Abstract
Subtilisin QK, a new fibrinolytic enzyme, could cleave directly cross-linked fibrin in vitro. To verify the thrombolytic function of Subtilisin QK in vivo, the thrombolytic effect of purified Subtilisin QK on tail-thrombus of mouse was investigated. After injected with carrageenan, the tail-thrombus of Subtilisin QK treated group were shorter than the physiological saline treated group. Moreover, the tail-thrombus decreased correlate with Subtilisin QK in a dose-dependent manner. Thrombus nearly disappeared while the mice were treated with 12000 IU Subtilisin QK. The result indicated that Subtilisin QK significantly inhibited thrombus formation in mouse tail. This study made more foundation for further development of Subtilisin QK as a novel bifunctional thrombolytic agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intravascular thrombosis is one of the main causes of multiple cardiovascular diseases. The formation of thrombus in blood vessel is a very complicated physiological process participated by many factors [1]. The initial stage of artery thrombus formation is mainly caused by blood vessel injury, platelet adhesion and aggregation, but slowly blood stream and blood blockage usually cause vein thrombus [2, 3]. Besides many inhibitors of coagulation factors were developed to prevent and cure thrombosis, thrombolytic therapy is now designed to achieve recanalization in these diseases. There are many thrombolytic agents that are currently either approved for clinical use or under clinical investigation: urokinase, streptokinase, and tissue plasminogen activator (tPA), etc. [4]. However, these agents function to active plasminogen to plasmin, and extra plasmin activity can easily result in bleeding through its proteolytic degradation of other factors in the blood. So the pursuit of high quality thrombolytic agent never stopped [5].
Two fibrinolytic enzymes were recently obtained from Bacillus subtilis QK02 (CCTCC, M203078), a strain with highest activity of fibrinolytic enzyme, and the enzyme with higher activity was named Subtilisin QK. Subtilisin QK is composed of 274 amino acid residues (MW = 27.8 kDa) and shows high efficiency of directly cross-linked fibrin degradation by SDS-PAGE and fibrin plate method in vitro [6]. In addition, Subtilisin QK has the ability of inhibiting tyrosine nitration induced by nitrite, hydrogen peroxide and hemoglobin in vitro and in vivo and protecting human umbilical vein endothelial cell (ECV-304) from the damage caused by nitrite and hydrogen peroxide [7]. But there were no researches evaluating the thrombolytic effect of Subtilisin QK in vivo up to now.
Several experimental models of thrombosis have been established in the past. Various techniques, including physical (mechanical, electrical current and ligation) and chemical (trypsin, FeCl3, and H2O2) approaches have been employed to destroy blood vessel endothelial, induce platelet adhesion and aggregation, slow down and block blood stream to startup endogenesis coagulation [8]. Nearly all these approaches need complicated surgery to exposure blood vessel except the carrageenan induced thrombus in the tail of animals. The advantages of carrageenan induced thrombosis model lies in that easily manipulation, accurately and continuously measurement of thrombus range and extent without killing the animals, and lesser cost because of small scale animal used [9]. One explanation for the thrombotic activity of carrageenan is that activation of Hageman factor which is followed by intravasal coagulation. Histological study indicated that thrombocyte aggregates and leukostasis participated in disseminated intravasal coagulation [9, 10].
Carrageenan induced thrombosis model is first introduced in 1984, when mouse is treated by carrageenan, there appears wine-colored region in end of the tail of mouse, and the length of pathological increases with the time elapsed [11]. Carrageenan has also been used to induce arthritis in the rat, rabbit, dog, and pig [12]. This model has been used to test many clinical used anti-thrombus and thrombolytic agent (heparin, UK, Aspirin, etc.). These results verify the thrombus attribute of animal tail in another point of view [13–15]. In the present study, thrombolytic function of purified Subtilisin QK was evaluated in carrageenan induced thrombosis model.
Materials and methods
Strains, reagents and animals
Lumbrokinase (earthworm fibrinolytic enzyme, Beijing Biology Company) was purchased from Middle-south Hospital of Wuhan University with the standard fibrinolytic activity of 300000 IU per capsule. Carrageenan (Type I, C1013) from Sigma; Human fibrinogen and thrombin from Chinese Medicine Testing Institute were purchased. Other common used reagents are all analytical grade made in P.R. China.
Male Kunming Mouse with mean weight about 20 g were used and provided by Chinese Traditional Medicine University of Hubei province. All procedures were performed with the approval of the Committee on the Use of Live Animals in Teaching and Research of the Wuhan University. Food pellets were available from Disease Control Center in Hubei province, P.R. China.
Protein identification and fibrin plate
Subtilisin QK was purified by chromatography from B. subtilis QK02 as described in our previous work. Quantitative analysis of fibrinolytic activity was conducted by the standard fibrin plate method [6, 16].
