Abstract
Thrombosis is the common mechanism of various diseases of heart and vasculature and their major morbility and mortality. An efficient, safe and easy thrombolysis method is needed. We tried to develop a new type of ultrasound microbubbles carrying thrombolytics and simultaneously targeting to thrombus, which could bind with thrombus specifically and release the encapsulated drug locally under the ultrasound exposure. Microbubbles carrying tissue plasminogen activator (tPA) and Arg-Gly-Asp-Ser tetrapeptide (RGDS) were prepared by lyopyilization. Their properties were detected, including morphology, particle size, surface potential and pH. The results showed that the microbubbles were suitable for intravenous injection. The envelope rate of tPA, detected by ELISA, was (81.12 ± 2.44%), and the conjugate rate of RGDS, detected by flow cytometer, was (94.49 ± 6.19%). The tPA encapsulated in microbubbles kept fibrinolysis activity under the conditions of both natural releasing and ultrasound exposure, checked by agarose fibrin plate process. The contrast-enhanced ultrasonography (CEU) in rabbit liver showed that they were good for enhanced ultrasound imaging. The in vitro thrombolysis of the microbubbles to the blood clots from healthy human was detected with a mimical flowing model propelled by peristaltic pump. The drug-loaded microbubbles plus ultrasound irradiation got higher thrombolysis with the lowest dosage. The tPA-loaded microbubbles targeting to thrombus can be prepared by lyopyilization, which will bring out a novel way for the targeting drug-released thrombolysis therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thrombosis in blood vessels is the common mechanism of various diseases and the major cause of their morbility and mortality, such as myocardial infarction, ischemic stroke, systemic embolism, pulmonary embolism and phlebothrombosis. The interventional transcatheter therapy is limited because of the strict requirement of technique and hardware, and transvenous drug thrombolysis such as tissue plasminogen activator (tPA), though easy to operate, is still doubted by clinicians for its short half-life period, high price and the hemorrhagic complication [1]. So a non-invasive, safe, cheap and easy-operating thrombolysis method is urgently needed.
It has been proved by a large number of in vivo or in vitro researches [2–4] that ultrasound irradiation can enhance the effect of thrombolytics and it can be further improved with the introduction of ultrasound contrast microbubbles because of the enhanced cavitation effect accelerated by the interaction between the ultrasound radiation and microbubbles. Furthermore, microbubbles-mediated drug delivery and target imaging are the hot spots of the researches on microbubbles. Drugs or genes can be adhered to the bubble surface, encapsulated in the shell or carried inside the inner cavity of microbubbles, being protected from the degradation of blood [5, 6], and local ultrasound irradiation can improve the interaction between the drug and the target tissue or cells [7]. Targeted imaging can be achieved through the binding of some special legends and the surface of microbubbles, which leads the microbubbles to combine with the molecules or cells in interest and fulfil the target molecular imaging [8–10].
Then, can the technologies mentioned above be integrated and applied in thrombolysis? If the microbubbles carrying thrombolytics can bind with the thrombus and release the drugs by the enhanced cavitation under the ultrasound exposure, the goals of both less drugs and higher effects can be reached and the above-mentioned limits may be broken. So the construction of a novel type of ultrasound contrast microbubbles was designed, which were simultaneously carrying tissue plasminogen activator (tPA) and tetrapeptide of Arg-Gly-Asp-Ser (RGDS) capable of binding with the activated platelet in thumbus. The microbubbles were expected to deliver tPA to thrombus, avoiding the degradation of blood, bind with thrombus specifically, release tPA by ultrasound irradiation, and achieve an easier, safer and improved thrombolysis. Its thrombolytic efficacy was evaluated with human blood clot under a condition of simulated extracorporeal circulation.
