Abstract
Background Age-related changes in blood coagulation and fibrinolytic factors are associated with an increase in risk of thrombotic events. The purpose of this study was to assess the effects of age, regular aerobic exercise and detraining on blood coagulation and fibrinolytic factors in men. Methods Initially, 41 sedentary and 42 physically active men (20–64 years) were analyzed for plasma levels of coagulation and fibrinolytic factors. Twelve sedentary men were then subjected to 16-week aerobic exercise training and subsequent 2-week detraining. Their blood samples taken at rest were assayed for activity levels of prothrombin, coagulation factor (F) V, VII, VIII, IX, X, XI and XIII, antithrombin III, protein C and plasminogen, and for antigen levels of fibrinogen, prothrombin fragment 1 + 2 (F1 + 2), FIX, protein C, tissue-type plasminogen activator (tPA), plasminogen activator inhibitor 1 (PAI-1) and tPA/PAI-1 complex. Results Plasma levels of most coagulation factors, particularly for fibrinogen and FIX antigens as well as FXIII activity significantly increased with aging in sedentary men, while that tendency disappeared in physically active men. By the exercise training, plasma antigen and/or activity levels of most blood coagulation factors except for prothrombin and FIX decreased. These training-effects, however, disappeared after detraining, and in some cases even rebounded to higher levels than those of pre-training. Plasma antigen levels of tPA, PAI-1 and tPA/PAI-1 complex decreased with the training and remained low even after detraining. Conclusion Regular aerobic exercises give complex effects on expression of hemostatic factors, overall favoring the hemostatic balance to less thrombotic, partly cancelling out the age effects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plasma concentrations or activated forms of most blood coagulation factors gradually increase with advancing age, while fibrinolytic and anticoagulant factors stay more or less constant or even slightly lower [1–3]. This disparity between procoagulant and anticoagulant/fibrinolytic activities apparently results in age-related increase in the overall blood coagulation activity, and the first molecular mechanism presumably responsible in part for this phenomenon was recently discovered [4]. Age-related changes in coagulation and fibrinolytic factors may be associated with an increase in risk of atherothrombotic events [5–7]. Thrombosis plays a critical role in the pathogenesis of cardiovascular diseases including acute myocardial infarction, unstable angina and sudden cardiac death [5–7].
Cross-sectional studies with large patient cohorts indicated that physically active people have lower plasma concentration levels of coagulation factors [8], and higher concentration levels of fibrinolytic factors [2, 9], than those of sedentary counterparts. However, these cross-sectional analyses failed to make a meaningful correlation of the differences observed among groups with regular physical exercises, separating from the effects of lifestyles or constitutional and genetic influences. Several interventional studies carried out to date with middle-age and elderly subjects actually produced inconsistent results, either positive [9–11] or none-positive [3] effects of aerobic exercise training on coagulation and fibrinolytic factors in relation to lowering the risk of cardiovascular diseases. To our knowledge, impacts of regular physical activity on several coagulation factors (i.e., V, X, XI, XIII) have never been extensively studied.
In this study, by taking cross-sectional and intervention experimental approaches, we tested our hypothesis that regular aerobic exercise training may attenuate age-related adverse changes in coagulation and fibrinolytic factors in men. We also tested whether short-term detraining moderates the effects obtained through training on blood coagulation and fibrinolytic factors.
Methods
Subjects of study
In a cross-sectional study, 41 sedentary and 42 physically active men (20–64 years of age) were included. Men with at least 30 min of moderate intensity physical activity, more than 2 times per week, were defined as a physically active subject group. Sedentary and physically active groups were age-wise divided into three subgroups including 20–34, 35–49, and 50–64 years of age. Their physiological characteristics were shown in Table 1. Twelve sedentary healthy middle-age men (50 ± 3 years of age) were also subjected to an interventional study. All subjects were normotensive (<140/90 mmHg) and free of overt chronic heart diseases as assessed by medical history and physical examination. All subjects were non-medicated. All subjects gave their written informed consents for participating in this study, which were reviewed and approved by the local Institutional Review Board before commencement.
