Abstract
In the present study we evaluated the presence of cysteine protease from the latex of four plants Asclepias curassavica L., Calotropis gigantea R.Br., Pergularia extensa R.Br. and Cynanchum puciflorum R.Br. belongs to the family Asclepiadaceae. Cysteine proteases from these plants latex exhibited both thrombin and plasmin like activities. Latex enzyme fraction in a concentration dependent manner induced the formation of clot in citrated blood plasma. Direct incubation of fibrinogen with latex enzyme fraction resulted in the formation of fibrin clot similar to thrombin enzyme. However prolonged incubation resulted in degradation of the formed fibrin clot suggesting plasmin like activity. Latex enzyme fraction preferentially hydrolyzed Aα and Bβ chains of fibrinogen to form fibrin clot. Latex enzyme fraction also hydrolyzed the subunits of fully cross linked fibrin efficiently, the order of hydrolysis was α-polymer > α-chains > β-chain and γ–γ dimer. Cysteine proteases from all the four Asclepiadaceae plants latex exhibited similar action on fibrinogen and fibrin. This study scientifically validate the use of plant latex in stop bleeding and wound healing by traditional healers all over the world.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The plant family Asclepiadaceae includes more than 2,000 species classified under 280 genera are distributed world wide in the tropical and sub-tropical regions [1]. Most of the Asclepiadaceae members are perennial herbs and their latex is exclusively used as a common remedy for wound healing and to stop bleeding on fresh cuts by traditional healers [2–5]. Latex from these plants is a mixture of several hydrolytic enzymes, in which proteases are the key enzymes responsible for the observed pharmacological actions [6]. Fibrin formations during blood coagulation and hydrolysis of fibrin in the later stages are the important events associated with hemostasis and wound healing. Proteases interfere in blood coagulation and fibrin hydrolysis was isolated and characterized from several plant latexes. Ficin, a cysteine protease from Ficus carica and a serine protease from Synadenium grantii were shown to involve in blood coagulation and fibrin hydrolysis [7, 8]. Such reports suggested the involvement of latex proteases in coagulation and fibrinolysis. In this communication we are reporting that latex of Asclepiadaceae members mainly contained cysteine proteases with both thrombin and plasmin like activities. These cysteine proteases with dual activities are therapeutically important in would healing process.
Materials and methods
Materials
Fibrinogen, thrombin and plasmin were purchased from Sigma Chemical Company (St. Louis, MO, USA). Latexes were collected from Asclepiadaceae plants found in and around Manasagangothri Campus, University of Mysore, Mysore, India. All other chemicals and reagents purchased were of analytical grade.
Plant materials, latex collection and preparation of crude enzyme source
Asclepias curassavica L., Calotropis gigantea R.Br., Pergularia extensa R.Br. and Cynanchum puciflorum R.Br. plants were identified by Prof. Shivamurthy, Dept. of Studies in Botany, University of Mysore, Mysore, India. A voucher specimen of each plant was deposited at the Herbarium, Dept. of Studies in Botany, University of Mysore, Mysore, India. The latex from each plant was collected separately in a clean glass test tube by breaking petioles. Latex enzyme fractions were prepared as described in our previous publication [9]. The protein concentrations of the prepared enzyme fractions of the plant latexes were estimated according to the method of Lowry et al. [10].
Caseinolytic activity
Caseinolytic activity was assayed according to the method of Murata et al. [11], using denatured casein as substrate. Briefly, 0.4 ml casein (2%) in 0.2 M Tris–HCl buffer pH 8.5 was incubated separately with latex enzyme fraction in a final volume of 1 ml for 1 h at 37°C. The reaction was stopped by adding 1.5 ml of 0.44 M trichloroaceticacid and allowed to stand for 15 min. The reaction mixture was centrifuged at 1,500 g for 15 min. An aliquot (1 ml) of the supernatant was mixed with 2.5 ml of 0.4 M sodium carbonate and 0.5 ml of Folin reagent (1:2, v/v). The color developed was read at 660 nm. Activity was expressed as units/h. One unit of enzyme activity was defined as the amount of enzyme required to increase an absorbance of 0.01 at 660 nm/h at 37°C. Inhibition studies were carried out after preincubating the latex enzyme fractions with or without specific protease inhibitors separately for 15 min. Further, the assay was carried out as described above.