Methods
Construction of microbubbles carrying tPA and RGDS
The tPA (CALBIOCHEM, US) and RGDS (CHINESE PEPTIDE, China) were labelled with 5FAM and FITC before preparation, which radiated red and green fluorescence, respectively. The activator carbodiimide muriate and RGDS were mixed and reacted for 30 min under the condition of pH 7.4 and 4°C. Certain quantities of Dipalmitoyl phosphatidylcholine (DPPC), Distearoyl phosphatidylcholine (DSPC) (Both from Genzyme Pharmaceuticals, Sweden), d-glucose, Amino-terminated Polyethylene Glycol (AT-PEG) (Hercules, US), tPA and RGDS were misce bene with deionized water. The mixture was freeze-dried according to the presetted procedure. A certain quantity of dissolvent liquid composed of glycerine, propylene glycol and deionized water was added pro rata into the freeze-dried half-finished product, the air in the vial was substituted by perfluoropropane subsequently, and then sterilized by irradiation of Co60. To suspend the mixture, the vial was closed tightly and oscillated with a mechanical shoker according to the presetted frequency and duration. In this way, the primary product of the microbubbles was acquired. After centrifugation of 1000 rpm for 10 min, the underlayer liquid was abandoned, by which the free tPA and RGDS fragments were removed. The supernatant was moved into a new vial, kept at the low temperature, away from light.
Detection of microbubbles’ properties
Prepared drug-loaded microbubbles were dropped on the glass slide, mounted with buffer glycerine and the morphology of the microbubbles was observed under light microscope and fluorescence microscope (Leica, Germany).
The microbubbles carrying drug and the normal microbubbles without tPA and RGDS prepared in the same way were both diluted to 200 times with saline, the size, surface potential and pH value of which were measured with Coulter particle counting instrument (Guangdong, China), zeta sizer 3000 (MALVERN, England) and pH detector (P5500, Germany), respectively.
Envelope rate of tPA was detected by ELISA (SUNBIO, China). Fifty microliter drug-loaded microbubbles were diluted to 5 ml and 2 ml 10% Trion X-100 was added and swirl mixed, by which the shell of microbubbles was broken and the tPA encapsulated in the bubbles was released thereby. The mixture was centrifuged for 15 min at 2500 rpm and the supernatant emulsion was taken and diluted with deionized water to 1000 times. Another 50 μl microbubbles were diluted to 5 ml and centrifuged at 4°C and 20,000×g for 1 h, the subnatant was moved out and diluted to 1000 times. ELISA was performed according to the kit strictly. A490 was taken as Y axis and tPA concentration (ng/ml) was taken as X axis, by which, an linear regression equation was acquired: Y = 0.014X + 0.1225 (r = 0.998, P < 0.01). Then the concentrations of all the samples were calculated and the envelope rate was got from the following equation accordingly: Envelope rate = (Total tPA – free tPA)/Total tPA × 100%.
Conjugating rate of RGDS was detected by flow cytometer (BECKMAN, US). One milliliter lipid microbubbles carrying tPA and RGDS were diluted to 5 ml, and 1 ml normal microbubbles carrying tPA without RGDS was diluted to 5 ml as the contrast, both of which were use to detect the conjugating rate of RGDS with flow cytometer.
The thrombolytic activity of the microbubbles carrying tPA was detected by agarose fibrin plate process. All the materials for plate preparation and the standard urokinase were purchased from National Institute for the Control of Pharmaceutical and Biological Products of China, 0.5 g agarose was soluted in 50 ml ultrapure water, which was autoclaved. Then bovine fibrinogen (72 mg) and bovine thrombin (190 µ) were added, the containing bottle was oscillated, and the plate was planked immediately. Six wells were bored in the plate with the diameter of 5 mm, and eight samples of 80 μl were dropped in them, including normal microbubbles without tPA (A), standard urokinase preparation (1280 IU) (B), tPA-encapsulated microbubbles (C), upper layer of post-centrifuge tPA-encapsulated microbubbles (D), lower layer (E) and ultrasound-irradiated tPA-encapsulated microbubbles (F), in which the ultrasound irradiation was performed with the ultrasound transducer (0.5 MHz and 1.8 W/cm2) immersed in water 3 cm apart from the polyethylene tube containing microbubbles. After incubation at 37°C for 12 h, the plate was stained for 5 min with Coomassie brilliant blue and decolorized for 10 min with eluent twice. At last, the processes of imaging and image collection were performed to the agarose fibrin plate with the transmitted light in gel imaging device.