Experimental procedures
All measurements were performed after an abstinence of caffeine and an overnight fast. To avoid effects of acute exercises, subjects were instructed not to perform any training and other vigorous physical activities on the day prior to that when measurements were done. After the measurements of height, body mass, and body mass index (BMI), phlebotomies were performed with minimal venostasis. The first 6 ml aliquots of blood were collected in three syringes containing 3.0 mg of EDTA-2Na, and used for determining levels of plasma cholesterol and lipoprotein(a) [Lp(a)]. Blood sample for determining plasma glucose was collected in 2.0 ml syringes containing 2.5 mg of sodium fluoride and 7.4 mg of EDTA-2Na. Blood samples for determining blood coagulation and fibrinolytic variables were collected in 1.8 ml syringes containing 3.8% sodium citrate. Within 30 min of phelebotomy, all blood samples were centrifuged for 20 min at 3,000g at 4°C. Serum and platelet-poor plasma samples were aliquoted and stored at −80°C until further analyses. After blood sampling, hemodynamic variables and maximal oxygen consumption were measured.
Measurements
Coagulation, anticoagulation, and fibrinolytic factors
Plasma activity levels of coagulation factors (F) including prothrombin, FV, FVII, FVIII, FIX, FX, and FXI were analyzed by one-stage clotting assays according to the standard methods with minor modifications [12]. Plasma antigen levels of fibrinogen, FIX, prothrombin fragment 1 + 2 (F1 + 2), tissue-type plasminogen activator (tPA), plasminogen activator inhibitor 1 (PAI-1) and tPA/PAI-1 complex were analyzed by enzyme-linked immunosorbent assay (ELISA) as described [13, 2]. Plasma activity level of FXIII was measured by a photometric assay using a synthetic substrate [14]. Plasma activity levels of plasminogen and antithrombin III (ATIII) were analyzed by using a chromogenic substrate assay [15, 16]. Plasma protein C (PC) antigen and activity levels were measured by a latex agglutination immunoassay and activated partial thromboplastin time method, respectively [17].
Metabolic risk factors
Fasting plasma levels of total cholesterol, LDL-cholesterol and glucose were determined by standard enzymatic methods [18]. Fasting plasma HDL cholesterol level was evaluated by a synthetic polymer method [18], while Lp(a) was measured by using a latex immunoassay [19].
Hemodynamic variables
After at least 15 min of supine rest in a quiet, temperature-controlled room, electrocardiogram and brachial blood pressures were simultaneously measured with a vascular testing device (form PWV/ABI, Colin Medical Technology, Komaki, Japan).
Maximal oxygen consumption
Subjects were subjected to an incremental-graded cycling exercise for 2 min at 20 Watt followed by 15 Watt increase every 1 min, until they reached 85% age-predicted maximal heart rate (208–0.7 × age) [20]. Oxygen consumption and heart rate were monitored with an online computer-assisted circuit spirometry and electrocardiogram (AE300S, Minato Medical Science, Tokyo, Japan). Maximal oxygen consumption (VO2max) was estimated by the age-predicted maximum heart rate and the linear regression line between heart rate and oxygen consumption during cycle exercise as we previously described [21].
Aerobic exercise training and detraining intervention
Interventional studies were carried out by subjecting sedentary men to 16 weeks of aerobic exercise training and subsequent 2 weeks of detraining. Subjects were first provided with an orientation, followed by exercise training on their own. During the first 2 weeks, subjects performed a relatively low intensity exercise training composed of brisk walking or jogging with 60% heart rate reserve, 30 min/day and 3–4 days/week. Thereafter, exercise intensity and duration of training were increased to 75% heart rate reserve, 45 min/day and 4–5 days/week. Exercise intensity during the training was monitored using a heart rate monitor (Polar, Tokyo, Japan) and physical activity logs. All subjects reported these records to us by e-mail. Subjects were instructed not to perform exercise other than scheduled trainings during the training period, and to limit other exercises to daily living actions during the detraining period.
Statistical analysis
To assess the results obtained from the cross-sectional and interventional approaches, two-way ANOVA (age and physical activity) and repeated measures of ANOVA were used. All data are reported as mean (SE). Statistical significance was set a priori at P < 0.05 for all comparisons.
Results
Age cross-sectional study
Physical characteristics
As shown in Table 1, heart rates were lower in physically active men than those of sedentary peers. VO2max significantly decreased with aging in both sedentary and physically active men. The youngest physically active group showed a significantly higher VO2max than their sedentary peers. Significant increases in plasma levels of total cholesterol and fasting glucose with aging were observed in sedentary men. In physically active groups, plasma total cholesterol and LDL cholesterol significantly increased with aging. Plasma levels of Lp(a) significantly increased with aging in sedentary subjects but not in physically active men.
