Abstract
Coronary plaque rupture is associated with a systemic inflammatory response. The relationship between baseline N-terminal pro B-type natriuretic peptide (NT-proBNP), a prognostic marker in patients with acute coronary syndromes, and systemic inflammatory mediators in patients with ST-segment elevation myocardial infarction (STEMI) treated with primary percutaneous coronary intervention (PCI) is not well described. Of 5,745 STEMI patients treated with primary PCI in the APEX-AMI trial, we evaluated the relationship between baseline NT-proBNP levels and baseline levels of inflammatory markers and markers of myonecrosis in a subset of 772 who were enrolled in a biomarker substudy. Spearman correlations (r s) were calculated between baseline NT-proBNP levels and a panel of ten systemic inflammatory biomarkers. Interleukin (IL)-6, a pro-inflammatory cytokine, was significantly positively correlated with NT-proBNP (r s = 0.317, P < 0.001). In a sensitivity analysis excluding all heart failure patients, the correlation between baseline IL-6 and NT-proBNP remained significant (n = 651, r s = 0.296, P < 0.001). A positive association was also observed with high sensitivity C-reactive protein (r s = 0.377, P < 0.001) and there was a weak negative correlation with the anti-inflammatory cytokine IL-10 (r s = −0.109, P = 0.003). No other significant correlations were observed among the other testes inflammatory cytokines and chemokines. In STEMI patients undergoing primary PCI, the pro-inflammatory cytokine IL-6 was modestly correlated with baseline NT-proBNP levels. This relationship remained significant in patients without heart failure. This finding is consistent with pre-clinical and clinical research suggesting that systemic inflammation may influence NT-proBNP expression independently of myocardial stretch.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
B-type natriuretic peptide (BNP) is an important prognostic biomarker in patients with acute coronary syndrome (ACS) [1–4]. N-terminal pro B-type natriuretic peptide (NT-proBNP) and markers of myocardial cell necrosis add prognostic value to established risk prediction scores [5, 6]. In non ST-segment elevation myocardial infarction (NSTEMI) baseline NT-proBNP values have been shown to correlate with troponin T; however, little is known about its relationship with markers of myocardial necrosis in the ST-elevation myocardial infarction (STEMI) population treated with primary percutaneous coronary intervention (PCI) [7].
There is emerging evidence that coronary plaque rupture triggers both a pro- and anti-inflammatory cytokine response [8–13]. BNP expression is commonly thought to result from myocardial stretch; however, it may also be regulated by systemic inflammatory mediators [14]. In pre-clinical studies, the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha (TNFα) all stimulated cardiocyte BNP expression [15–17]. Further, BNP can be elevated in the presence of normal cardiac function in inflammatory conditions, such as sepsis and burns [18, 19]. IL-10, an anti-inflammatory cytokine, can transiently rise in ACS [20]. BNP has been shown to increase IL-10 release in vitro, suggesting that BNP can stimulate an anti-inflammatory response; however, this relationship has not been established in vivo [21]. Although, BNP and both pro- and anti-inflammatory mediators can be elevated after plaque rupture, the relationship between baseline N-terminal pro-brain natriuretic peptide (NT-proBNP) and baseline inflammatory biomarkers in STEMI patients treated with primary PCI is not well described.
The purpose of this study was to describe the relationship between baseline NT-proBNP and inflammatory serum biomarkers in STEMI patients treated with primary PCI. Additionally, the relationship between NT-proBNP with markers of cell necrosis, time to revascularization, and sum ST-segment deviation were also explored.
Methods
The assessment of pexelizumab in acute myocardial infarction (APEX-AMI) trial was a multicenter, double blind, placebo-controlled, randomized clinical trial comparing pexelizumab versus placebo in 5,745 STEMI patients undergoing primary (PCI) [22, 23]. Patients over the age of 18 were eligible if they presented for primary PCI within 6 h of symptom onset with high-risk electrocardiographic features. Treatment groups were pooled for this analysis since no treatment effect on outcomes was observed.
