Abstract
Background Interest in the biology of endogenous progenitor cells (EPCs) continues to grow as evidence of their role in vascular repair mounts. EPC enumeration requires specialized laboratory techniques and is performed immediately after sample acquisition, limiting the clinical contexts in which EPC enumeration can be performed and the ability to increase sample sizes through multi-center participation. Methods We compared the numbers of EPCs enumerated in samples processed immediately after acquisition (n = 36) with EPCs enumerated in specimens stored for 24 hours or after cryopreservation of mononuclear cells (MNC) using two EPC identification strategies: cell surface marker expression (CD133/CD34) and aldehyde dehydrogenase activity (ALDHbr cells). Results EPCs assessed in fresh samples correlated with EPCs enumerated after whole blood storage (r = 0.699 for CD133+CD34+ cells, r = 0.880 for ALDHbr cells, P < 0.005 and P < 0.0001, respectively) or mononuclear cryopreservation (r = 0.590 for CD133+CD34+ cells, r = 0.894 for ALDHbr cells, P < 0.0001 for each); however, correlation based on assessment of ALDHbr cells was higher (P < 0.0003 for comparison of correlation coefficients). Initial results from a multi-site clinical trial suggest that EPC enumeration after mononuclear cell cryopreservation is feasible. Conclusion EPC analysis based on ALDH activity is reproducible, even after extended whole blood storage or MNC cryopreservation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Measurement of EPCs may represent a novel risk stratification strategy, given recent demonstrations that EPC numbers may predict susceptibility to subsequent cardiovascular events [1, 2]. Furthermore, given the growing evidence that EPCs play important roles in vascular repair, the study of these cells as contributors during novel angiogenic and regenerative therapeutics is growing in importance. For instance, REVEAL (Reduction of Infarct Expansion and Ventricular Remodeling with Erythropoietin After Large Myocardial Infarction) is a multi-site phase II clinical trial of erythropoietin (EPO) in acute myocardial infarction, assessing the effect of EPO administration within hours of acute MI treated with PCI on infarct size (clinicaltrials.gov identifier: NCT00378352). Proposed mechanisms for the salutary effects of EPO in pre-clinical models of acute myocardial infarction include EPC mobilization [3–5]. As the effects of EPO administration on EPC mobilization after myocardial infarction in human subjects is unknown, EPC mobilization as a secondary end-point is of key clinical interest.
While accurate assessment of circulating EPCs is important, there exists no standard for this determination. EPC enumeration in studies has been commonly performed using culture assays [6, 7] or using fluorescence activated cell sorting (FACS) methodologies based on expression of specific cell surface markers [1, 2, 7, 8]. Both methodologies to date call for immediate (≤2 h) sample processing, and the reliability with which EPCs can be measured using these techniques after storage of peripheral blood samples has not been systematically assessed. However, such immediate processing is frequently not convenient or feasible within multi-center clinical trials. Ideally for such studies, EPC methodologies should (a) allow sample acquisition on a 24 h a day basis at time of subject presentation, (b) require minimal sample manipulation by clinical personnel to minimize inconvenience and study coordinator time commitment, (c) require minimal specialized training not commonly available amongst clinical personnel, and (d) allow for storage of such samples for future batch analysis or shipment to third party core laboratories for central EPC enumeration.
As such, we explored the feasibility of two approaches to EPC enumeration: (a) storage of whole blood specimens or (b) cryopreservation of the cellular blood component. Either of these techniques might allow for shipment of samples to central core facilities which might improve reliability of EPC assessment between sites. We specifically compared the test performance of two EPC identification strategies after each sample processing strategy: cell surface marker expression of progenitor cell epitopes (CD133 and CD34) and EPC identification based on aldehyde dehydrogenase (ALDH) activity [9]. We compared EPC numbers in immediately processed samples (standard) versus those that been stored as whole blood samples or frozen (MNC cryopreservation) samples Finally, we report our initial experience with implementation of these assay strategies in the National Institute on Aging-sponsored Reduction of Infarct Expansion and Ventricular Remodeling with Erythropoietin After Large Myocardial Infarction (REVEAL) trial, a multi-site phase II clinical trial of erythropoietin administration after acute ST-elevation myocardial infarction.