Coagulation variables
As summarized in Fig. 1, resting plasma antigen levels of fibrinogen, F1 + 2, FIX and PC, and activity levels of FIX and FXIII showed significant age-related increases for sedentary men, while physically active men showed significantly lower levels of plasma FX and FXI activity than sedentary men (ANOVA: P < 0.05 for both). Particularly, plasma level of FX activity was significantly higher in physically active men aged 35–49 than physically active men aged 20–34, whereas that in physically active men aged 50–64 was significantly lower than their sedentary peers. Plasma activity level of FXI was significantly lower in physically active men aged 20–34 than those in their sedentary peers and in physically active men aged 50–64. Plasma FVIII activity significantly increased with aging in physically active men but not in sedentary men. Significant effects of aging were not observed in plasma activity levels of prothrombin and ATIII.
Fibrinolytic variables
Effects of aging and physical activity on fibrinolytic factors were shown (Fig. 1). Plasma antigen levels of tPA and tPA/PAI-1 complex increased with aging in sedentary and physically active men. However, physically active men showed significantly lower plasma antigen levels of tPA and tPA/PAI-1 complex than those in sedentary men (ANOVA: P < 0.05 for both). Plasma PAI-1 antigen level did not change with aging in sedentary and physically active men. Plasma level of plasminogen activity was significantly higher in physically active men aged 35–49 than physically active men aged 20–34, whereas that in physically active men aged 50–64 was significantly lower than their sedentary peers.
Interventional study
Physical characteristics
As shown in Table 2, body mass and BMI significantly decreased by exercise training, and remained low even after detraining. VO2max significantly increased by exercise training. With exercise training, systolic, diastolic and mean blood pressures and plasma levels of LDL cholesterol and total cholesterol tended to decrease, albeit not significantly (ANOVA: P < 0.10 for all). Heart rate and plasma levels of HDL cholesterol and Lp(a) did not change significantly throughout the observation period. Fasting plasma glucose levels significantly increased after the training program, and remained at significantly elevated levels even after the detraining period.
Coagulation variables
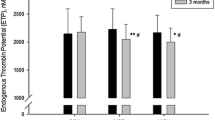
As shown in Fig. 2, coagulation factors showed significant changes with exercise training and detraining. Plasma activity levels of FV, FVIII, FX, FXI, and FXIII decreased significantly by exercise training. After detraining, FX and FXI activity levels returned to the pre-training levels, whereas FV, FVIII and FXIII activity rebounded by detraining reaching levels significantly higher than the pre-training levels. The plasma FIX activity level increased significantly by training and returned to the pre-training levels after detraining. Although the plasma prothrombin activity level increased significantly by training and remained high even after detraining, plasma F1 + 2 concentration did not change significantly. Plasma protein C activity showed slight increases with training and detraining. Plasma levels of FVII activity and FIX antigen showed no significant changes with training, but rebounded to significantly higher levels after detraining. FIX activity showed an increase and stayed elevated even after detraining, although the latter level was not as high as that of the FIX antigen. Plasma levels of fibrinogen, PC antigen and ATIII activity did not change significantly either with training or detraining.
Fibrinolytic variables
In Fig. 2, changes in fibrinolytic factors by exercise training and detraining were also shown. Plasma antigen levels of tPA, PAI-1, and tPA/PAI-1 complex significantly decreased by training, and remained low even after detraining. Plasma plasminogen activity level significantly decreased by exercise training, and returned to the baseline levels after detraining.
Discussion
In spite of significant studies including these, few comprehensive studies have been done on the impacts of regular physical activity on other coagulation factors in relation to age [2, 3, 8–11]. Particularly, no systematic studies have been done on coagulation factors, FV, FX, FXI and FXIII. This situation explains the current limited understanding of age-related influences of aerobic exercise training on hemostatic factors of middle-aged and elderly people. In the present studies, we carried out inclusive studies aiming to make a comprehensive understanding of effects of physical exercises on hemostatic parameters.