The study population for our analyzes comprised of a subset of patients presenting to an APEX-AMI biomarker study center and consented to provide blood samples for future biomarker research. Blood samples were drawn in participating patients after randomization, but prior study drug administration and PCI [24]. Samples were allowed to clot, then centrifuged and the resultant serum frozen to −20°C then to −70°C the following day (if available). Samples were bulk shipped on dry ice to a central collection facility (Duke Center for Human Genetics, Durham, NC, USA) then to a central laboratory (Montreal Heart Institute, Montreal, Quebec, Canada) for batched biomarker analysis.
N-terminal pro B-type natriuretic peptide was measured using electrochemiluminescence immunoassay with Elecsys instrument and reagent kit (Roche Diagnostics, Indianapolis, IN). The analytical range was 5–35,000 pg/ml with an inter and intra-assay variability of 4–8%, respectively [25]. The remaining ten biomarkers measured in up to 772 patients were: the acute phase reactant high sensitivity C-reactive protein (hsCRP), pro-inflammatory cytokines (IL-1β, Il-6, IL-12), anti-inflammatory cytokines [IL-1 receptor antagonist (IL-1ra), IL-4, and IL-10], chemokines interferon gamma (IFNγ) and interferon inducible protein 10 (IP-10). These were measured using phase sandwich ELISA tests, cytometric beads assays, multiple fluorescent beads assays, and immunonephelometry.
Baseline markers of myocardial cell necrosis [creatinine kinase (CK), CK-MB, troponin I] were drawn after prior to study drug administration and analyzed at APEX-AMI study centers. The method for measuring sum ST-segment deviation (Σ STD) has been previously described [26]. Briefly, blinded central electrocardiogram evaluation took place at two core laboratories (Canadian VIGOUR Centre, Edmonton, Canada; Duke Clinical Research Institute, Durham, NC). Σ STD was calculated by adding the sums of ST-segment elevation and reciprocal depression.
Statistical analysis
Categorical variables are reported using frequencies and counts and continuous variables are represented as medians (25th, 75th percentiles). Testing of differences between those in the biomarker substudy and those who were not, used the Wilcoxon rank-sum test for continuous variables and the χ2 test for categorical variables.
Statistical dependence between variables was tested with the Spearman rank correlation test (r s). Since NT-proBNP is elevated in heart failure, a clinical and a hemodynamic sensitivity analysis were performed on the correlation between NT-proBNP and IL-6 [27]. In the first analysis, patients with either a history of heart failure and those who developed clinical heart failure within 90 days of the index STEMI presentation were excluded. In the second, the correlation was controlled for patients with a left ventricular end diastolic pressure or pulmonary capillary wedge pressure >16 mmHg at the time of PCI [28]. Analyzes were performed with SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Study population
Baseline patient characteristics of the 772 patient included in this analysis are presented in Table 1. Compared with the overall trial population, the substudy population had a higher percentage of female patients, were more likely to be older, hypertensive, have prior coronary artery disease, stroke, a chronic inflammatory condition, and have a smoking history. Patients enrolled in the biomarker substudy tended to be sicker with more anterior infarctions, higher Killip class, lower blood pressure at hospital presentation, and higher baseline creatinine.
NT-proBNP and inflammatory biomarkers
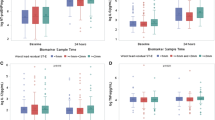
Table 2 shows the correlations between baseline inflammatory marker levels and quartiles of baseline NT-proBNP. There was significant positive correlation between hsCRP and NT-proBNP [Spearman correlation (r s) = 0.377, P < 0.001]. Among all pro-inflammatory cytokines and chemokines, only IL-6 had a moderate significant positive correlation with NT-proBNP (r s = 0.317, P < 0.001). No significant correlations were observed with either IL-1β or TNFα. There was a weak negative correlation between NT-proBNP and the anti-inflammatory cytokine IL-10 (r s = −0.109, P = 0.003). The relationship between NT-proBNP quartiles and IL-6, IL-10, and hsCRP is shown in Fig. 1.
