Abstract
Major bleeding is a serious and potentially fatal complication of treatment with vitamin K antagonists (VKAs). Prothrombin complex concentrates (PCCs) can substantially shorten the time needed to reverse VKA effects. To determine the efficacy and safety of 3-factor PCCs for the rapid reversal of VKAs in patients with major bleeding. Patients receiving VKAs and suffering from acute major bleeding were eligible for this prospective cohort study if their international normalized ratio (INR) was higher than or equal to 2.0. Stratified 35–50 IU kg−1 PCC doses were infused based on initial INR. A total of 126 patients (62 males; mean age: 74 years, range 37–96 years) were enrolled. The mean INR at presentation was 3.3 (range 2–11). At 30 min after PCC administration the mean INR was 1.4 (range: 0.9–3.1), declining to less than or equal to 1.5 in 75 % of patients. The benefit of PCC was maintained for a long time, since in 97 % of all post-infusion time points through 96 h the mean INR remained lower than or equal to 1.5 (mean: 1.19; range: 0.9–2.3). During hospitalization neither thrombotic complications nor significant adverse events were observed and 12 patients died (10 %); none of the deaths was judged to be related to PCC administration. 3-factor PCC administration is an effective, rapid ad safe treatment for the urgent reversal of VKAs in patients with acute major bleeding. Broader use of PCC in this clinical setting appears to be appropriate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Therapy with vitamin K antagonists (VKAs) is an effective and commonly used treatment for long-term primary and secondary prophylaxis of arterial (including atrial fibrillation, mechanical heart valves) and venous thrombosis (such as deep vein thrombosis and pulmonary embolism) [1]. Despite meticulous surveillance of the treatment by regular international normalized ratio (INR) monitoring, bleeding events are frequent and major bleeding remains the most serious and potentially fatal complication of VKAs [2–7]. In large-scale epidemiological studies involving patients receiving VKAs, the annual incidence of major bleeding complications has ranged from 1 to 3 % [2, 3]. Sites accounting for the highest proportions of such complications were gastrointestinal (30–60 %) and intracranial (17–30 %) [2, 3]. Moreover, in patients with VKAs-associated intracranial haemorrhage (ICH), the mortality is very high, ranging from 50 to 60 % [8, 9].
Timely and complete reversal of VKAs is required in patients suffering from major haemorrhages, in whom immediate replacement of functional coagulation factors is indicated. Fresh frozen plasma (FFP) is a possible option, even if the time needed for preparation and infusion may lead to a clinically important delay in achieving effective reversal. Moreover, the effect of FFP may be inadequate especially in patients with exceedingly high INR values [10] and volume overload is a frequent complication observed following rapid transfusion of large volumes of FFP [10, 11].
All the prothrombin complex concentrates (PCCs) are human plasma derived and contain vitamin K-dependent coagulation factors II, IX, X (with or without a variable amount of factor VII) in a concentrated form and in a well standardised amount. PCCs produce a rapid and adequate action and substantially shorten the time needed to reverse VKA effects [12–15]. Moreover, these products are virally inactivated and they can be administered very rapidly without the need for matching the blood group or thawing the product [16]. In addition, a number of studies enrolling small numbers of patients have suggested that PCCs are able to correct more quickly and completely warfarin-related coagulopathy than FFP [10, 11, 17–19] and to reduce the risk of haematoma growth in ICH patients [20]. For these considerations, several clinical guidelines recommend that PCC infusion should be preferred over FFP for urgent reversal of anticoagulation in patients with life-threatening bleeding [1, 21–23]. Despite this, there remain limited evidences in the literature about the efficacy and safety of PCC administration in terms of recurrent bleeding, thromboembolic events, and other possible adverse events. Furthermore, little information exists on the ability of 3-factor PCCs to rapidly and effectively correct coagulopathy.
Aim of this multicentre, prospective cohort study was to evaluate the efficacy and safety of a 3-factor PCC infusion for the rapid reversal of VKAs and bleeding control in patients with acute major bleeding.