Thrombosis model
Male Kunming mice were subcutaneously injected with sterile carrageenan dissolved in physiological saline at the concentration of 0.2% (w/v). Different doses of carrageenan were subcutaneously injected and the most suitable concentration was determined. Mice were observed at an interval of 8 h, which included the length, pathological degree and the appearance ratio of wine-colored thrombus formation tail.
Thrombolytic reagents injection
A total of 30 Male Kunming mice were randomly divided into 5 groups (n = 6). Group 1 served as control with saline water; Groups 2, 3, 4 were, respectively, given 3000, 6000 and 12000 IU purified Subtilisin QK dissolved in physiological saline; Group 5 were given 12000 IU lumbrokinase as positive control. After mouse was subcutaneously injected with carrageenan, lumbrokinase or Subtilisin QK were respectively injected through celiac into mice. These thrombolytic reagents injection was at 0, 8, 24, 32, 48, and 56 h, the thrombus length were measured at 72 h. Each experiment was done at a minimum in triplicate.
Statistical analysis
Statistical comparisons were made using GraphPad Prism version 4.0 for Windows software (GraphPad Software, San Diego, CA, USA). Data are presented as means ± SEM. Statistical analysis was performed by using ANOVA or two-tailed student’s t test when appropriate. P < 0.05 was considered as statistically significant. All experiments were performed at least three times.
Results
Fibrinolytic activity of Subtilisin QK
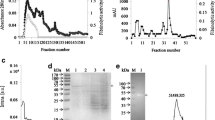
To quantify the fibrinolytic activity of Subtilisin QK, we made a standard fibrinolytic activity curve of lumbrokinase by fibrin plate method (data not shown). Purified Subtilisin QK protein was added into the fibrin plate, after incubation at 37°C for 18 h, the dissolving area was measured (Fig. 1). The fibrinolytic activity was calculated when comparing its dissolving area to the standard curve. By calculating, the fibrinolytic activity of the purified Subtilisin QK used in this experiment was 38000 IU/mg.
Establishment of tail-thrombus model
Carrageenan-induced tail thrombosis model is greatly affected by environment temperature. When temperature is lower than 18°C, thrombus formation ratio reaches 100%. So the thrombosis model was established at 18°C.
First we designed experiments to determine the best dose of carrageenan and optimize the tail thrombosis model. As shown in Fig. 2, carrageenan dose was correlated with the thrombus length in mouse tail in determined range (5–15 mg/kg). When the concentration of carrageenan exceeded 15 mg/kg and the length of thrombus would not increase. So the concentration of carrageenan was fixed on 15 mg/kg in this study, which could guarantee all the carrageenan treated group to appear thrombus and have a suitable thrombus length in the tail. In our work it was showed that 24 h after carrageenin injection infarction in the tail is not visible, 48 h after carrageenin injection there is a little infarction in the tip end of mouse tail, and the length of infarction in the tail would be stable 72 h after carrageenin injection (data not shown).
Thrombolytic effect of Subtilisin QK in mouse thrombosis model
In order to test the thrombolytic activity of Subtilisin QK in vivo, Carrageenan-induced tail thrombus model was utilized. After mice were subcutaneously injected with carrageenan, different dose Subtilisin QK were injected through celiac into mice and length of tail thrombus were measured at 72 h. It was showed in Fig. 3 that there were no obvious differences when Subtilisin QK at 3000 IU, but there were significant differences when it reached 6000 IU, the effect was more obvious with the concentration increasing. We could conclude that Subtilisin QK significantly inhibited carrageenan-induced mouse tail thrombus formation in vivo. It was also concluded that the inhibition activity increased along with the injection amount of Subtilisin QK, which presented linearity relationship. When the amount of Subtilisin QK reached 12000 IU, thrombus nearly disappeared in mouse tail and its thrombolytic effect was a little better than 12000 IU lumbrokinase treated group. The representative actual results of each group were showed in Fig. 4. The average thrombus length in Group 1 was 5.8 cm; and the average length of thrombus decreased gradually in Groups 2 and 3, respectively, 5.2 and 3.4 cm with the increase of the amount of Subtilisin QK. The thrombus nearly disappeared in the tip of mousse tail in Group 4. From these results, it was concluded that Subtilisin QK could significantly inhibit thrombus formation in carrageenan induced mouse tail thrombus model.