In a sensitivity analysis excluding all patients with a history of heart failure (n = 29) and those who developed heart failure within 90 days of study enrolment (n = 92), the correlation between baseline IL-6 and NT-proBNP remained significant (r s = 0.296, P < 0.001). In a second sensitivity analysis controlling for left ventricular end diastolic pressure or pulmonary capillary wedge pressures recorded at the time of PCI, the relationship remained significant (r s = 0.184, P < 0.001) in the subset of patients in whom data was available (n = 315).
NT-proBNP correlation with markers of myocardial necrosis and time from symptom onset
The relationship between baseline markers of myocardial cell necrosis and baseline NT-proBNP are presented in Table 3. Total CK (r s = 0.113, P = 0.011) was weakly positively correlated with baseline NT-proBNP levels, while moderate positive correlations were observed with CK-MB (r s = 0.338, P < 0.001) and troponin I (r s = 0.420, P < 0.001).
Weak positive correlations with symptom onset to randomization time (r s = 0.275, P < 0.001) and symptom onset to presentation time (r s = 0.197, P < 0.001) were observed. There was no significant correlation with Σ STD (r s = −0.071, P = 0.051).
Discussion
This analysis from a contemporary international trial of STEMI patients undergoing primary PCI has three main findings that build upon pre-existing biomarker knowledge. First, although pre-clinical studies have reported that lL-1β, IL-6, and TNFα can stimulate BNP expression, IL-6 was the only cytokine with a modest positive correlation with baseline NT-proBNP levels [15–17]. Importantly, the relationship remained significant after excluding patients with clinical heart failure and after controlling for elevated filling pressures. Secondly, the small inverse relationship between NT-proBNP and IL-10 suggests there is not a clinically meaningful correlation between NT-proBNP and anti-inflammatory cytokine activation in ACSs. Finally, baseline NT-proBNP levels were only moderately correlated with baseline CK-MB and troponin I levels.
Coronary plaque rupture triggers an inflammatory cascade [8–12]. Three pro-inflammatory cytokines have been reported to stimulate BNP release. In separate studies using rat myocytes, Tanaka et al. [16] and Kuwahara et al. [17] showed that IL-6 stimulates BNP expression, while a study by Ma et al. [15] showed that IL-1β and TNFα stimulate BNP mRNA expression. In the present analysis, IL-6 was the only pro-inflammatory cytokine IL-6 with a significant positive correlation with NT-proBNP, though the association was only of modest strength. Importantly, this relationship remained significant after excluding patients with a history of heart failure and those who developed clinical heart failure. This is notable given the established relationship between NT-proBNP and clinical heart failure and elevated filling pressures [27]. This latter sensitivity analysis should be interpreted with caution given that less than half of the study patients had filling pressures measured. This observation supports the hypothesis that, in addition to release in response to myocardial stretch, BNP expression may, in part, also be regulated by systemic inflammatory mediators [14].
The hypothesis that cytokines can stimulate BNP release has not been well studied in humans with ACS, but evidence supporting this postulate exists. BNP rises have been reported during temporary coronary artery occlusion when cardiac filling pressures have remained unchanged [29]. Studies of critically ill septic and burn patients with normal echocardiograms have reported elevated BNP levels [18, 19]. These studies did not include measurements of inflammatory cytokines, but the results are supportive of the hypothesis given that the study patients’ had conditions with a known systemic inflammatory response. Additionally, IL-6 and BNP levels were correlated in 15 unstable angina patients in a single center study; however, the study did not sample baseline values. Our study is the largest to report a significant positive correlation between baseline NT-proBNP and IL-6 in the STEMI population in the contemporary era. The moderate correlation supports pre-clinical studies where IL-6 has been shown to stimulated NT-pro BNP expression although we cannot infer causation from this study as other possible pathophysiologic mechanisms exist. In addition to plaque rupture, myocardial stretch may also stimulate IL-6 expression [30]. This explanation would be consistent with the observation that both IL-6 and BNP are elevated in heart failure patients [31, 32]. Future studies should be directed at elucidating the potentially complex interaction between myocardial stretch, IL-6 and NT-proBNP synthesis.