Patients and methods
Study population
Patients admitted to three Italian centers with objectively diagnosed acute symptomatic major bleeding during VKAs treatment and with an index INR ≥2.0 were eligible for inclusion. Other inclusion criteria were: age ≥18 years and the obtainment of a written informed consent. If a candidate was unable to sign informed consent, then the consent could be obtained from a legal representative or a family member of the patient. Exclusion criteria were: concomitant acute ischemic cardiovascular disorder diagnosed prior to the administration of PCCs, disseminated intravascular coagulation, sepsis, pregnancy, breast feeding, mental retardation. Patients were recruited 24/24 h a day and 7 days a week.
Treatment
All included patients received 35–50 IU kg−1 body weight of Uman Complex DI 500 IU/20 ml (Kedrion S.p.A., Castelvecchio Pascoli, Italy). Uman Complex DI 500 IU/20 ml is a human PCC and nominally contains the following IU of the human coagulation factors: factor II (25 IU/ml), factor IX (25 IU/ml), factor X (20 IU/ml). In addition the product contains the inhibitor protein C and its cofactor protein S (~9 IU/ml). Uman Complex DI is manufactured by two ion-exchange chromatography steps with no albumin added as a stabilizer. Safety of plasma used for the manufacturing of Uman Complex DI is achieved by using a robust safety program which includes also two viral inactivation methods validated: a solvent-detergent treatment (TnBP-TWEEN 80 to 25–26 °C for not <8 h) of the non-lyophilized preparation plus a dry heat treatment (99.5 ± 1 °C for 30 min) of the lyophilized final product.
Prothrombin complex concentrates were administered within 6 h from the diagnosis of major bleeding at different doses depending on baseline INR levels: 35–39, 40–45 or 46–50 IU kg−1 body weight doses were infused to patients with baseline INRs of 2.0–3.9, 4.0–6.0, or >6.0, respectively. Prior to PCC infusion, all patients were also treated with intravenous infusion of 10 mg of vitamin K. Concomitant therapy with whole blood, plasma, or plasma fraction was not allowed within the first 30 min after PCC infusion, unless urgently required as judged by the attending clinician. Conversely, an additional infusion of PCCs was allowed at intervals of 6 h after the administration of the first dose, depending on the INR level reached.
Study outcomes
The primary end-point of the study was the rate of INR values of equal to or lower than 1.5 after 30 min from the infusion of PCCs; pre-specified secondary end-points included the rate of INR levels equal to or lower than 1.5 at 6, 24, 48, 72 and 96 h after infusion.
Clinical end-points included mortality, bleeding recurrences, thromboembolic complications, viral infections and adverse events. The occurrence of clinical end-points was monitored throughout hospital stay and within 90 days of follow-up. The adjudication of the endpoints was done locally.
Laboratory and clinical assessment
Blood samples were collected for determination of INR prior to infusion and at intervals of 0.5, 6, 24, 48, 72 and 96 h afterward. Prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, haemoglobin, platelet count, d-dimer were determined at baseline and after 0.5, 6, and 24 h. The INR and the haematology parameters were measured at the local laboratories of the study centers.
At enrolment, all patients underwent a complete clinical assessment that included medical history, physical examination and determination of vital signs.
The occurrence of any adverse events (including death, thromboembolic complications, bleeding recurrences and allergic reactions) was monitored after 7, 30 and 90 days. Viral exposure was evaluated at baseline and 7, 30 and 90 days post-infusion of PCCs.
Sample size and statistical analysis
All statistical analyses were performed with the use of SPSS software version 11.0. Continuous variables such as INR values were analysed using ANOVA test for repeated measurements with the Dunnett multicomparison test. The (95 % CI) were also calculated for categorical variables expressed as percentage. All statistical tests were two-sided and p values <0.05 were considered statistically significant. For sample size calculation the following considerations were performed. The primary efficacy endpoint of this prospective cohort study was considered as the percentage of patients achieving an INR value <1.5 after at least 30 min after the PCC infusion. We hypothesized that at least 90 % of VKA treated patients receiving PCC could achieve an INR value of less than 1.5, 6 h after the infusion of PCC. Therefore, with a population of at least 90 patients, a percentage of 90 % of “successes” would result in 95 % CI of 83–96 %. Hence, a sample size of at least 90 subjects was considered sufficient to show an acceptable success rate after PCC infusion.
The study was performed in accordance with the International Conference on Harmonization Good Clinical Practice guidelines and the 1996 Declaration of Helsinky. The study was approved by local ethic committees at each participating center.