The effect of Subtilisin QK and lumbrokinase on carrageenan induced tail thrombus model. Each column standard for one group. Group 1: Carrageenan and saline water; Group 2: carrageenan and 3000 IU Subtilisin QK; Group 3: carrageenan and 6000 IU Subtilisin QK; Group 4: carrageenan and 12000 IU Subtilisin QK; Group 5: carrageenan and 12000 IU lumbrokinase. Results were statistically significant using Student’s test. *p < 0.05 vs. Group 1. **p < 0.01 vs. Group 1. Bars indicate means ± SEM. n = 6. The analysis is based on three independent experiments
The effect of Subtilisin QK and lumbrokinase on carrageenan induced mouse tail thrombus length (72 h after carrageenan injection). Arrows indicate thrombus formation region (wine-colored). Group 1: Carrageenan and saline water; Group 2: carrageenan and 3000 IU Subtilisin QK; Group 3: carrageenan and 6000 IU Subtilisin QK; Group 4: carrageenan and 12000 IU Subtilisin QK; Group 5: carrageenan and 12000 IU lumbrokinase. Data represents one of three experiments
Discussion
Carrageenan is a kind of amylose sulfate substance extracted from algae and it can be used to induce several kinds of tissue inflammation and tail thrombus animal model [10, 12, 17, 18]. When giving hypodermic injection of carrageenan on back of mouse, there appears wine-colored region at the tip of mouse tail, then the pathogen region experience typical dry necrosis. Since thrombus formation occurred in mouse tail and has obvious border with normal region, we can observe appearing time, developing process, spreading range and length of the formed thrombus. This provides an easy intuitionistic and manipulating animal model for research the whole process of thrombus formation in vivo. After proved by clinically affirmed effective medicaments like heparin, urokinase and aspirin, thrombus character of the animal model was further confirmed and this indicate that we can easily test and select latent anti-thrombus reagents with this animal model.
It has been broadly used to test antithrombotic substances including recombinant human tissue factor pathway inhibitor (rTFPI) [19], prostacycline and propionyl carnitine [20], low molecular weight heparan sulphate [13], flavonoids and lanthanides [21], heparin and phenprocoumon [14]. Some researchers speculate that thrombus formation of carrageenan induced mouse tail may due to local blood vessel inflammation and endothelial cell injury based on the pathological slice up observation [15], blood vessel inflammation could release massive inflammation factors, like interleukin-1, tumor necrosis factor, and these inflammation factors may destroy the function of normal endothelial cells to maintain the balance between hemagglutination and fibrinolysis, which thus induce the formation of thrombus. In addition, when the endothelial cells of blood vessel damage, secretion of factors relaxing blood vessel will reduce; on the other hand, factors shrinking blood vessel increase, which causes the partial vasospasm and further promotes the thrombus to form [22]. Though recent study has showed that mitochondrial lesion in cardiomyocytes in rat when injecting carrageenan to induce thrombosis in the tail [10], our previous work indicated that 72 h after carrageenan injection some mouse tail broke at the infarcted portion, but the tail-broken mouse lived externally no difference with normal mouse (data not shown), the adverse effects of carrageenan on internal systems should be tested in the following work.
Our previous results had shown that Subtilisin QK have strong ability of dissolving fibrin in vitro, which was the main basement of thrombus. These results make the development of this fibrinolytic enzyme into a novel thrombolytic reagent become possible. In order to test the thrombolytic effect of Subtilisin QK in vivo, we utilized carrageenan induce mouse tail thrombus model. Our studies demonstrated that Subtilisin QK displayed strong thrombolytic ability in mouse, and its effect is better than clinically used lumbrokinase. Our result makes it further to exploit Subtilisin QK as a novel thrombolytic reagent. In addition, due to the enzyme is extracted from B. subtilis, it has the advantage of extracting material source abundance, lower cost of finished product, unambiguous and onefold effective fraction, which compared with lumbrokinase. Therefore, Subtilisin QK is a kind of effective thrombolytic reagent with great exploitable potential.
Based on our results, we confirmed that Subtilisin QK, as a fibrinolytic enzyme, could execute its thrombolytic function effectively in vivo. From the angle of investigation, we can utilize it to exploit antibody targeted thrombolytic reagent. Moreover we will combine Subtilisin QK with our previously obtained fibrin specific single-chain variable fragment (scFv) [23] together to develop a more advanced thrombus targeting thrombolytic molecular.