Ultrasound contrast imaging
Five healthy rabbits were involved in the process, weighing 2.5 ± 0.3 kg (2.3–2.8 kg) and purchased from the experimental animal center of Third Military Medical University. All the operations were approved by the Animal Care and Use Committee of the Third Military Medical University. The rabbits were anesthetized by intraperitoneally injecting 2% pentobarbital sodium of 2 ml/kg and fixed. The hair on liver region of which was depilated with 8% sodium sulfide and the venous pathway was constructed with the 5th scalp acupuncture through the ear edge vein.
An ATL 5000 ultrasound imaging device (Philips, Holland) with the L12-5 transducer was employed for the contrast imaging. As the satisfactory image of rabbit liver was got, the contrast condition (GI Con/Gen) affiliated with the instrument was applied and held during the experiment, including the transducer frequency of 5 MHz, the mechanical index of 0.6, depth of 4 cm, focus at 2 cm and gain range of 43–69 dB.
The microbubbles carrying tPA and RGDS were injected by 0.02 ml/kg, followed by the injection of 1 ml saline to wash the tube. All the processes of contrast imaging were recorded and stored as AVI files in hardware. When the video was replayed, images were collected with software (Adobe Premiere) by 1 frame/10 s along the timeline and stored as BMP files. All the files were analyzed with Adobe Photoshop software. The region of interest in liver with constant size was selected and the mean grey scale was detected with histogram analysis. The time–intensity curve was produced with Excel, according to which, the peak intensity (PI), peak time (PT) and mean transit time (MTT) were measured.
In vitro thrombolysis
Whole blood was obtained by antecubital venipuncture from three healthy volunteers in the morning after a 12-h fast. The blood was allowed to coagulate in a glass test tube at room temperature for approximately 10 min. The clots were incubated at T = 37°C in a temperature controlled water bath (DKB-8A, Shanghai, China) for 3 h to ensure clot retraction. The clots were stored at 5°C prior to use to prevent spontaneous clot degradation. After serum was aspirated, the clots were cut into pieces 189–287 mg in weight. Each clot was weighed on a electronic balance (Bp221S, SATORIUS, Germany) prior and posterior to the thrombolysis. The thrombolysis rate was calculated by the percentage ratio of the difference value to the initial weight. The experiment was approved by the volunteers and the Ethics Committee of Xinqiao Hospital affiliated to Third Military University.
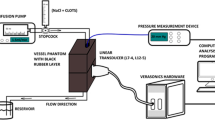
An ultrasound transducer (CSY-2, Puji, China), immobilized in a 37°C water bath, was positioned with the focal zone centered on the blood clot within the tank. Phosphate-buffered saline solution was pumped through the tubing via a peristaltic pump (BT100-2J, Langer, China). Microbubbles and tPA were injected upstream of the blood clot and were washed through the region of the clot within the tubing. The clots were then washed via sustained flow with saline solution propelled by the peristaltic pump (Fig. 1).
All the clots were divided into seven groups: (1) US, treated with ultrasound exposure only; (2) tPA, treated with tPA only; (3) tPA + US, irradiated by ultrasound as tPA was added; (4) tPA + US + MB, irradiated by ultrasound as tPA and mormal microbubbles were added simultaneously; (5) tMB-tPA + US, irradiated with ultrasound as the microbubbles carrying tPA and RGDS were added; (6) MB-tPA + US, ultrasound inosation was performed as the microbubbles carrying tPA without RGDS were added; (7) control, no treatment.
In the experiment, ultrasound of 2 MHz, 1.8 W/cm2 and duty cycle of 95% was employed, 1.5 ml tPA (0.1 mg/ml) was injected, and 50 μl microbubbles, including MB, MB-tPA and tMB-tPA, were injected as bolus. The treating time in each group was 10 min.
Statistical analysis
All the data were expressed as mean ± SD (\( \overline{x} \pm s \)). One-way ANOVA was applied to compare the thrombolysis rate between groups, which was performed by the software SPSS 10.0 in computer. Values of P < 0.05 were considered statistically significant.