Results
Between October 2005 and September 2011, 126 patients were enrolled; 102 patients were admitted to emergency departments and 24 to internal medicine departments. Baseline characteristics of the patients are reported in Table 1 and indications for VKAs use are summarized in Table 2.
Seventy-two events (57 %) were hemorrhages at the central nervous system: 50 patients presented with intracerebral haemorrhage, 14 with subdural haemorrhage and 8 with subarachnoidal haemorrhage. Forty-eight patients (38 %) presented with gastrointestinal bleeding and two with post-traumatic haemoperitoneum. One patient each presented with severe haemoptysis, large spontaneous chest wall haematoma, hemothorax after recent lung biopsy and retroperitoneal post-traumatic bleeding.
According to the baseline INR levels (Table 1), a single PCC dose of 35–39, 40–45 and 46–50 IU kg−1 body weight was infused in 102, 15 and 9 patients, respectively [25–27]. The mean rate of PCCs administration was 2,518 IU per patient (SD: ±788 UI) (range 500–4,200 IU), over a mean infusion time of 30 min (SD = 0.8) (range 15–60 min). All patients received concomitant vitamin K (10 mg intravenously).
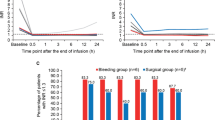
The mean INR at presentation was 3.3 (range 2–11). At 30 min after PCCs administration the mean INR was significantly reduced to 1.4 (range: 0.9–3.1) (p < 0.0001), declining to less than or equal to 1.5 in 75 % of the patients. In details, the rate of the patients with an INR 30 min after PCC infusion less than or equal to 1.5 was 91 % in the group with baseline INR of 2.0–3.9, 36 % in the group with baseline INR of 4–6, 11 % in the group with baseline INR >6. Only 5 patients (3.9 %) with INR exceeding 2.0 after the first administration of PCC received a second infusion of the concentrates.
The benefit of PCC was maintained for a long time, since in 97 % of all post-infusion time points through 96 h the mean INR remained lower than or equal to 1.5 (mean: 1.19; range :0.9–2.2) (Fig. 1). In details, the mean INR values at pre-treatment, 30 min, 6, 24, 48, 72 and 96 h post-treatment were 3.3, 1.4, 1.2, 1.2, 1.2, 1.1, respectively.
During hospitalization neither thrombotic complications nor severe adverse events were observed; only one patient suffered from non-fatal ICH recurrence and INR at the time of recurrence was 1.4.
After discharge, three patients (2.4, 95 % CI: from 0.8 to 8 %) suffered from thromboembolic complications. A 56-year old man died because of the occurrence of an ischemic stroke 37 days after PCC infusion; he had restarted anticoagulant treatment 5 days after ICH. The patient was at increased risk of thrombosis because of the concomitant presence of a prosthetic mechanical mitral valve and atrial fibrillation. A 79-year old female was hospitalized for an acute myocardial infarction 47 days after PCCs infusion, while taking antiplatelet drugs; her history included arterial embolism and severe cardiomyopathy. A 69-year old female was hospitalized for an acute left leg proximal deep vein thrombosis 42 days after PCCs infusion, during treatment with prophylactic dose of low-molecular-weight heparin.
Overall, 19 patients (15, 95 % CI: from 12 to 20 %) died: 12 patients died during hospitalization and 7 died after discharge. The causes of the death were as follows: heart failure (6 cases), pneumonia (3 cases), respiratory failure (3 cases), renal failure (2 cases), cancer (2 cases), sepsis (1 case), ischemic stroke (1 case), head injury (1 case). None of the deaths was judged to be related to PCCs administration.
In no patients there was evidence of viral transmission or other adverse events at the end of follow-up. No patients had a diagnosis of fluid overload associated with the reversal process.
Discussion
Bleeding is the most frequent complication of VKAs [1], but only few studies have focused on treatment options available for the emergency reversal of anticoagulation in case of acute major bleeding. The results of our study suggest that 3-factor PCCs infusion produces an effective and long-lasting reversal of VKAs by rapidly normalizing INR levels in nearly all the cases. In our cohort of patients, we did not observe either thrombotic complications or significant adverse events in the immediate post-infusion period. The long-term safety of our management strategy was also supported by the low rate of adverse events at a 3-month follow-up.