Thrombin like activity
Fibrinogen-clotting assay
The assay was carried out according to the method of Denson [12]. Human fibrinogen 0.2 ml of 0.5% solution prepared in 0.01 M Tris–HCl buffer (pH 7.4) was pre-warmed to 37°C. Time taken for the formation of visible fibrin clot was recorded after the addition of different concentrations of latex enzyme fraction.
Spectrophotometric analysis of fibrin clot formation by latex enzyme fraction
Formation of fibrin clot and hydrolysis of fibrin clot by latex enzyme fraction were carried out by measuring absorbance at 540 nm using Spectrophotometer. Bovine fibrinogen 0.6 ml of 0.5% solution prepared in 10 mM Tris–HCl buffer pH 7.4 was pre-warmed to 37°C. Fibrin clot formation was initiated by adding 25 μg of the latex enzyme fractions. Increase in the turbidity due to fibrin formation and decrease in turbidity due to fibrin hydrolysis was recorded by measuring absorbance at 540 nm. Standard thrombin and plasmin were used as positive control.
Plasmin like activity
SDS-PAGE analysis of plasma clot hydrolysis
The assay was carried out as described by Rajesh et al. [6]. Briefly, the washed plasma clot (cross linked fibrin) was incubated with 1 μg of latex enzyme fraction separately in a reaction volume of 40 μl containing 10 mM Tris–HCl buffer pH 7.4 for 1 h. The reaction was terminated by adding 20 μl of sample buffer containing 4% β-mercaptoethanol and 1 M urea, boiled for 3 min and centrifuged to settle the debris of plasma clot. An aliquot (20 μl) of the supernatant was subjected to 7.5% SDS-PAGE to analyze fibrin hydrolyzing pattern.
Statistics
The results of biochemical and pharmacological experiments were expressed as the mean ± SD of atleast four independent experiments.
Results
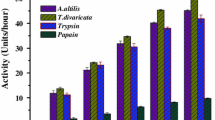
The latex enzyme fractions prepared from four different plants belongs to Asclepiadaceae were assessed for proteolytic activity using denatured casein as substrate. The enzymatic fractions from A. curassavica, C. gigantea P. extensa and C. puciflorum showed strong proteolytic activities when compared to trypsin (Table 1). The proteolytic activities of the four latex enzyme fractions were inhibited over 90% by IAA, a specific cysteine protease and not by any other specific protease inhibitors.
Pharmacologically several plant latex proteases have been shown to exert procoagulant activity when tested with human citrated plasma. Similarly latex prepared from Asclepiadaceae members containing cysteine proteases showed pronounced procoagulant activity. The latex enzyme fraction induced coagulation in the citrated blood plasma and the time taken for the induction of plasma clot reduced as the concentration of latex enzyme fraction increases. This procoagulant activity of latex protease was further substantiated using pure fibrinogen as the substrate. The latex protease enzyme fraction hydrolyzes fibrinogen to form fibrin clot suggesting that these latex cysteine protease has thrombin like activity. The time taken for the formation of fibrin clot was reduced as the concentrations of latex protease fraction were increased and attained a plateau (Fig. 1a). At 10 μg concentration, A. curassavica, C. gigantea, P. extensa and C. puciflorum latex cysteine proteases convert fibrinogen to fibrin in 108, 110, 70 and 60 s, respectively.