Results
Properties of microbubbles carrying tPA and RGDS
The finished product of the microbubbles carrying tPA and RGDS was a kind of white curd suspension. Observed under light microscope, the microbubbles took on conferted small smooth circles (Fig. 2a), in which the center half-lucent area was surrounded by the outer round shell, with the concentration of 7–9 × 109/ml. Under fluorescence microscope, red and green fluorescence, emitted by the 5FAM-labaled tPA (Fig. 2b) and FITC-labaled RGDS (Fig. 2c), respectively, were both observed as color circles, indicating the tPA and RGDS were located on the shell surface or in the shell of microbubbles, while no fluorescence was observed in the normal microbubbles.
The morphology of the microbubbles carrying tPA and RGDS. a The microbubbles took on conferted small smooth circles under light microscope (×200). b Red fluorescence emitted by 5FAM-labeled tPA carried by microbubbles under fluorescence microscope (×200). c Green fluorescence emitted by FITC-labeled RGDS carried by microbubbles (×200)
The mean size, Zeta potential and pH value of the drug-loaded microbubbles and the normal were presented in Table 1, which indicated that the prepared microbubbles fit the requirement of intravenous injection.
The envelope rate of tPA in drug-loaded microbubbles was (81.12 ± 2.44%). And tested by flow cytometer, the conjugating rate of RGDS was (94.49 ± 6.19%).
The imaging of agarose fibrin plate shows (Fig. 3): The deep blue resolved circles were observed around the sampling well of standard substance of urokinase, the drug-carrying microbubbles radiated by ultrasound, the tPA-encapsulated microbubbles and its upper layer post-centrifuged, among which the former two were larger than the latter two, while no resolved circles was observed at the other wells. The results indicated that the activity of tPA encapsulated in microbubbles was maintained as it was released after 12-h self-lysis or under ultrasound exposure, and the latter got higher thrombolysis activity, which may be attributed to the rupture of microbubbles by the sonication and the subsequent release of most of encapsulated tPA.
Ultrasound contrast imaging with drug-loaded microbubbles
The echo of rabbit liver was enhanced obviously after the microbubbles was injected intravenously (Fig. 4). The time-intensity curve (Fig. 5) showed a good imaging effect with PI of 86.54 ± 5.09, PT of (46 ± 8.94 s) and MTT of (690 ± 61.64 s). During the experiment, there was no untoward reaction and death in the rabbits. All the animals waked up in the following 24 h, normal in moving and food processing.
The thrombolysis rates of all the groups were presented in Table 2. Compared with the control, the thrombolysis rates were improved significantly (P < 0.05), among which the highest were the group of tPA + US + MB and tMB-tPA + US, and there was no significant difference between them.
Discussion
TPA is a serine protease, which can activate the plasminogen to turn to plasmin, and the latter can degrade fibrin, the fundamental substance of thrombus, and recanalize the embolic blood vessels. TPA belongs to the second generation thrombolytics, which is the choice drug in clinical drug thrombolysis. However, hemorrhage is the most common complication in its clinical application all along [11], and some other factors such as short half life and high price also limit its popularization. Delivering tPA with drug carrier may be helpful to avoid the early degradation in blood, which can lower the drug consumption and reduce the incidence of hemorrhage, but the appropriate carrier with satisfactory encapsulation and thrombolysis efficacy is still in research [12–14].
It’s been approved that the microbubbles combined with ultrasound irradiation can improve gene transfer and drug delivery [15, 16], which is a new technique of potential for its high performance, non-invasion and easy operation. In 1998, Unger et al. [17] prepared the lipid microbubbles carrying paclitaxel the first time by floating paclitaxel with soybean oil, which also enhanced the ultrasonographic imaging well, proving the feasibility that the microbubbles could be good drug delivery vector. Lipid microbubbles can encapsulate both liposoluble and water-soluble drugs while tPA is a kind of water-soluble protein. Taking lipid as the shell, microbubbles has several qualities such as good deformability, stability, biological compatibility, safety, no immunogenicity and no danger of infection, which endow it with the wide prospect of clinical application. Compared with the other ways of preparation of thrombolytics-loaded microbubbles [14, 18, 19], the lyopyilization we employed to encapsulate tPA into the lipid shell is operated under the condition of low temperature, which is suited for the temperature sensitive material such as tPA, and can embed the drugs in the shell, which can effectively protect them from the outside disturbance. The results showed that the envelope rate of tPA is satisfactory, and the agarose fibrin plate process proved that the encapsulated tPA kept good activity under the condition of both self-lysis and ultrasound exposure. Furthermore, the freeze-dried powder containing shell materials and drugs, prepared by this technique, is suited to mass production and long-term storage, so taking a long view, it is favorable for the clinical application and business development.