Our experience compares favourably with previously published series describing the use of PCCs for urgent reversal of warfarin in patients with major bleedings [17, 24–33].
Yasaka et al. [17] enrolled 42 anticoagulated patients admitted to an emergency department for major haemorrhagic complications, 35 of whom involving the central nervous system; this trial showed the efficacy of 4-factor PCC to rapidly normalize the INR values in almost all the cases. Imberti et al. [27] included in a prospective cohort study 92 patients suffering from acute intracerebral haemorrhage while receiving VKAs. A 3-factor PCC was administered in all the patients, at a dose depending on baseline INR levels and ranging between 35 and 50 IU/kg−1. The median INR at presentation was 3.3 (range 2–9). At 30 min after PCC administration the median INR was 1.4 (range 0.9–3.1), declining to 1.5 or less in 75 % of patients. The benefit of PCC was maintained for a long time, since in 98 % of all post-infusion time points through 96 h the median INR remained equal or less than 1.5 (median 1.19; range 0.9–2.3). Lankiewicz and colleagues [30] retrospectively investigated the feasibility, efficacy and safety of administering 4-factor PCCs to urgently reverse the anticoagulant effect of warfarin in 58 patients enrolled in a single center; 36 of them (62 %) presented with ICH. PCC doses were determined according to baseline INR levels, ranging between 25 and 50 IU/kg−1. PCC administration was very effective, since immediately after infusion 76 % of the patients had INR levels of lower than 1.5 and 96.5 % had INR levels of lower than 2.0. Pabinger and coworkers [31] prospectively evaluated whether balanced 4-factor PCCs allow INR normalization (defined as INR levels of equal to or lower than 1.3) in 43 anticoagulated patients requiring either an emergency surgical or urgent invasive diagnostic intervention or suffering from an acute major bleeding. The study demonstrated that PCCs treatment was an effective rapid hemorrhage control resource in the emergency anticoagulant reversal setting, since 30 min after treatment infusion the INR levels declined to equal to or less than 1.3 in 93 % of the treated patients. Viguè and co-workers [32] investigated the efficacy and safety of a 4-factor PCC for ultra-rapid INR normalization in 18 anticoagulated patients with ICH requiring urgent surgery. This study demonstrated that a bolus infusion of PCCs (1 min) was able to completely reverse anticoagulation within 3 min in all the patients.
In our study, the overall mortality during hospitalization and within 90 days of follow-up was 15 %; this figure is much lower than that shown in historical series in untreated patients [8, 9]. Of interest, the mortality rates observed in our trial were similar to those found in a recently published meta-analysis about safety of PCCs for rapid anticoagulation reversal of VKAs, in which 10.6 % of the cases died during follow-up [34].
In our study we have used a 3-factor PCC, which contains only factors II, IX, X in approximately equal quantities, with no detectable factor VII activity. The administration of PCCs with significant factor VII content may not be necessary to reverse warfarin-induced bleeding complications, although PCCs with significant factor VII content may be more efficient in correcting remarkably increased INRs [35]. Thus far, no direct head to head comparisons between 3- and 4-factor PCCs are available to address this issue. However, it is possible that the favourable results obtained in our study with 3-factor PCCs may be, at least in part, driven by the relatively low INR baseline levels of our patients. As a matter of fact, only 1 of the 9 patients presenting with an INR level >6 achieves a INR <1.5 within 30 min after administration of PCC. This data fit with the results of the study by Holland and colleagues, showing that a 3-factor PCC (Profilnine) given at a dose of 50 UI/kg was able to reduce the median INR from 8.6 (range 5.3–15) only to 4.7 (range 1.4–15) [36]. In such patients with extremely high INR values, if the goal is a better and faster normalisation of the INR, a 4-factor PCC is probably better, since it is well known that the results of INR test strongly depends on factor VII content. On the other hand, we do not have any evidence from available studies of a greater efficacy on strong clinical end-point of 4-factor PCC compared to 3-factor products. Finally, the argument in favour of 4-factor PCC, i.e. better and faster normalisation of extremely high INR is not clinical and could be counterbalanced by recently published data showing greater safety of the 3-factors PCC; Dentali in his meta-analysis reported a thrombotic incidence for 4-factor PCC of 2.3 versus 0.7 % for 3-factor products, even if the 95 % confidence interval overlap (1.2–3.8 vs. 0.0–2.4 %) and the difference was not statistically significant [34].