In the present study, our training–detraining program successfully detected the effects of aerobic exercise training on plasma levels of coagulation as well as fibrinolytic factors. Plasma levels of most coagulation factors, particularly for fibrinogen and FIX antigens as well as FXIII activity show significant age-related increases in sedentary men, but not in physically active men. In both cross-sectional evaluation and interventional testing, men with regular physical activities and aerobic exercise trainings showed lower levels of plasma FX and FXI activities as well as of tPA and tPA/PAI-1 complex levels. Exercise training also lowered plasma activity levels of FV, FVIII and FXIII, although detraining resulted in rebounds, driving their levels to higher than pre-training levels. Interestingly, physical exercises significantly lowered the plasma F1 + 2 level without rebounding with detraining, while prothrombin activity showed a training-dependent slight increase. The reason for this apparent contradiction requires further study.
We also found that age-related augmentation of several coagulation factors, including plasma activity level for FXIII, is significantly suppressed in physically active men. Through cross-sectional evaluation and interventional testing, we confirmed such suppressing effects of regular physical activity and exercise training on plasma FX and FXI activity levels. Similarly, plasma FV activity level was also significantly lowered by aerobic exercise training in the interventional experiment, while no significant differences were found in these activity levels between physically active men and sedentary peers. This is the first comprehensive study suggesting that the regular aerobic exercise training may work to counter the age-related increase in activities of coagulation factors such as FV, FX, FXI, and FXIII.
Interestingly, FVIII and FIX activities showed a different pattern from other coagulation factors. Plasma FVIII and FIX activity levels were found in the cross-sectional analysis to be significantly higher and lower in physically-activity men than in the sedentary peers, respectively. However, for sedentary middle-aged men in the interventional evaluation, FVIII and FIX activities decreased and elevated, respectively, by aerobic exercise training. These findings support that each coagulation factor is uniquely controlled in a specific manner in regard to physical exercises, warranting further testing of effects of prolonged and various intensity exercises on individual factors.
Plasma antigen levels of tPA, PAI-1, and tPA/PAI-1 complex in physically active men are lower than in sedentary peers, and similarly that plasma levels of tPA and PAI-1 antigens and tPA/PAI-1 complex significantly lowered by 4 months of moderate-intensity aerobic training compared to the pre-training levels. These findings suggest that regular moderate-intensity physical activities have general effects of lowering plasma levels of fibnolytic factors, supporting the previous cross-sectional observations reported by others [2, 9].
The training–detraining program used in this study successfully detected the effects of aerobic exercise training on plasma levels of coagulation as well as fibrinolytic factors. Plasma FX and FXI activity levels, once decreased by exercise training, are recovered by detraining to the pre-training baseline levels. Decreased plasma levels of tPA activity, PAI-1 antigens and tPA/PAI-1 complex antigen by exercise training also showed a tendency of returning to the baseline level by detraining. They, however, still remained at significantly low levels than pre-training levels. Unexpectedly, plasma levels of FV, FVIII, and FXIII activities and FIX antigen rebounded by detraining beyond the pre-training baseline levels. This may imply that habitual, and not non-habitual, aerobic exercise or physical activity is important for ensuring a low risk condition for cardiovascular diseases. Specific mechanisms for inducing such effects of regular physical exercise on coagulation and fibrinolytic factors remain to be determined.
Our cross-sectional studies also showed that regular physical exercises reduce age-related increases of plasma Lp(a) level, an emerging important risk factor for cardiovascular disease [22]. More investigations of the effects of regular exercise trainings on the plasma concentration of Lp(a) are needed for clearing conflicting conclusions described by others [23].
Together with the beneficial effect of regular aerobic physical activities on cholesterol, these effects of regular aerobic exercise observed on hemostatic factors may contribute to lower the risk of atherothrombosis and cardiovascular diseases. Importantly, moderate intensity and brisk walking/jogging mode of exercise trainings examined in our interventional studies are simple, safe, and practical for an individual to continue for ling term, and easily incorporated into an ordinary lifestyle as a regular exercise protocol.
In conclusion, through cross-sectional and interventional examinations, we showed that regular aerobic physical activities give complex, but mostly lowering effects of plasma antigen and activity levels of blood coagulation as well as fibrinolytic factors.