IL-10 is an anti-inflammatory cytokine that promotes coronary plaque stability by inhibiting macrophage function, suppressing cytokines production, and inhibiting of metalloproteinases [33, 34]. Relatively low levels of IL-10 are thought to be a risk for coronary plaque rupture, yet levels can also transiently rise in some patients following an ACS [20, 35]. Pre-clinical studies showed that BNP stimulates macrophage IL-10 expression leading to the hypothesis that BNP may induce an anti-inflammatory cytokine response [21]. The small inverse relationship between NT-proBNP and IL-10 observed in this study suggests that the transient rise in IL-10 levels during the acute phase of ACS is not likely due to NT-proBNP. This result is consistent with animal and human studies showing that IL-10 is decreased in heart failure following acute myocardial infarction [36, 37]. Heeschen et al. [20] have shown that found elevated IL-10 levels at the time of hospital discharge were associated with lower mortality. Given the contrasting outcomes associated with elevated levels it of NT-proBNP and IL-10, it appears unlikely that there is a clinically significant correlation between these biomarkers.
Both BNP and markers of myocardial necrosis have been shown to improve baseline clinical prognostication in STEMI patients, but less is known about the relationship between these biomarkers in STEMI patients [6]. Moderate correlations between baseline BNP and troponin were reported in STEMI patients and in a single center series of 126 STEMI patients undergoing primary PCI [7, 38]. Additionally, correlations between peak CK-MB and BNP levels have been have been described previously, whereas similar correlations have not been reported for baseline levels [39]. The present study re-affirms the previously observed correlations former study’s findings in an international population and to our knowledge, this is the first study show a modest correlation with between baseline NT-proBNP and CK-MB in STEMI patients treated with primary PCI.
Limitations
Limitations of this APEX-AMI biomarker analysis warrant consideration. First, measures of left ventricular end diastolic pressure (LVEDP) and left ventricular ejection fraction were not routinely collected. A sensitivity analysis using clinical heart failure as reflection of elevated LVEDP was performed; however, subclinical heart failure cannot be excluded. Second, BNP levels are inversely related to glomerular filtration rate and this study did not exclude patients with renal failure; however, the median creatinine in this study was within the normal range [40]. Lastly, these analyzes should be considered hypothesis-generating, and they have not been validated in an independent data set.
Conclusions
In cohort of contemporary STEMI patients treated with primary PCI, the pro-inflammatory cytokine IL-6 was modestly correlated with baseline NT-proBNP levels. This relationship remained significant after excluding patients with clinical heart failure and after controlling for elevated filling pressures. These findings support pre-clinical and clinical studies that suggest that BNP may be expressed in response to systemic inflammatory stimuli. Additionally, the small inverse relationship between NT-proBNP and IL-10 suggests that a clinically relevant correlation between NT-proBNP and anti-inflammatory cytokine activation in ACS is unlikely. Finally, moderate correlations were observed between baseline NT-proBNP and CK-MB and troponin I levels. Future research should be directed at elucidating the potentially complex relationships between pro-inflammatory cytokines and BNP expression while controlling for measures of myocardial stretch and exploring how these relationships impact clinical outcomes.