Thrombin and plasmin like activities of latex enzyme fraction. a Fibrinogen-clotting assay. Fibrinogen 0.2 ml of 0.5% was pre-warmed to 37°C. Time taken for the formation of visible fibrin clot was recorded after adding different concentrations of plants latex protein ranging from 2 to 40 μg. A. curassavica (♦), C. gigantea (■), P. extensa (▲) and C. puciflorum (×). Values represent mean ± SD (n = 4). b Spectrophotometric analysis of fibrin clot formation and its hydrolysis (plasmin like activity) by latex enzyme fraction. Plants latex protein (25 μg) was added to 0.6 ml of 0.5% fibrinogen. Appearance of turbidity due to fibrin formation and disappearance of turbidity due to fibrin hydrolysis was recorded by measuring absorbance at 540 nm. As controls 0.1 unit thrombin and plasmin was used instead of latex extract for comparison. Data represents the best of the four individual experiments. A—Thrombin, B—A. curassavica, C—C. gigantea, D—P. extensa, E—C. puciflorum and F—plasmin
Further, the thrombin like activity was determined spectrophotometrically by measuring the absorbance of fibrinogen at 540 nm. The latex enzyme fraction increased the absorbance of clear fibrinogen solution as a consequence of fibrin formation with time and attained maximum absorbance within 13 min. However, prolonged incubation with latex enzyme fraction resulted in decreased absorbance due to the hydrolysis of formed fibrin clot suggesting the plasmin like activity. Compared to latex enzyme fraction, thrombin increased the absorbance of fibrinogen substrate and attained plateau after 25 min. whereas, the standard plasmin used did not account for any absorbance when incubated with fibrinogen substrate (Fig. 1b). Hydrolysis of fibrin clot by latex enzyme fractions were analyzed by SDS-PAGE. At 1 μg concentration, latex enzyme fraction hydrolyzed α-polymer, α-chain and β-chains of fully cross linked fibrin within 1 h of incubation. γ–γ dimer appeared to be resistant for hydrolysis (Fig. 2). However, at higher concentrations and prolonged incubation showed complete degradation of all the subunits. Similar to caseinolytic activity, thrombin like and plasmin like activities of all the four latex enzyme fractions were inhibited by IAA.
Electrophoretic pattern of plasma clot by latex enzyme fraction. Washed plasma clot was incubated with 1 μg of plants latex fraction in Tris–HCl buffer (10 mM; pH 7.6) for 1 h at 37°C. The hydrolyzed product was resolved on 7.5% SDS-PAGE for analysis. A—control (Plasma clot), B—plasma clot + 2 μg A. curassavica, C—plasma clot + 2 μg C. gigantea, D—plasma clot + 2 μg of P. extensa and E—plasma clot + 2 μg of C. puciflorum
Discussion
The plants A. curassavica, C. gigantea, P. extensa and C. puciflorum are the individual members belong to Asclepiadaceae gained importance because of their enormous application in traditional medicine [1, 2]. Presence of latex is one of the important characteristic features of these plants. Proteolytic enzymes constitute the major fraction in these plants latex exhibiting varied potency and specificity to hydrolyze different protein substrates [9]. The proteolytic activities of these four latex enzyme fractions were inhibited by IAA suggested the presence of unique type of cysteine proteases. These data and the available literature suggested that the protease activity found in the latex of Asclepiadaceae members belongs to the class cysteine proteases.
Application of plant latex on fresh cuts to stop bleeding is the general practice followed by tribal and rural people of India [4]. Recent reports suggested that observed pharmacological activity of plant latex is due to the presence of proteases [6, 7]. These latex enzyme fraction exhibited pronounced procoagulant activity when tested on platelet poor plasma and induce formation of fibrin clot even in the absence of calcium. This data suggested that latex enzyme fraction can bypass the calcium requiring steps and act on the terminal step of blood coagulation cascade (fibrinogen to fibrin). However, the possibility of other actions and interactions of these latex enzyme fractions with plasma components might not be ruled out. Since direct incubation of latex enzyme fraction with purified human fibrinogen induced fibrin clot formation similar to that of thrombin and the time taken for initiation of fibrin formation decreased with increasing concentrations of latex enzyme fraction, we may propose that the pro-coagulant activity triggered by latex enzyme fraction was the consequence of thrombin like activity. This implies that latex enzyme fraction might cleave fibrinogen at similar sites as that of thrombin. The observed thrombin like activity could be the basis for the use of plant latex to stop bleeding on fresh cuts. Apart from its usage to stop bleeding, plants latexes have been using from hundreds of years in folk medicine as remedy for wound healing [5]. Fibrinolysis is one of the important proteolytic events associated with wound healing process. During physiological event, plasmin is the key enzyme that dissolves fibrin clot and hemostatic plug along with MMPs facilitating wound healing [13]. Apart from clot inducing activity (thrombin like activity), latex enzyme fraction exhibited clot dissolving properties (plasmin like activity). Latex enzyme fraction hydrolyzed all the subunits of fully cross linked fibrin as efficiently as plasmin. These observations suggested the dual actions of latex enzyme fraction as clot inducing and clot dissolving activities which might be due to the presence of thrombin and plasmin like enzymes or a single enzyme responsible for the dual actions.