Experimental
Materials and methods
Patient enrollment
To do our initial assay comparison, we enrolled a total of 90 patients undergoing elective cardiac catheterization at Duke University Medical Center. Patients were approached before their procedure to obtain informed consent. After insertion of an arterial sheath, 30 cc of peripheral blood was removed and placed in EDTA containing tubes. Control samples were processed within 4 h of acquisition. This protocol was reviewed and approved by the Duke Institutional Review Board.
Mononuclear cell isolation and storage
Mononuclear cells were recovered by spinning the sample at 2,000g for 20 min. Serum was removed and the buffy coat transferred to another tube, where it was mixed with a 10× excess of erythrocyte lysis buffer (Aldagen, Inc.) for 15 min at room temperature. MNCs were recovered by spinning at 250g for 5 min and were washed extensively with PBS containing 1% BSA. Cells were re-suspended in PBS-1% BSA, counted, and recovered by centrifugation.
Mononuclear cell samples were divided into aliquots. A “fresh” sample was promptly analyzed (<4 h from time of acquisition). For frozen samples, MNCs were washed with PBS and cryopreserved in buffer containing RPMI medium supplemented with fetal calf serum (20%) and DMSO (10%). The sample was aliquoted into individual cryovials, and the cryovials placed in a Nalgene 5100 Cryo 1°C Freezing Container, and the container placed in a −80°C freezer, thereby effectively freezing cells at a rate of 1°C per minute. Samples were stored at −80°C, At time of analysis, cells were rapidly thawed by immersion of cryovials into a 37° bath, followed by transfer of the thawed cell solution into a large volume (20 cc) of PBS-1.0% BSA, and serial washing of the cells to remove DMSO.
Whole blood samples stored for 24 h were maintained at room temperature or 4°C in EDTA-containing vacutainers.
Analysis of EPCs based on ALDH activity
Mononuclear fractions were incubated with freshly prepared BODIPY-aminoacetaldehyde (B-AAD, Aldagen Inc., Durham, NC) at a concentration of 1 μM at 37°C for 30 min in a freshly activated Aldecount tube after which cells were maintained on ice to retain the fluorescent byproduct. Baseline activity levels were established based on control samples incubated with B-AAD in the presence of diethylaminobenzaldehyde (DEAB), a potent inhibitor of ALDH activity according to the manufacturer’s instructions.
Analysis of EPCs based on cell surface marker expression
Mononuclear fractions (4 × 106 cells) were incubated with selected antibodies to determine the expression of cell surface markers. Cells were washed with Iscove’s modified Dulbecco’s medium supplemented with 2% fetal calf serum (IMDM+2%), concentrated to 107 cells/ml in IMDM+2%, and blocked with FcR blocking reagent (Miltenyi Biotec) for 10 min, then incubated with CD133-APC (Miltenyi Biotec), and CD34-FITC (Miltenyi Biotec) for 30–60 min at 4°. Dead and dying cells were excluded using staining with 7-AAD (1 μg/106 cells, Molecular Probes).
After appropriate staining, cells were subjected to FACS analysis and sorting on a FACS Calibur (BD Biosystems) machine and analyzed using FlowJo software (Treestar, Costa Mesa, CA). The percentage of cells within the mono- and lymphocyte gate was calculated.
REVEAL study
The REVEAL (Reduction of Infarct Expansion and Ventricular Remodeling with Erythropoietin After Large Myocardial Infarction, clinicaltrials.gov identifier: NCT00378352) trial is a NIA sponsored phase II study of erythropoietin (EPO) administration within 4 h after successful percutaneous intervention in the setting of ST-elevation myocardial infarction. As part of the dose escalation phase of this study, the initial 91 patients were randomized in a 2:1 fashion to treatment with 15,000 units (patients 1–30), 30,000 units (patients 31–61) or 60,000 units (patients 62–91) of erythropoietin versus placebo within 4 h of restoration of normal blood flow in the infarct related artery. A key secondary endpoint in the trial is the effect of EPO on EPC mobilization (Clinical Trial # NCT00378352).