RGDS, the 95–98th and 572–575th amino acid residue of Aa chain of fibrinogen (Fg), is the special ligand for GPIIb/IIIa on platelet membrane, and the bind of Fg and activated platelet is the common way of thrombosis, so RGDS is often chosen as the target ligand for thrombus [20, 21]. AT-PEG, as the bridging agent, was added into the materials to construct the shell of microbubbles, which made the hydrophilic end of phospholipid carrying amino group. The amino group was activated by carbodiimide during mechanical oscillation and could bind with the carboxy group of RGDS via covalent bond. The conjugation by this way is firm and the carrying rate detected by flow cytometry is about 94%, guaranteeing the effective combination of microbubbles with thrombus.
Observed under fluorescence microscope, the microbubbles radiate both red and green fluorescence that were labelling tPA and RGDS, respectively, proving tPA and RGDS were carried in the lipid membrane of microbubbles simultaneously. Whether they are located inside microbubble, in the shell, or on the outer surface still needs to be confirmed, however, presumed from the preparation process, drug is likely encapsulated in shell. Compared with the normal microbubbles, the tPA-loaded microbubbles have similar properties, which makes the in vivo application possible, and the capability of ultrasonic contrast imaging potentially endues the microbubbles a special quality of evaluating the thrombolysis effects during therapy. The in vitro thrombolysis showed that the drug-loaded targeted microbubbles had good thrombolysis efficacy. Although it was similar to that of the combined using of tPA, normal microbubbles and ultrasound, the drug amount entering the flow model was much less. Calculated from loading amount of tPA in microbubbles and its usage in experiment, the tPA entering model was about 0.03 mg, which was 1/5 of that in tPA + US + MB (1.5 ml tPA of 0.1 mg/ml, i.e. 0.15 mg). We speculate that the drug-loaded microbubbles combined with thrombus via the RGDS and thereby were settled there, and ultrasound insonation caused the rupture of microbubbles and released the tPA, bringing about a relative high drug concentration locally. Hope is pinned on this novel type of microbubbles that they can protect the encapsulated tPA from the early deactivation by degradation of blood as they enter circulation, gather at the thrombus via target conjugation, release the drug by ultrasound-induced microbubbles destruction, and enhance the thrombolysis by the cavitation produced simultaneously. By this new non-invasive approach, it becomes possible to reach the aim of improving thormbolysis rate, and meanwhile decreasing the drug dosage and lower the costs.
There are still some limitations in the research. First, it’s just a primary step of the target thrombolysis of drug-loaded microbubbles under ultrasound exposure, and the realization of the hypothesis still needs more rigorous studies, including the confirmation of in vivo experiment. The in vivo circulation is much more complicated, so whether the technology can get satisfactory efficacy is uncertain, and its safety is another important problem for it has been widely reported that the biophysical effects induced by the combined application of ultrasound irradiation and microbubbles will affect adjacent tissues such as endothelium of blood vessels [22], both of which will be the emphases of our follow-up work. Second, the technique of preparing microbubbles needs to be further improved in some aspects such as drug-loading rate, stability, etc. Third, the device and conditions of ultrasound irradiation used in study are for therapy, then whether the diagnostic ultrasound has similar effect also needs to be answered in the future research.