Conclusion
This study provides the scientific validation for the use of plant latex to stop bleeding and wound healing as exploited in folk medicine. The study explains the similar involvement of cysteine proteases from the four Asclepiadaceae plants latex in blood coagulation and fibrinolysis. A more detailed analysis of these plant latex cysteine proteases and their action on fibrinogen and fibrin would find their usefulness for the medical and pharmacological applications.
Abbreviations
- Latex enzyme fraction:
-
Asclepiadaceae plants latex enzyme fractions
- IAA:
-
Iodoaceticacid
- PMSF:
-
Phenylmethylsulphonyl fluoride
- EDTA:
-
Ethylene diaminotetraaceticacid
- EGTA:
-
Ethylene glycol–N, N, N′, N′-tetraaceticacid
References
Pankaj O (2003) Doomar or Gular (Ficus glomerata) as medicinal herbs in Chattishgarh, India. Research Note. botanical.com/site/column_poudhia/127_doomar.html
Mueen Ahmed KK, Rana AC et al (2005) Calotropis species (Ascelpediaceae)—A comprehensive review. Pharmacognosy Mag 1:48–52
Muthu C, Ayyanar M, Raja N et al (2006) Medicinal plants used by traditional healers in Kancheepuram District of Tamil Nadu, India. J Ethnobiol Ethnomed 2:43. doi:10.1186/1746-4269-2-43
Kumar A (1999) Ayurvedic medicines: some potential plants for medicine from India. A meeting of the International Forum on traditional medicines. Toyama Medical and Pharmaceutical University, Toyama, Japan
Thankamma (2003) L. Hevea latex as wound healer and pain killer. Curr Sci 84:971–972
Rajesh R, Raghavendra gowda CD, Nataraju A et al (2005) Procoagulant activity of Calotropis gigantia latex associated with fibrin (ogen) olytic activity. Toxicon 46:84–92. doi:10.1016/j.toxicon.2005.03.012
Richter G, Hans PS, Friedrich D et al (2002) Activation and inactivation of human factor X by proteases derived from Ficus carica. Br J Haematol 119:1042–1051. doi:10.1046/j.1365-2141.2002.03954.x
Rajesh R, Nataraju A, Raghavendra gowda CD et al (2006) Purification and characterization of a 34-kDa, heat stable glycoprotein from Synadenium grantii latex: action on human fibrinogen and fibrin clot. Biochimie 88:1313–1322. doi:10.1016/j.biochi.2006.06.007
Rajesh R, Shivaprasad HV, Raghavendra gowda CD et al (2007) Comparative study on plant latex proteases and their involvement in hemostasis: a special emphasis on clot inducing and dissolving properties. Planta Med 73:1061–1067. doi:10.1055/s-2007-981575
Lowry OH, Rosenbrough NJ, Farr A et al (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Murata J, Satake M, Suzuki T (1963) Studies on snake venom. XII. Distribution of proteinase activities among Japanese and Formosan snake venoms. J Biochem 53:431–443
Denson KWE (1969) Coagulant and anticoagulant actions of snake venoms. Toxicon 7:5–11. doi:10.1016/0041-0101(69)90154-8
Lijnen HR (2002) Matrix metalloprotenases and their cellular fibrinolytic activity. Biochemistry 67:92–98. doi:10.1023/A:1013908332232
Acknowledgments
We thank Dr. P. Sharanappa, DOS in Bioscience, Hemagangothri, University of Mysore for giving information about latex bearing plants. Authors also thank Prof. Shivamurthy, DOS in Botany, Manasagangothri, University of Mysore for plants identification. H·V. Shivaprasad thanks University of Mysore, Mysore, India for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shivaprasad, H.V., Riyaz, M., Venkatesh Kumar, R. et al. Cysteine proteases from the Asclepiadaceae plants latex exhibited thrombin and plasmin like activities. J Thromb Thrombolysis 28, 304–308 (2009). https://doi.org/10.1007/s11239-008-0290-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-008-0290-2