Even if PCCs are actually considered the optimal therapeutic option for the acute reversal of VKAs in patients with major bleeding [21, 22], there is a paucity of studies comparing their efficacy with other available haemostatic interventions, such as FFP and recombinant activated factor VII (rVIIa). In the studies that compared PCCs with FFP, PCCs showed a substantially more rapid and stable effect than FFP [10, 11, 18, 20]. rVIIa has shown promising results in this setting [37–40], but no randomized clinical trials have yet compared its efficacy and safety against those of PCCs or FFP. In a sustained anticoagulation animal model designed to simulate standard long-term oral coumarin therapy in patients, PCCs were shown to be more effective than rVIIa in restoring hemostatic function [39]. Moreover, in an experimental study using in vivo rat and in vitro human models of anticoagulation, both PCCs and rVIIa were associated with a reversal of prothrombin time, but only PCCs restored overall thrombin generation [40]. The very short half-life of rVIIa can be a serious drawback for the treatment of bleeding in anticoagulated patients, with the risk of exposing the patients to a potentially dangerous time window of persistent anticoagulant effect [16].
The results of our trial add important information on the use of PCCs for the urgent reversal of warfarin in patients with acute major bleeding. Firstly, our population was quite homogenous when compared to those enrolled in other similar trials. In fact, we have included only patients requiring reversal of warfarin because of an acute major haemorrhagic event, while all the other published trials have recruited anticoagulated patients requiring urgent reversal for surgical or invasive diagnostic interventions or INR normalization because of acute bleeding in different sites [30–32]. Second, in most previous studies the INR values were monitored for a short time, usually not exceeding 24 h post-infusion hours [31, 41]. In our study we have evaluated the long-term time course of change in INR, showing that the benefit of PCCs was maintained for a long time; in fact, in 96 % of all post-infusion time points through 96 h median INR remained lower than or equal to 1.5.
A potential complication of PCCs administration is the occurrence of venous and arterial thromboembolism, even if recently published data have clearly shown that the risk of thromboembolic events in VKA-treated patients receiving PCCs for anticoagulation reversal is quite low [34, 42, 43]. In our series, no case of thrombosis occurred during the initial hospitalization, while we observed three late thromboembolic events during the follow-up period. Given the broad time frame between PCCs administration and the occurrence of thrombotic events, it is unlikely that all the observed events can be related to the use of PCCs. Finally, no case of viral transmission was registered at the end of the follow-up.
This study has some limitations. First, we did not include a control group. The use of an untreated control group was obviously not ethical; moreover, since several European clinical guidelines recommend PPCs infusion as the treatment of choice for the urgent reversal of anticoagulation in patients with life-threatening bleeding [21, 22], a comparison with other haemostatic agents (such as FFP or rVIIa) was unfeasible. Second, because of the relatively small sample size, the patients included in this study may not be representative of the overall population and, therefore, definitive conclusions cannot be drawn from these data. However, the practical difficulties associated with obtaining suitable patients in this clinical setting make our results, albeit limited, of interest. Finally, the clinical significance of our findings could be considered questionable, since the primary end-point of the study was based on a surrogate marker of efficacy, that was reduction of INR levels. However, the association between INR levels and the risk of bleeding complications is well established.
In conclusion, PCCs administration is an effective, rapid and safe treatment for the urgent reversal of VKAs in patients with major bleeding. A broader use of PCCs in this clinical setting should be encouraged.