Blood specimens were obtained at baseline before EPO administration and 24 ± 12 and 48° ± 12 h after drug delivery. Blood specimens were processed according to an outlined technique and the MNC fraction was cryopreserved, stored at −80°C, and shipped on dry ice to our laboratory. Samples were subsequently thawed, the cells washed and recovered, and EPC analysis performed as previously described.
Statistical analysis
Endogenous progenitor cells identified in duplicate at the same or serial time points were plotted, and correlation co-efficients determined. For correlation between individual determinations, Pearson correlation coefficients are reported. For all other analysis non-parametric Spearman coefficients are reported.
Results
Patient enrollment and sample processing
We enrolled 88 patients between July of 2005 and June 2006 at Duke University. These patients were reflective of patients undergoing cardiac catheterization at Duke (Table 1), with an average age of 61 years, 54% male, 80% Caucasian, and having a significant burden of hypertension (62%), hyperlipidemia (64%), diabetes (40%), and ongoing smoking (17%). Notably, these are the types of patient profiles commonly encountered in clinical trials of cardiovascular therapeutics in which EPC measurement may be of interest.
Correlation of EPC numbers amongst assays
On freshly processed samples, EPCs were identified on the basis of expression of CD133 and CD34 (CD133+CD34+ cells) and based on aldehyde dehydrogenase activity (ALDHbr cells). Median EPC numbers [intraquartile ranges] were 0.01862% [0.00989, 0.03685%) for CD133+CD34+ cells and 0.05849% [0.022, 0.0878%) for ALDHbr cells. As we have previously reported [9], there is a strong correlation between the numbers of CD133+CD34+ cells and ALDHbr cells (Fig. 1, r = 0.85, P < 0.0001).
Effect of sample processing on stability of EPC numbers
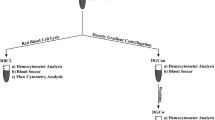
We next determined the effect of sample storage or processing on the reliability of EPC enumeration. We identified two approaches we felt might allow ready EPC enumeration at central facilities: overnight shipping of samples at either room temperature or under mild refrigeration, and cryopreservation of cellular blood components, allowing for later batch analysis of samples. Typical FACS data for samples analyzed immediately (fresh samples, top panels), after 24 h of storage (middle panels), or after cryopreservation (bottom panels) are demonstrated in Fig. 2.
Typical FACS data for cells assessed immediately after sample acquisition (top panels), after a 24-h storage period (middle panels), or after mononuclear cell cryopreservation and thawing (bottom panels). Analysis based on ALDH activity (SSC vs. ALDH activity plot) is shown in the left panels. Analysis based on cell surface expression of CD133 and CD34 [CD133 (y-axis) vs. CD34 (x-axis)] is shown in the right panels
We assessed the reliability of EPC enumeration after each technique by comparing EPCs enumerated after sample storage with EPCs enumerated in an identical sample analyzed immediately after acquisition, assessing EPCs as CD133+CD34+ cells (Fig. 3) or ALDHbr cells (Fig. 4). There is strong statistical correlation between these independent determinations by each approach; (r = 0.68 and 0.59 for CD133+CD34+ EPCs between fresh versus whole blood storage or fresh versus cryopreserved mononuclear cell analysis, P < 0.005 and P < 0.0001, respectively, r = 0.88 and 0.89 for ALDHbr cells for fresh versus stored whole blood or fresh versus cryopreserved mononuclear cell analysis, P < 0.0001 for each). However, there was considerable scatter between EPC numbers in the fresh and processed samples when assayed based on cell surface marker expression. Additionally there were higher numbers of total EPCs as a percentage of total cells identified based on CD133/CD34 expression in stored samples with median [IQR] 0.0.0186 [0.0.0099, 0.0368%], 0.0713 [0.0.0338, 0.0.169%], 0.0.0388 [0.0.0218, 0.0.0588%] in fresh, frozen, or stored whole blood samples, respectively. In contrast, there was more consistent measures when EPCs were identified based on ALDH activity: Median [IQR] (0.0585 [0.0220, 0.878%], 0.062 [0.0352, 0.110%], 0.0776 [0.028, 0.115%] in fresh, frozen, or stored whole blood samples, respectively.
