1007/s11239-012-0827-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-012-0827-2