References
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E (2001) The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 345(14):1014–1021. doi:10.1056/NEJMoa011053
Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E (2002) Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes. Circulation 105(15):1760–1763. doi:10.1161/01.cir.0000015464.18023.0a
Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG (2003) B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 107(22):2786–2792. doi:10.1161/01.cir.0000070953.76250.b9
Mega JL, Morrow DA, De Lemos JA, Sabatine MS, Murphy SA, Rifai N, Gibson CM, Antman EM, Braunwald E (2004) B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE-TIMI-23 substudy. J Am Coll Cardiol 44(2):335–339
Grabowski M, Filipiak KJ, Malek LA, Karpinski G, Huczek Z, Stolarz P, Spiewak M, Kochman J, Rudowski R, Opolski G (2007) Admission B-type natriuretic peptide assessment improves early risk stratification by Killip classes and TIMI risk score in patients with acute ST elevation myocardial infarction treated with primary angioplasty. Int J Cardiol 115(3):386–390
Damman P, Beijk MAM, Kuijt WJ, Verouden NJW, van Geloven N, Henriques JPS, Baan J, Vis MM, Meuwissen M, van Straalen JP, Fischer J, Koch KT, Piek JJ, Tijssen JGP, de Winter RJ (2011) Multiple biomarkers at admission significantly improve the prediction of mortality in patients undergoing primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction. J Am Coll Cardiol 57(1):29–36
James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol EJ, Simoons ML, Wallentin L (2003) N-terminal pro-brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease. Circulation 108(3):275–281. doi:10.1161/01.cir.0000079170.10579.dc
Herskowitz A, Choi S, Ansari AA, Wesselingh S (1995) Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol 146(2):419–428
Pannitteri G, Marino B, Campa PP, Martucci R, Testa U, Peschle C (1997) Interleukins 6 and 8 as mediators of acute phase response in acute myocardial infarction. Am J Cardiol 80(5):622–625
Smith DA, Irving SD, Sheldon J, Cole D, Kaski JC (2001) Serum levels of the antiinflammatory cytokine interleukin-10 are decreased in patients with unstable angina. Circulation 104(7):746–749. doi:10.1161/hc3201.094973
Deten A, Volz HC, Briest W, Zimmer H-G (2002) Cardiac cytokine expression is upregulated in the acute phase after myocardial infarction. Experimental studies in rats. Cardiovasc Res 55(2):329–340
Nian M, Lee P, Khaper N, Liu P (2004) Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res 94(12):1543–1553
Koten K, Hirohata S, Miyoshi T, Ogawa H, Usui S, Shinohata R, Iwamoto M, Kitawaki T, Kusachi S, Sakaguchi K, Ohe T (2008) Serum interferon-gamma-inducible protein 10 level was increased in myocardial infarction patients, and negatively correlated with infarct size. Clin Biochem 41(1–2):30–37
Kinnunen P, Vuolteenaho O, Ruskoaho H (1993) Mechanisms of atrial and brain natriuretic peptide release from rat ventricular myocardium: effect of stretching. Endocrinology 132(5):1961–1970. doi:10.1210/en.132.5.1961
Ma KK, Ogawa T, de Bold AJ (2004) Selective upregulation of cardiac brain natriuretic peptide at the transcriptional and translational levels by pro-inflammatory cytokines and by conditioned medium derived from mixed lymphocyte reactions via p38 MAP kinase. J Mol Cell Cardiol 36(4):505–513. doi:10.1016/j.yjmcc.2004.01.001
Tanaka T, Kanda T, Takahashi T, Saegusa S, Moriya J, Kurabayashi M (2004) Interleukin-6-induced reciprocal expression of SERCA and natriuretic peptides mRNA in cultured rat ventricular myocytes. J Int Med Res 32(1):57–61