We also determined whether the variability in results seen when using the cell surface technique is due primarily to the reliability of the assay itself or due to changes in the cells which accompanies sample storage and/or processing. We assessed the precision of our EPC enumeration techniques by determining EPC numbers in paired samples analyzed from fresh whole blood. Enumeration of CD133+CD34+ cells in 19 duplicate samples yielded in a Pearson correlation coefficient of 0.944 (Fig. 5). This correlation coefficient is significantly higher that the correlation observed between EPCs enumerated in fresh samples versus frozen MNCs (r = 0.59, P < 0.0003 for comparison of correlation coefficients) or the correlation between EPCs in fresh versus whole blood stored samples (r = 0.679, P < 0.01 for comparison of correlation coefficients). Thus, we believe that the reliability of the CD 34/CD133 assay is not an issue but rather loss of assay performance when applied to stored samples.
In contrast the reliability of EPC enumeration based on ALDH activity in cryopreserved samples or after whole blood storage was highly preserved. Specifically, the correlation coefficient between duplicate samples (Fig. 4, r = 0.972) is not significantly different than that observed between EPC analysis on fresh samples versus frozen MNCs (r = 0.94, n = 36, P = 0.3 for comparison of correlation coefficients) or the correlation between samples analyzed immediately and those analyzed 24 h later (r = 0.89, P = 0.3 for comparison of correlation coefficients). Thus, ALDH activity can be used to enumerate EPCs as reliably in stored specimens as in freshly obtained samples.
Operationalization of MNC isolation and EPC enumeration in clinical protocols
To assess EPC measurement in an actual multi-center trial, a simplified protocol for MNC isolation, tailored to clinical personnel with minimal laboratory experience and access to only basic equipment, was implemented in the REVEAL trial. REVEAL sites, all of which agreed to the EPC sub-study, were provided a manual (see supplementary materials) as well as training by clinical monitors who underwent an internal orientation in our laboratory. Each enrolled patient was to have EPC specimens isolated at three separate time points.
Endogenous progenitor cell specimens from 101 of the initial 102 patients have been received and recorded, and a web-based system used to track shipments. Analysis was successfully conducted in all samples in which an adequate cellular component was present.
All 11 sites were able to isolate, cryopreserve, and ship MNCs to allow for core lab analysis; however, the consistency of adequately prepared specimens varied from site to site. Sufficient cells for EPC analysis were present in all EPC collections for 51 of the 101 patients (52%) from which samples have been received. In total, 181 out of a total of 284 received individual time points were analyzable for EPC numbers (63.7%). During the course of the study, the percentage of analyzable samples has improved from 58.6% (cohort 1, n = 87 samples from 30 patients), 51.7% (cohort 2, n = 89 samples from 31 patients), 65.9% (cohort 3, n = 85 samples from 30 patients), to 84.5% in the efficacy cohort (33 samples from 11 patients). We conclude that MNCs isolation and cryopreservation is feasible by clinical personnel commonly involved in the conduct of clinical studies. Success improved during the conduct of the study, and required significant study coordinator effort, capability, and commitment.
Discussion
Endogenous progenitor cell enumeration is of significant clinical interest, given the relationship of EPC numbers to cardiovascular events [1, 2] and the role EPC mobilization may play in novel angiogenic therapies. Yet, EPC enumeration has been limited by requirements for prompt sample processing and the significant expertise required to perform EPC identification reliably. Comparing two independent EPC analysis methodologies: cell surface marker expression and ALDH activity, we found that the ALDH assay gave more precise and reliable findings on stored samples.
