Abstract
Essential thrombocythemia is a disorder that causes persistent increase in the platelet count. The disease is associated with an elevated risk of thrombosis. A 71-year-old woman was diagnosed with left main coronary thrombosis after an angiogram due to stable angina. One week before the angiogram was taken the patient had also been diagnosed with essential thrombocythemia. After appropriate medical treatment for 5 days the patient underwent an excimer laser treatment, which failed in dissolving the thrombus. Before the patient underwent coronary surgery, thrombopheresis was performed in order to reduce the platelet count. After a successful coronary operation the patient improved completely.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential thrombocythemia (ET) is a rare myeloproliferative disorder with a predisposition to the development of thrombosis, bleeding, or myelodysplasia [1, 2]. Patients with ET have a clonal stem defect and are at higher risk for thrombosis when compared to those without clonal hematopoesis. It is difficult to estimate the risk of thrombosis from the published literature due to the various different definitions and the differing length of follow up in available series. The average rate of thrombosis is 6.6% of patient–year in series [3]. Most thrombotic episodes were transient and few patients experienced stroke, myocardial infarct or pulmonary embolism [4, 5]. There is limited data in medical literature regarding left main coronary thrombosis due to ET and the treatment options. We hereby report a case of left main coronary artery thrombosis secondary to ET, and discuss the possible therapeutic strategies.
Case Report
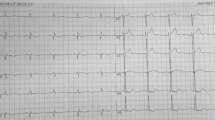
A 71-year-old woman with a history of stable angina pectoris since 6 months was admitted to our hospital due to left main coronary thrombosis diagnosed after an angiogram. Risk factors present in the subject for coronary artery disease included her age and hypertension. Physical examination revealed basillary pulmonary rales and a low intensity aortic systolic ejection murmur was detected on auscultation. Her blood pressure and heart rate were 140/90 mmHg and 100 bpm, respectively. The presenting ECG showed evidence of left ventricular hypertrophy with negative T waves on lead DI, aVL, V1-V3, and a left anterior hemi-block was also present. The chest radiograph showed an increased cardiothoracic ratio. The routine laboratory examination was normal except a thrombocytosis (Hgb: 12.6 mg/dl, Hct 37.4, Plt: 1,070 × 103/mm3). A degenerative aortic valve with mild stenosis was evident at two-dimensional echocardiography (peak velocity: 2.7 m/sn and maximal systolic gradient 36 mmHg). The left ventricle was also mildly hypertrophic (IVS: 13 mm). The patient had been diagnosed with essential thrombocythemia (ET) only 1 week before the admission to our hospital based on the findings of marked thrombocytosis (1,070 × 103/mm3), splenomegaly, and numerous megakaryocytes detected on bone marrow biopsy.
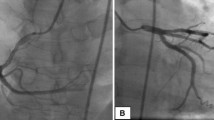
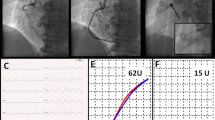
Because the coronary angiogram revealed a huge thrombosis obstructing 95% of the left main coronary artery and extending also into the main circumflex artery (CX) (Fig. 1a, b), the patient was consulted with the cardiac surgeon in charge who was concerned about the bleeding complications posed by thrombocytosis. We agreed to try to dissolve the thrombus at first by laser catheterization. Before the laser procedure, the patient was treated medically with aspirin 300 mg, enoxaparin, clopidogrel 150 mg, hydroxyurea (500 mg3 × 1) and platelet glycoprotein IIb/IIIa inhibitor (tirofiban at weight based atherectomy doses) for 5 days. The coronary angiography repeated on the day of the laser procedure showed no signs of regression regarding the size of the thrombus. The 0.14 intermediate guidewire crossed over the thrombus and it was not possible to get through the thrombus. The catheter was pushed gently to contact the thrombus. Initial lasing energy parameters were set at a fluency of 45 mJ/mm2 at 25 Hz, and were increased up to 60 mJ/mm2 at 40 Hz with a lasing cycle characterized by 5 s of active firing and 10 s of silence. At first we used a concentric (1.7) excimer laser catheter. The thrombosis persisted after the laser trains, and became complicated with an episode of ventricular fibrillation, which was treated with DC shock successfully. The same laser procedure was repeated with an eccentric 2.0 laser catheter. The thrombus regressed only by 4–5% (Fig. 2) and the catheter was easily pushed towards the left anterior descending artery with loss of contraction in the thrombus. The guidewire was steered to enter the circumflex artery in order to have a close contact with the remaining thrombus. Because the thrombus also extended and blocked the entrance of the circumflex artery, this attempt was also unsuccessful. Therefore, the patient was referred for coronary artery by-pass surgery. Based on the hematology consultation, thrombopheresis was performed prior to surgery whereby the platelet count decreased to its target level (400 × 103/mm3). Finally, coronary artery by-pass graft operation was performed with the right internal mammary - LAD and saphenous – CX anastomosis. The procedure went uneventful and no postoperative complications were observed.