Abbreviations
- tPA:
-
Tissue plasminogen activator
- MW:
-
Molecular weight
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- rTFPI:
-
Recombinant human tissue factor pathway inhibitor
- ScFV:
-
Single-chain variable fragment
References
Hsiao G, Yen MH, Lee YM, Sheu JR (2002) Antithrombotic effect of PMC, a potent alpha-tocopherol analogue on platelet plug formation in vivo. Br J Haematol 117:699–704. doi:10.1046/j.1365-2141.2002.03492.x
Jorgensen L (2006) The role of platelets in the initial stages of atherosclerosis. J Thromb Haemost 4:1443–1449. doi:10.1111/j.1538-7836.2006.02006.x
Boisseau MR (1997) Venous valves in the legs: hemodynamic and biological problems and relationship to physiopathology. J Mal Vasc 22:122–127
Selwyn A (2003) Prothrombotic and antithrombotic pathways in acute coronary syndromes. Am J Cardiol 91(12):3–11. doi:10.1016/S0002-9149(03)00428-4
Plow EF, Hoover-Plow J (2004) The functions of plasminogen in cardiovascular disease. Trends Cardiovasc Med 14:180–186. doi:10.1016/j.tcm.2004.04.001
Ko JH, Yan JP, Zhu L, Qi YP (2004) Identification of two novel fibrinolytic enzymes from Bacillus subtilis QK02. Comp Biochem Physiol C Toxicol Pharmacol 137:65–74. doi:10.1016/j.cca.2003.11.008
Ko J, Yan J, Zhu L, Qi Y (2005) Subtilisin QK, a fibrinolytic enzyme, inhibits the exogenous nitrite and hydrogen peroxide induced protein nitration, in vitro and in vivo. J Biochem Mol Biol 38:577–583
Verbeuren TJ (2006) Experimental models of thrombosis and atherosclerosis. Therapie 61:379–387. doi:10.2515/therapie:2006069
Bekemeier H, Hirschelmann R, Giessler AJ (1984) Carrageenan-induced thrombosis in the rat and mouse as a test model of substances influencing thrombosis. Biomed Biochim Acta 43(8–9):S347–S350
Bekemeier H, Hirschelmann R, Giessler AJ (1985) Carrageenin-induced thrombosis in rats and mice: A model for testing antithrombotic substances? Agents Actions 16:449–451. doi:10.1007/BF01982887
Kod’ousek R, Jezdinsk J, Krajci D (2007) Histological and ultrastructural changes of cardiomyocytes in experimental rats with tail thrombosis following subplantar application of carrageenin. Med Princ Pract 16:360–366. doi:10.1159/000104809
Hansra P, Moran EL, Fornasier VL, Bogoch ER (2000) Carrageenan-induced arthritis in the rat. Inflammation 24:141–155. doi:10.1023/A:1007033610430
Gervasi GB, Bartoli C, Carpita G (1991) A new low molecular weight heparan sulphate antagonizes kappa-carrageenan-induced thrombosis in rats. Pharmacol Res 24:59–63. doi:10.1016/1043-6618(91)90065-6
Hirschelmann R, Bekemeier H (1986) Heparin and phenprocoumon inhibit kappa-carrageenan induced thrombosis in rats. Folia Haematol Int Mag Klin Morphol Blutforsch 113:379–382
Hu S, Tian Q, Gu J, Sha J, Zhao D, Yan P et al (1993) A new kind of thrombus formation animal model in vivo. Zhonghua Xue Ye Xue Za Zhi 14:541–542
Astrup T, Mullertz S (1952) The fibrin plate method for estimating fibrinolytic activity. Arch Biochem Biophys 40:346–351. doi:10.1016/0003-9861(52)90121-5
Di Rosa M (1972) Biological properties of carrageenan. J Pharm Pharmacol 24:89–102
Liang AH, Ding XS, Li W, Xue BY, Wang JH, Yang HJ (2005) Development of an animal model of blood stasis syndrome and thrombosis. Zhongguo Zhong Yao Za Zhi 30:1613–1616
Elsayed YA, Nakagawa K, Kamikubo YI, Enjyoji KI, Kato H, Sueishi K (1996) Effects of recombinant human tissue factor pathway inhibitor on thrombus formation and its in vivo distribution in a rat DIC model. Am J Clin Pathol 106:574–583
Bertelli A, Bertelli AA, Giovannini L (1994) The potentiating effect of propionyl carnitine on prostacycline prevention of thrombosis induced by endothelin (ET-1) and K-carrageenan. Drugs Exp Clin Res 20:7–11
Bekemeier H, Gabor M, Hirschelmann R (1990) Influence of flavonoids and lanthanides on kappa-carrageenan rat tail thrombosis. Exp Pathol 40:61–63
Wang LL, Li ZC, Mei QB, Zhao DH (2000) MN9202 protection of tail artery in carrageenan induced thrombos is rats. J Fourth Mil Med Univ 21(2):214–216; Chinese
Yan JP, Ko JH, Qi YP (2004) Generation and characterization of a novel single-chain antibody fragment specific against human fibrin clots from phage display antibody library. Thromb Res 114:205–211. doi:10.1016/j.thromres.2004.06.013
Author information
Authors and Affiliations
Corresponding authors
Additional information
F. Yan and J. Yan contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yan, F., Yan, J., Sun, W. et al. Thrombolytic effect of Subtilisin QK on carrageenan induced thrombosis model in mice. J Thromb Thrombolysis 28, 444–448 (2009). https://doi.org/10.1007/s11239-009-0333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-009-0333-3