Several observations deserve merit. First, we confirmed that EPCs as enumerated by ALDH activity were highly correlated with cells expressing CD133 and CD34 in fresh samples [9, 10]. Second, EPC enumeration after a 24 h storage period in either whole blood or cryopreserved MNC specimens is feasible; however, the correlation with immediate EPC analysis is only modest when cell surface techniques are used. In contrast, ALDH activity can be used as a methodology for extremely reliable EPC enumeration in either whole blood stored for up to 24 h or cryopreserved MNC specimens. We [9], as well as others [11, 12], have demonstrated that ALDHbr cells from several sources demonstrate markedly enhanced endothelial differentiation potential. This makes the use of this technique for EPC enumeration attractive as a general methodology less dependent on prompt sample processing.
EPC analysis based on ALDH activity may be more reliable due to any of several factors. First, sample quality is degraded after prolonged storage or cell cryopreservation, and cell viability, while remaining high ( ≥95% after 24 h whole blood storage), does not approach that of freshly analyzed samples (≥99%). We used 7-AAD to exclude dead or dying cells for the antibody analysis, a step which is commonly taken in EPC analyses [8]. In contrast, analysis for ALDHbr cells does not require addition of a viability stain, since non-viable cells do not retain the fluorescent product. Adequate compensation for small amounts of fluorescence in this channel may have complicated the analysis of CD133+CD34+ cells. Furthermore, the requirement for gating in multiple independent channels, as opposed to a single color channel used for ALDH analysis, may lead to greater variability in the assay of CD133+CD34+ cells when sample quality is variable. This is especially true when one is enumerating very rare cell populations such as circulating EPCs.
Second, whole blood storage or mononuclear cell cryopreservation may lead to changes in the levels of cell surface expression of CD34 or CD133. In contrast, our data suggests that numbers of cells displaying high levels of ALDH activity is minimally affected.
Finally, ALDHbr cells form a distinct population well separated from ALDHlow cells (Fig. 2). This enhanced discrimination between ALDH positive and negative population may lead to less variability in EPC enumeration than occurs with analysis based on cell surface marker expression, especially if some sample degradation occurs.
To our knowledge, these findings represent the first time such correlation studies were performed. In the HEALING-II trial, EPC enumeration was undertaken to assess the relationship between EPC numbers and endothelialization of an EPC capture stent [13]. Given the elective nature of patient enrollment, the requirement for only a single baseline sample, and the small number of sites enrolling patients in this study, a strategy of shipping whole blood specimens to a central EPC laboratory was employed. However, this study did not publish any information on the reliability of these tests relative to fresh samples.
We further examined the feasibility of implementing a mononuclear cell isolation strategy in the REVEAL study of erythropoietin administration in acute myocardial infarction. We elected to isolate MNCs at the site level given that there were three samples to be obtained for each patient, substantial patient enrollment was expected at off hours, and a baseline sample needed to be obtained immediately after enrollment. Shipment and prompt analysis of whole blood samples on such a schedule might entail a 48 hour or greater delay before samples could be received at a central facility, and would require 3 individual EPC analyses for each patient enrolled. Given these constraints, we proposed a strategy of immediate MNC isolation and cryopreservation, allowing for immediate sample processing at the local site followed by shipment of all three samples for combined analysis.
Our early experience in the REVEAL study highlights both advantages and disadvantages to MNC isolation and cryopreservation. This strategy allows prompt processing of the specimen, elective deferral of EPC analysis, and batch analysis of multiple samples from the same patient, possibly improving intra-patient EPC count variability; however, MNC isolation requires a dedicated local staff willing to undertake a procedure with which they may be unfamiliar.