Kuwahara K, Saito Y, Ogawa Y, Tamura N, Ishikawa M, Harada M, Ogawa E, Miyamoto Y, Hamanaka I, Kamitani S, Kajiyama N, Takahashi N, Nakagawa O, Masuda I, Nakao K (1998) Endothelin-1 and cardiotrophin-1 induce brain natriuretic peptide expression by distinct transcriptional mechanisms. J Cardiovasc Pharmacol 31:S354–S356
Maeder M, Ammann P, Kiowski W, Rickli H (2005) B-type natriuretic peptide in patients with sepsis and preserved left ventricular ejection fraction. Eur J Heart Fail 7(7):1164–1167. doi:10.1016/j.ejheart.2005.03.003
Papp A, Uusaro A, Parviainen I, Hartikainen J, Ruokonen E (2003) Myocardial function and haemodynamics in extensive burn trauma: evaluation by clinical signs, invasive monitoring, echocardiography and cytokine concentrations. A prospective clinical study. Acta Anaesthesiol Scand 47(10):1257–1263. doi:10.1046/j.1399-6576.2003.00235.x
Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML, Zeiher AM, Investigators CS (2003) Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation 107(16):2109–2114
Chiurchiù V, Izzi V, D’Aquilio F, Carotenuto F, Di Nardo P, Baldini PM (2008) Brain natriuretic peptide (BNP) regulates the production of inflammatory mediators in human THP-1 macrophages. Regul Pept 148(1–3):26–32. doi:10.1016/j.regpep.2008.02.009
Armstrong PW, Adams PX, Al-Khalidi HR, Hamm C, Holmes D, O’Neill W, Todaro TG, Vahanian A, Van de Werf F, Granger CB (2005) Assessment of pexelizumab in acute myocardial infarction (APEX AMI): a multicenter, randomized, double-blind, parallel-group, placebo-controlled study of pexelizumab in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J 149(3):402–407. doi:10.1016/j.ahj.2004.12.015
The APEX-AMI investigators (2007) Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention. JAMA 297(1):43–51. doi:10.1001/jama.297.1.43
Mahaffey KW, Reist CJ, Fu Y, Brener SJ, Theroux P, Patel MR, Stebbins A, Westerhout CM, Todaro TG, Adams PX, Granger CB, Armstrong PW (2008) Integrating ancillary studies in a large clinical trial: the design and rationale of the APEX library. Contemp Clin Trials 29(6):887–895. doi:10.1016/j.cct.2008.06.003
Ezekowitz JA, Armstrong PW, Granger CB, Theroux P, Stebbins A, Kim RJ, Patel MR (2010) Predicting chronic left ventricular dysfunction 90 days after ST-segment elevation myocardial infarction: an assessment of pexelizumab in acute myocardial infarction (APEX-AMI) substudy. Am Heart J 160(2):272–278. doi:10.1016/j.ahj.2010.05.035
Buller CE, Fu Y, Mahaffey KW, Todaro TG, Adams P, Westerhout CM, White HD, van’t Hof AWJ, Van de Werf FJ, Wagner GS, Granger CB, Armstrong PW (2008) ST-segment recovery and outcome after primary percutaneous coronary intervention for ST-elevation myocardial infarction. Circulation 118(13):1335–1346. doi:10.1161/circulationaha.108.767772
Januzzi JL, van Kimmenade R, Lainchbury J, Bayes-Genis A, Ordonez-Llanos J, Santalo-Bel M, Pinto YM, Richards M (2006) NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1,256 patients. Eur Heart J 27(3):330–337. doi:10.1093/eurheartj/ehi631
Zile MR, Gaasch WH, Carroll JD, Feldman MD, Aurigemma GP, Schaer GL, Ghali JK, Liebson PR (2001) Heart failure with a normal ejection fraction. Circulation 104(7):779–782. doi:10.1161/hc3201.09422629
Tateishi J, Masutani M, Ohyanagi M, Iwasaki T (2000) Transient increase in plasma brain (B-type) natriuretic peptide after percutaneous transluminal coronary angioplasty. Clin Cardiol 23(10):776–780. doi:10.1002/clc.4960231016