References
Ageno W, Gallus AS, Wittkowsky A et al (2012) Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (9th Edition). Chest 141:e44S–e88S
Palareti G, Leali N, Coccheri S et al (1996) Bleeding complications of oral anticoagulant treatment: an inception-cohort, prospective collaborative trial (ISCOAT). Lancet 348:423–428
Ageno W, Garcia D, Aguilar MI et al (2009) Prevention and treatment of bleeding complications in patients receiving vitamin K antagonists, part 2: treatment. Am J Hematol 84:584–588
Nicolini A, Ghirarduzzi A, Iorio A et al (2002) Intracranial bleeding: epidemiology and relationships with antithrombotic treatment in 241 cerebral hemorrhages in Reggio Emilia. Haematologica 87:948–956
Baldi G, Altomonte F, Altomonte M et al (2006) Intracranial haemorrhage in patients on antithrombotics: clinical presentation and determinants of outcome in a prospective multicentric study in Italian emergency departments. Cerebrovasc Dis 22:286–293
Garcia DA, Regan S, Crowther M, Hylek EM (2006) The risk of hemorrhage among patients with warfarin-associated coagulopathy. J Am Coll Cardiol 47:804–808
Go AS, Hylek EM, Chang Y et al (2003) Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA 290:2685–2692
Rosand J, Eckman MH, Knudsen KA et al (2004) The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 164:880–884
Aguilar MI, Hart RG, Kase CS et al (2007) Treatment of warfarin-associated intracerebral hemorrhage: literature review and expert opinion. Mayo Clin Proc 82:82–92
Makris M, Greaves M, Phillips W et al (1997) Emergency oral anticoagulant reversal: the relative efficacy of infusion of fresh frozen plasma and clotting factor concentrate on correction of the coagulopathy. Thromb Haemost 77:477–480
Boulis NM, Bobek MP, Schmaier A, Hoff JT (1999) Use of factor IX complex in warfarin-related intracranial hemorrhage. Neurosurgery 45:1113–1118
Yasaka M, Sakata T, Minematsu K, Naritomi H (2002) Correction of INR by prothrombin complex concentrate and vitamin K in patients with warfarin related hemorrhagic complications. Thromb Res 108:25–30
Riess HB, Meier-Hellmann A, Motsch J et al (2007) Prothrombin complex concentrate (octaplex) in patients requiring immediate reversal of oral anticoagulation. Thromb Res 121:9–16
Lorenz R, Kienast J, Otto U et al (2007) Successful emergency reversal of phenprocoumon anticoagulation with prothrombin complex concentrate: a prospective clinical study. Blood Coagul Fibrinolysis 18:565–570
Leissinger CA, Blatt PM, Hoots WK, Ewenstein B (2008) Role of prothrombin complex concentrates in reversing warfarin anticoagulation: a review of the literature. Am J Hematol 83:137–143
Marietta M, Pedrazzi P, Girardis M, Torelli G (2007) Intracerebral haemorrage: an often neglected medical emergency. Intern Emerg Med 2:38–45
Yasaka M, Sakata T, Naritomi H, Minematsu K (2005) Optimal dose of prothrombin complex concentrate for acute reversal of oral anticoagulation. Thromb Res 115:455–459
Fredriksson K, Norrving B, Stromblad LG (1992) Emergency reversal of anticoagulation after intracerebral hemorrhage. Stroke 23:972–977
Cartmill M, Dolan G, Byrne JL, Byrne PO (2000) Prothrombin complex concentrate for oral anticoagulant reversal in neurosurgical emergencies. Br J Neurosurg 14:458–461
Huttner HB, Schellinger PD, Hartmann M et al (2006) Hematoma growth and outcome in treated neurocritical care patients with intracerebral hemorrhage related to oral anticoagulant therapy: comparison of acute treatment strategies using vitamin K, fresh frozen plasma, and prothrombin complex concentrates. Stroke 37:1465–1470
Guidelines of the FCSA (2003) A guide to oral anticoagulant treatment. Haematologica 88(suppl .2): 1–47
Baglin TP, Keeling DM, Watson HG (2006) Guidelines on oral anticoagulation (warfarin): third edition—2005 update. Br J Haematol 132:277–285
Steiner T, Kaste M, Forsting M et al (2006) Recommendations for the management of intracranial haemorrhage—part I: spontaneous intracerebral haemorrhage. The European Stroke Initiative Writing Committee and the Writing Committee for the EUSI Executive Committee. Cerebrovasc Dis 22:294–316