We attempted to simplify the technique, providing all reagents and equipment required for EPC isolation. This precluded the use of equipment such as Pipetteman, pipettes, and other disposables which are taken for granted in more basic laboratories but which might not be readily available in the clinical setting. Although a majority of sties were able to reliably produce cellular specimens of high quality, this may in part explain the difficulty some sites experienced in MNC processing. Currently, attempts are being made to identify specific deficits that might be responsible for the poor quality of specimens from a minority of sites. Based on our previous experience at successful sites, a >80% rate of successful sample acquisition might be expected.
Conclusions
Endogenous progenitor cell enumeration on the basis of ALDH activity is more accurate and reliable than identification based on cell surface markers, even after extended whole blood storage or MNC cryopreservation. This technique allows assessment of EPCs in wider clinical contexts by permitting local sample procurement followed by central batch analysis. Implementation of such a strategy in a multi-site trial is feasible and may have wider application as part of mechanistic substudies in future multicenter trials.
References
Schmidt-Lucke C, Rossig L, Fichtlscherer S et al (2005) Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: proof of concept for the clinical importance of endogenous vascular repair. Circulation 111:2981–2987. doi:10.1161/CIRCULATIONAHA.104.504340
Werner N, Kosiol S, Schiegl T et al (2005) Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med 353:999–1007. doi:10.1056/NEJMoa043814
George J, Goldstein E, Abashidze A et al (2005) Erythropoietin promotes endothelial progenitor cell proliferative and adhesive properties in a pi 3-kinase-dependent manner. Cardiovasc Res 68:299–306. doi:10.1016/j.cardiores.2005.06.022
Prunier F, Pfister O, Hadri L et al (2007) Delayed erythropoietin therapy reduces post-mi cardiac remodeling only at a dose that mobilizes endothelial progenitor cells. Am J Physiol Heart Circ Physiol 292:H522–H529. doi:10.1152/ajpheart.00357.2006
Zen K, Okigaki M, Hosokawa Y et al (2006) Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol 40:799–809. doi:10.1016/j.yjmcc.2006.03.012
Hill JM, Zalos G, Halcox JPJ et al (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med 348:593–600. doi:10.1056/NEJMoa022287
Vasa M, Fichtlscherer S, Aicher A et al (2001) Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res 89:e1–e7. doi:10.1161/hh1301.093953
Scheubel RJ, Zorn H, Silber R-E et al (2003) Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol 42:2073–2080. doi:10.1016/j.jacc.2003.07.025
Povsic T, Zavodni K, Kelly F et al (2007) Circulating endogenous progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol 53:2243–2248. doi:10.1016/j.jacc.2007.08.033
Povsic T, Zavodni K, Vainorius E, et al. (2008) Common endothelial progenitor cell assays identify discrete epc populations. Am Heart J. doi:10.1016/j.ahj.2008.10.010
Gentry T, Foster S, Winstead L et al (2007) Simultaneous isolation of human bm hematopoietic, endothelial and mesenchymal progenitor cells by flow sorting based on aldehyde dehydrogenase activity: implications for cell therapy. Cytotherapy 9:259–274. doi:10.1080/14653240701218516
Nagano M, Yamashita T, Hamada H et al (2007) Identification of functional endothelial progenitor cells suitable for the treatment of ischemic tissue using human umbilical cord blood. Blood 110:151–160. doi:10.1182/blood-2006-10-047092
Duckers H, Silber S, de Winter R et al (2007) Circulating endothelial progenitor cells predict angiographic and intravascular ultrasound outcome following percutaneous coronary interventions in the healing-ii trial: evaluation of an endothelial progenitor cell capturing stent. EuroIntervention 3:67–75
Acknowledgements
This study was funded in part by National Heart, Lung, and Blood Institute grant K-18 HL081419-01A1 (TJP), a Society of Geriatric Cardiology Merck Geriatric Cardiology Research Award (TJP), and a grant from the Duke Clinical Research Institute (TJP).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Povsic, T.J., Adams, S.D., Zavodni, K.L. et al. Aldehyde dehydrogenase activity allows reliable EPC enumeration in stored peripheral blood samples. J Thromb Thrombolysis 28, 259–265 (2009). https://doi.org/10.1007/s11239-009-0306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-009-0306-6