Pan J, Fukuda K, Saito M, Matsuzaki J, Kodama H, Sano M, Takahashi T, Kato T, Ogawa S (1999) Mechanical stretch activates the JAK/STAT pathway in rat cardiomyocytes. Circ Res 84(10):1127–1136
Tsutamoto T, Hisanaga T, Wada A, Maeda K, Ohnishi M, Fukai D, Mabuchi N, Sawaki M, Kinoshita M (1998) Interleukin-6 spillover in the peripheral circulation increases with the severity of heart failure, and the high plasma level of interleukin-6 is an important prognostic predictor in patients with congestive heart failure. J Am Coll Cardiol 31(2):391–398. doi:10.1016/s0735-1097(97)00494-4
Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AHB, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347(3):161–167. doi:10.1056/NEJMoa020233
Wang P, Wu P, Siegel MI, Egan RW, Billah MM (1995) Interleukin (IL)-10 inhibits nuclear factor B (NFB) activation in human monocytes. J Biol Chem 270(16):9558–9563. doi:10.1074/jbc.270.16.9558
Lacraz S, Nicod LP, Chicheportiche R, Welgus HG, Dayer JM (1995) IL-10 inhibits metalloproteinase and stimulates TIMP-1 production in human mononuclear phagocytes. J Clin Invest 96(5):2304–2310
Waehre T, Halvorsen B, Damas JK, Yndestad A, Brosstad F, Gullestad L, Kjekshus J, Froland SS, Aukrust P (2002) Inflammatory imbalance between IL-10 and TNF-alpha in unstable angina potential plaque stabilizing effects of IL-10. Eur J Clin Invest 32(11):803–810
Stumpf C, Lehner C, Yilmaz A, Daniel WG, Garlichs CD (2003) Decrease of serum levels of the anti-inflammatory cytokine interleukin-10 in patients with advanced chronic heart failure. Clin Sci 105(1):45–50. doi:10.1042/cs20020359
Lopes RD, Batista ML, Rosa JC, Lira FS, Martins EJ, Shimura AY, Brum PC, Lancha AH, Seelaender MC, Lopes AC (2010) Changes in the production of IL-10 and TNF-alpha in skeletal muscle of rats with heart failure secondary to acute myocardial infarction. Arq Bras Cardiol 94(3):293–300
Grabowski M, Filipiak KJ, Karpinski G, Wretowski D, Rdzanek A, Huczek Z, Horszczaruk GJ, Kochman J, Rudowski R, Opolski G (2004) Serum B-type natriuretic peptide levels on admission predict not only short-term death but also angiographic success of procedure in patients with acute ST-elevation myocardial infarction treated with primary angioplasty. Am Heart J 148(4):655–662. doi:10.1016/j.ahj.2004.04.023
Gill D, Seidler T, Troughton RW, Yandle TG, Frampton CM, Richards M, Lainchbury JG, Nicholls G (2004) Vigorous response in plasma N-terminal pro-brain natriuretic peptide (NT-BNP) to acute myocardial infarction. Clin Sci 106(2):135–139. doi:10.1042/cs20030131
Anwaruddin S, Lloyd-Jones DM, Baggish A, Chen A, Krauser D, Tung R, Chae C, Januzzi JL Jr (2006) Renal function, congestive heart failure, and amino-terminal pro-brain natriuretic peptide measurement: results from the proBNP investigation of dyspnea in the emergency department (PRIDE) study. J Am Coll Cardiol 47(1):91–97. doi:10.1016/j.jacc.2005.08.051
Acknowledgments
The APEX-AMI trial from which this work was derived, was supported by a research grant jointly funded from Procter & Gamble and Alexion Pharmaceuticals.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was conducted on behalf of the APEX-AMI investigators.
Rights and permissions
About this article
Cite this article
van Diepen, S., Roe, M.T., Lopes, R.D. et al. Baseline NT-proBNP and biomarkers of inflammation and necrosis in patients with ST-segment elevation myocardial infarction: insights from the APEX-AMI trial. J Thromb Thrombolysis 34, 106–113 (2012). https://doi.org/10.1007/s11239-012-0691-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-012-0691-0