Freilone R, Polio B, Delios G et al (2009) Efficacy and safety of prothrombin complex concentrate (Uman complex) and vitamin K in a cohort of 56 patients with anticoagulation-related acute intracranial haemorrhage: clinical features and outcomes at three months. Haematologica 94(s4):114–115
Tiraferri E, Galletti M, Argento A (2004) Emergency use of prothrombin complex concentrates in oral anticoagulant therapy. Rapid reversal. Haematologica 89:177
Van Aart L, Eijkhout HW, Kamphuis JS et al (2007) Individualized dosing regimen for prothrombin complex concentrate more effective than standard treatment in the reversal of oral anticoagulant therapy: an open, prospective randomized controlled trial. Thromb Res 119:15–16
Imberti D, Barillari G, Biasioli C et al (2009) Prothrombin complex concentrates for urgent anticoagulation reversal in patients with intracranial haemorrhage. Pathophysiol Haemost Thromb 36:259–265
Evans G, Luddington R, Baglin T (2001) Beriplex P/N reverses severe warfarin induced overcoagulation immediately and completely in patients presenting with major bleeding. Br J Haematol 115:998–1001
Preston FE, Laidlaw ST, Sampson B, Kitchen S (2002) Rapid reversal of oral anticoagulation with warfarin by a prothrombin complex concentrate (Beriplex): efficacy and safety in 42 patients. Br J Haematol 116:619–624
Lankiewicz MW, Hays J, Friedman KD et al (2006) Urgent reversal of warfarin with prothrombin complex concentrate. J Thromb Haemost 4:967–970
Pabinger I, Brenner B, Kalina U et al (2008) Beriplex P/N anticoagulation reversal study group. Prothrombin complex concentrate (Beriplex P/N) for emergency anticoagulation reversal: a prospective multinational clinical trial. J Thromb Haemost 6:622–631
Viguè M, Ract C, Tremey B et al (2007) Ultra-rapid management of oral anticoagulant therapy-related surgical intracranial haemorrhage. Intensive Care Med 33:721–725
Imberti D, Barillari G, Biasioli C et al (2011) Emergency reversal of anticoagulation with a three-factor prothrombin complex concentrates in patients with intracranial haemorrhage. Blood Transfus 9:148–155
Dentali F, Marchesi C, Pierfranceschi MG et al (2011) Safety of prothrombin complex concentrates for rapid anticoagulation reversal of vitamin K antagonists. A meta-analysis. Thromb Haemost 106(3):429–438
Kessler CM (2006) Urgent reversal of warfarin with prothrombin complex concentrate: where are the evidence-base data? J Thromb Haemost 4:963–966
Holland L, Warkentin TE, Refaai M et al (2009) Suboptimal effect of a three-factor prothrombin complex concentrate (Profilnine-SD) in correcting supratherapeutic international normalized ratio due to warfarin overdose. Transfusion 49:1171–1177
Sorensen B, Johansen P, Nielsen GL et al (2003) Reversal of the International Normalized Ratio with recombinant activated factor VII in central nervous system bleeding during warfarin thromboprophylaxis: clinical and biochemical aspects. Blood Coagul Fibrinolysis 14:469–477
Freeman WD, Brott TG, Barrett KM et al (2004) Recombinant factor VIIa for rapid reversal of warfarin anticoagulation in acute intracranial hemorrhage. Mayo Clin Proc 7:1495–1500
Brody DL, Aiyagari V, Shackeford AM, Diringer MN (2005) Use of recombinant factor VIIa in patients with warfarin-associated intracranial hemorrhage. Neurocrit Care 2:263–267
Dickneite G (2007) Prothrombin complex concentrate versus recombinant factor VIIa for reversal of coumarin anticoagulation. Thromb Res 119:643–651
Tanaka KA, Szlam F, Dickneite G, Levy JH (2008) Effects of prothrombin complex concentrate and recombinant activated factor VII on vitamin K antagonist induced anticoagulation. Thromb Res 122:117–123
Sørensen B, Spahn DR, Innerhofer P et al (2011) Clinical review: prothrombin complex concentrates–evaluation of safety and thrombogenicity. Crit Care 15(2):409
Franchini M, Lippi G (2010) Prothrombin complex concentrates: an update. Blood Transfus 8:149–154
Conflict of interests
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Imberti, D., Magnacavallo, A., Dentali, F. et al. Emergency reversal of anticoagulation with vitamin K antagonists with 3-factor prothrombin complex concentrates in patients with major bleeding. J Thromb Thrombolysis 36, 102–108 (2013). https://doi.org/10.1007/s11239-012-0817-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-012-0817-4