References
Abbate R, Prisco D, Rostagno C, Boddi M, Gensini GF (1993) Age-related changes in the hemostatic system. Int J Clin Lab Res 23:1–3
DeSouza CA, Jones PP, Seals DR (1998) Physical activity status and adverse age-related differences in coagulation and fibrinolytic factors in women. Arterioscler Thromb Vasc Biol 18:362–368
van den Burg PJ, Hospers JE, Mosterd WL, Bouma BN, Huisveld IA (2000) Aging, physical conditioning, and exercise-induced changes in hemostatic factors and reaction products. J Appl Physiol 88:1558–1564
Kurachi K, Kurachi S (2005) Molecular mechanisms of age-related regulation of genes. J Thromb Haemost 3:909–914
Kannel WB, Wolf PA, Castelli WP, D’Agostino RB (1987) Fibrinogen and risk of cardiovascular disease. The Framingham Study. Jama 258:1183–1186
Danesh J, Collins R, Appleby P, Peto R (1998) Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. Jama 279:1477–1482
Lowe GD, Yarnell JW, Sweetnam PM, Rumley A, Thomas HF, Elwood PC (1998) Fibrin D-dimer, tissue plasminogen activator, plasminogen activator inhibitor, and the risk of major ischaemic heart disease in the Caerphilly study. Thromb Haemost 79:129–133
Wannamethee SG, Lowe GD, Whincup PH, Rumley A, Walker M, Lennon L (2002) Physical activity and hemostatic and inflammatory variables in elderly men. Circulation 105:1785–1790
Smith DT, Hoetzer GL, Greiner JJ, Stauffer BL, DeSouza CA (2003) Effects of ageing and regular aerobic exercise on endothelial fibrinolytic capacity in humans. J Physiol 546:289–298
Stratton JR, Chandler WL, Schwartz RS, Cerqueira MD, Levy WC, Kahn SE, Larson VG, Cain KC, Beard JC, Abrass IB (1991) Effects of physical conditioning on fibrinolytic variables and fibrinogen in young and old healthy adults. Circulation 83:1692–1697
Ghiu IA, Ferrell RE, Kulaputana O, Phares DA, Hagberg JM (2004) Selected genetic polymorphisms and plasma coagulation factor VII changes with exercise training. J Appl Physiol 96:985–990
Adachi S (1996) Practical coagulation factor assays. Med Technol 24:629–633
Ranby M, Nguyen G, Scarabin PY, Samama M (1989) Immunoreactivity of tissue plasminogen activator and of its inhibitor complexes. Biochemical and multicenter validation of a two site immunosorbent assay. Thromb Haemost 61:409–414
Fickenscher K, Aab A, Stuber W (1991) A photometric assay for blood coagulation factor XIII. Thromb Haemost 65:535–540
Friberger P, Knos M, Gustavsson S, Aurell L, Claeson G (1978) Methods for determination of plasmin, antiplasmin and plasminogen by means of substrate S-2251. Haemostasis 7:138–145
Okajima K (1995) [Antithrombin III (AT III)]. Nippon Rinsho 53(Su Pt 2):43–47
Goto S, Handa S, Abe S, Takahashi E, Kawai Y, Watanabe K, Yoshikawa T, Hori S, Ikeda Y (1993) Serial changes in hemostatic and fibrinolytic states induced by coronary thrombolytic therapy. J Cardiol 23:335–341
Tanaka H, Clevenger CM, Jones PP, Seals DR, DeSouza CA (1998) Influence of body fatness on the coronary risk profile of physically active postmenopausal women. Metabolism 47:1112–1120
Miwa K, Nakagawa K, Yoshida N, Taguchi Y, Inoue H (2000) Lipoprotein(a) is a risk factor for occurrence of acute myocardial infarction in patients with coronary vasospasm. J Am Coll Cardiol 35:1200–1205
Tanaka H, Monahan KD, Seals DR (2001) Age-predicted maximal heart rate revisited. J Am Coll Cardiol 37:153–156
Hayashi K, Sugawara J, Komine H, Maeda S, Yokoi T (2005) Effects of aerobic exercise training on stiffness of central and peripheral arteries in middle-aged sedentary men. Jpn J Physiol 55:235–239
Bostom AG, Cupples LA, Jenner JL, Ordovas JM, Seman LJ, Wilson PW, Schaefer EJ, Castelli WP (1996) Elevated plasma lipoprotein(a) and coronary heart disease in men aged 55 years and younger. A prospective study. Jama 276:544–548
Mackinnon LT, Hubinger LM (1999) Effects of exercise on lipoprotein(a). Sports Med 28:11–24
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sugawara, J., Hayashi, K., Kurachi, S. et al. Age-related effects of regular physical activity on hemostatic factors in men. J Thromb Thrombolysis 26, 203–210 (2008). https://doi.org/10.1007/s11239-007-0092-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-007-0092-y