References
Caplan LR (2006) Stroke thrombolysis. Circulation 114:187–190
Siegel RJ, Atsr S, Fishbein MC, Brasch AV, Peterson TM, Nagai T, Pal D, Nishioka T, Chae J, Birnbaum Y, Zanelli C, Luo H (2000) Noninvasive, transthoracic, low-frequency ultrasound augments thrombolysis in a canine model of acute myocardial infarction. Circulation 101:2026–2029
Medel R, Crowly RW, McKisic MS, Dumont AS, Kassell NF (2009) Sonothrombolysis: an emerging modality for the management of stroke. Neurosurgery 65:979–993
Perren F, Loulidi J, Poglia D, Landis T, Sztajzel R (2008) Microbubble potentiated transcranial duplex ultrasound enhances IV thrombolysis in acute stroke. J Thromb Thrombolysis 25:219–223
Pitt WG, Husseini GA, Staples BJ (2004) Ultrasonic drug delivery—a general review. Expert Opin Drug Deliv 1:37–56
Lindner JR (2004) Microbubbles in medical imaging: current applications and future directions. Nat Rev Drug Discov 3(6):527–532
Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R (2002) Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation 105:1233–1239
Kaufmann BA, Lindner JR (2007) Molecular imaging with targeted contrast ultrasound. Curr Opin Biotechnol 18:11–16
Alonso A, Martina AD, Stroick M, Fatar M, Griebe M, Pochon S, Scheider M, Hennerici M, Allemann E, Meairs S (2007) Molecular imaging of human thrombus with novel abciximab immunobubbles and ultrasound. Stroke 38:1508–1514
Klibanov AL (2007) Ultrasound molecular imaging with targeted microbubble contrast agents. J Nucl Cardiol 14:876–884
Graham GD (2003) Tissue plasminogen activator for acute ischemic stroke in clinical practice: a meta-analysis of safety data. Stroke 34:2847–2850
Neik SS, Liang JF, Park YJ, Lee WK, Yang VC (2005) Application of “ATTEMPTS” for drug delivery. J Controlled Release 101:35–45
Xie Y, Kaminski MD, Torno MD, Finck MR, Liu XQ, Rosengart AJ (2007) Physicochemical characteristics of magnetic microspheres containing tissue plasminogen activator. J Magn Magn Mater 311:376–378
Tiukinhoy-Laing SD, Huang S, Klegerman M, Holland CK, McPherson DD (2007) Ultrasound-facilitated thrombolysis using tissue-plasminogen activator-loaded echogenic liposomes. J Drug Target 15:109–114
Unger EC, Matsunaga TO, McCreery T, Schumann P, Sweitzer R, Quigley R (2002) Therapeutic applications of microbubbles. Eur J Radiol 42:160–168
Hernot S, Klibanov AL (2008) Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev 60:1153–1166
Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y (1998) Acoustically active lipospheres containing paclitaxel: a new therapeutic ultrasound contrast agent. Invest Radiol 33:886–892
Mu Y, Li L, Ayoufu G (2009) Experimental study of the preparation of targeted microbubble contrast agents carrying urokinase and RGDS. Ultrasonics 49:676–681
Smith DAB, Vaidya SS, Kopechek JA, Huang S, Klegerman ME, McPherson DD, Holland CK (2010) Ultrasound-triggered release of recombinant tissue-type plasminogen activator from echogenic liposomes. Ultrasound Med Biol 36:145–157
Xie F, Lof J, Matsunaga T, Zutshi R, Porter TR (2009) Diagnostic ultrasound combined with glycoprotein b/a after coronary thrombotic occlusions. Circulation 119:1378–1385
Wang B, Wang L, Zhou XB, Liu YM, Wang M, Qin H, Wang CB, Liu J, Yu XJ, Zang WJ (2008) Thrombolysis effect of a novel targeted microbubble with low-frequency ultrasound in vivo. Thromb Haemost 100:356–361
Karshafian R, Bevan PD, Williams R, Samac S, Burns PN (2009) Sonoporation by ultrasound-activated microbubble contrast agents: effect of acoustic exposure parameters on cell membrane permeability and cell viability. Ultrasound Med Biol 35:847–860
Acknowledgements
The experimental work described in this article was supported by the national natural science foundation of China (No. 30500478 and No. 30670545). We wish to thank Prof. Zhong-Xiong Zhuo, Dr. Ping Zhang, Dr. Hong-Ting Zheng and Dr. Wei Wu for expert technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Xing Hua and Ping Liu have contributed to the work equally.
Rights and permissions
About this article
Cite this article
Hua, X., Liu, P., Gao, YH. et al. Construction of thrombus-targeted microbubbles carrying tissue plasminogen activator and their in vitro thrombolysis efficacy: a primary research. J Thromb Thrombolysis 30, 29–35 (2010). https://doi.org/10.1007/s11239-010-0450-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-010-0450-z