Discussion
Patients with ET have a higher risk of thrombosis. The predictive factors of thrombosis have been reported as history of thrombosis, a platelet count >600 × 103/mm3, and advanced age [2, 3]. Our patient had two risk factors for thrombosis, which were age and platelet count. Abnormalities in the number and function of platelets contribute to thromboembolic complications in patients with ET. Thrombomodulin, platelet factor 4, beta-thromboglobulin, prothrombin fragment and total degradation products of fibrinogen, increased in vivo thromboxane biosynthesis, and antiphospholipid antibodies were also suggested as a possible culprit of the increased thrombotic risk in ET [4–7]. Thrombotic complications occur in the form of cerebral, pulmonary and microvascular thrombotic complications [2].
With regard to coronary thrombosis there are two reports available in medical literature. Daya et al [8] and Michaels et al [5] reported cases with ST segment elevation myocardial infarction. Different from these cases our patient presented with stable angina pectoris since the last 6 months. The patient reported by Daya et al [8] underwent emergency surgery due to left main thrombosis with acute ST segment elevation myocardial infarction and suffered acute pulmonary embolism in the early postoperative period. Knowing the success of excimer laser in the removal of extensive thrombus [9], we decided to treat our patient by laser. The case reported by Michalis et al underwent primary angioplasty for the totally occluded right coronary artery, and abciximab treatment for 5 days resulted in complete resolution of the thrombus seen in left main and LAD. We were unable to dissolve the thrombus by laser, which means either the lesion was not a thrombus or it was heavily calcified. The latter seems more plausible because our patient had been suffering stable angina and had also been diagnosed with ET. Likewise, we also know the success of laser in dissolving fresh thrombus [9]. Our expectation was that the laser would dissolve at least a part of the thrombus whereby the catheter could have contact the remaining thrombus and dissolve it step by step. Disappointingly, it did not happen. Different from Michalis et al. our thrombus was also resistant to treatment with a glycoprotein IIb/IIIa receptor–inhibitor given for 5 days before the performance of the laser procedure, suggesting a calcified thrombus.
Taking into account these two cases and our experiences, treatment with a glycoprotein IIb/IIIa receptor–inhibitor seems appropriate in a patient with ET and a fresh thrombus in the coronary artery, provided that there is no emergent need for revascularization. Besides, a laser procedure seems not to be a good option in a patient with ET experiencing chronic angina, suggesting the presence of an old thrombus. However, the role of laser needs to be investigated in patients having ET and experiencing acute coronary syndrome due to a fresh thrombus.
In conclusion, in a patient with left main coronary artery thrombosis due to ET associated with stable angina, the appropriate treatment seems to be thrombopheresis followed by coronary artery by-pass surgery.
References
Passamonti F, Rumi E, Pungolino E, Malabarba L, Bertazzoni P, Valentini M, et al (2004) Life expectancy and prognostic factors for survival in patients with polycythemia vera and essential thrombocythemia. Am J Med 117:755–761
Cortelazzo S, Viero P, Finazzi G, D’Emilio A, Rodeghiero F, Barbui T (1990) Incidence and risk factors for thrombotic complications in a historical cohort of 100 patients with essential thrombocythemia. J Clin Oncol 8:556–562
Harrison CN, Gale RE, Machin SJ, Linch DC (1999) A large proportion of patients with a diagnosis of essential thrombocythemia do not have a clonal disorder and may be at lower risk of thrombotic complications. Blood 93:417–424
van Genderen PJ, Lucas IS, van Strik R, Vuzevski VD, Prins FJ, van Vliet HH, et al (1996) Erythromelalgia in essential thrombocythemia is characterized by platelet activation and endothelial cell damage but not by thrombin generation. Thromb Haemost 76:333–338
Michaels AD, Whisenant B, MacGregor JS (1998) Multivessel coronary thrombosis treated with abciximab (ReoPro) in a patient with essential thrombocythemia. Clin Cardiol 21:134–138
Rocca B, Ciabattoni G, Tartaglione R, Cortelazzo S, Barbui T, Patrono C, et al (1995) Increased thromboxane biosynthesis in essential thrombocythemia. Thromb Haemost 74:1225–1230
Harrison CN, Donohoe S, Carr P, Dave M, Mackie I, Machin SJ (2002) Patients with essential thrombocythaemia have an increased prevalence of antiphospholipid antibodies which may be associated with thrombosis. Thromb Haemost 87:802–807
Daya SK, Gowda RM, Landis WA, Khan IA (2004) Essential thrombocythemia-related acute ST-segment elevation myocardial infarction. A case report and literature review. Angiology 55:319–323
Topaz O, Ebersole D, Das T, Alderman EL, Madyoon H, Vora K, et al (2004) (the CARMEL multicenter trial). Excimer laser angioplasty in acute myocardial infarction. Am J Cardiol 93:694–701
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nurkalem, Z., Uslu, N., Gorgulu, S. et al. Left main coronary thrombosis with essential thrombocythemia. J Thromb Thrombolysis 22, 165–167 (2006). https://doi.org/10.1007/s11239-006-9016-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-006-9016-5