The photocatalytic conversion of lignin, obtained from camelina (Camelina sativa), over titania and iron titanate films has been studied with analysis of the products using laser desorptiopn/ionization and high-performance liquid chromatography. The photocatalytic reaction over titania films leads to the formation of the mixture of compounds, such as phenol, vanillic acid, resorcinol, and p-coumaryl alcohol. In the presence of iron titanate films, the predominant reaction products are vanillic acid and p-coumaryl. The highest antioxidant activity has been revealed in the case of lignin conversion products obtained over nitrogen-containing iron titanate films under visible light exposure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Effective use of renewable plant resources is one of the pathways for the implementation of the strategy of the European Union “European Green Deal”, which contributes to environment preservation and human biosafety increase. Biowastes containing polyphenolic compounds exhibit antioxidant and/or reducing properties and can be used, for example, as a biodiesel stabilizers in energetics, as pharmacological agents with antioxidant and antimicrobial properties in medicine, as a reducing agents in the “green” synthesis of metal nanoparticles, and as additives for the production of active polymer packaging films in the food industry. Additionally, valuable chemical substances can be obtained in the result of their processing. Biologically active substances can be isolated by extraction, pyrolysis [1, 2], and bioconversion [3]. They can be obtained by catalytic [4, 5], electrochemical [6], and photocatalytic processes [7,8,9,10,11,12].
Lignin is one of the most common natural polymers among cellulose and protein. Its content is 20% in grasses and grains, 25% in poplar fibers, and 30% in softwood. Lignin is found to be present as a by-product of the cellulose chemical cooking process as well as cellulosic ethanol production in large quantity [1]. Lignin is a branched natural polymer composing of phenylpropanoid monomeric units (coniferyl, sinapyl, and p-coumaryl alcohols) with common bonds in the β-O-4′, α-O-4′, 5-5′, β-β′, 4-O-5′, β-5′, and β-1′ [5]. The chemical composition and structure of the polymer depend on the origin of raw materials, methods of production and processing.
Thermochemical process [2], hydrodepolymerization, homogeneous catalysis [4, 5], and microbiological treatment [10] are used for the chemical conversion of lignin, other by-products of wood processing, and paper production waste. However, every method contain some disadvantages, such as a low yield of the target product, insufficient selectivity, and high estimated cost of the process [1,2,3,4,5,6].
Photocatalysis is one of the alternative ways of converting bio-raw materials into aliphatic and/or aromatic organic substances promoting the chemical conversion of lignin under the action of UV light at room temperature and atmospheric pressure [9, 10].
Depolymerization efficiency, product yield, the effect of process conditions, the nature of a photocatalyst (usually titanium dioxide or zinc-based powder), and the irradiation range have been analyzed in the literature. Authors of the work [9] observed the formation of vanillin after photocatalysis with TiO2/PEO; while several substances, namely 4-ethoxymethyl-2-methoxyphenol, succinic and vanillic acid, and vanillin were obtained in the work [10]. Vanillin, vanillic acid, palmitic acid, biphenyl, and 3,4,4-trimethoxybenzaldehyde structures have been detected in [11] after irradiation of lignin in the presence of TiO2 P25 for 420 min. Carbonyl derivatives, vanillin, and vanillic acid were obtained by a combined electro-photocatalytic system [6].
The use of photocatalysts in the form of powders results in certain difficulties associated with the separation of solid particles of a suspension from reaction products by centrifugation and ultrafiltration. The use of photocatalysts in the form of films avoids additional energy consumption and partial loss of reaction products during their extraction from the reaction mixture. Recently, researchers are focused on the creation of the photocatalysts that capable of absorbing visible light, which would promote the use of sunlight. The possibility of photocatalytic conversion of sodium lignosulfonate in the presence of titanium dioxide and iron titanate films under simulated sunlight irradiation at different pH values was shown in [12].
The main aim of the present study is to identify products of the photocatalytic conversion of lignin obtained from the extracts of camelina seeds (Camelina sativa) in the presence of titania and iron titanate film photocatalysts. The antioxidant activity of the obtained photocatalytic conversion products was determined by a test reaction with the stable free radical 2,2-diphenyl-1-picrylhydrazyl.
Experimental
Water-soluble lignin was obtained according to the following procedure. Crushed plant material, 30 g (an extract from camelina seeds) was treated with 500 mL of 96% ethanol for 6 h in a Soxhlet extraction apparatus to extract low molecular weight compounds. The purified extract was transferred to a 2-liter round-bottomed flask with a magnetic stirrer, 500 mL of 5% nitric acid was added and kept under stirring (300 rpm) in a water bath at 80°C for 3 h. The cooled reaction mixture was filtered and washed with distilled water on a Schott filter under reduced pressure. The sediment that remained on the filter was dissolved with 25% ammonia to convert lignin into a water-soluble form of ammonium salt. After filtration and evaporation, the lignin fraction was obtained in the form of oil.
The synthesis of TiO2, unmodified (TixFeyOz), and nitrogen-modified (N/TixFeyOz) iron titanate films was performed according to the procedure developed by us [13, 14].
Photocatalytic experiments were performed in an open-type quartz reactor with a volume of 40 mL, which contained a film and a lignin solution of a specified concentration (0.04 mL per 100 mL of solution) at pH 2. The stirring was performed at a constant temperature of the reaction medium of 293±1 K. The light source was a 300 W xenon lamp (Sciencetech 101-9118, Canada) with a simulated sunlight (SSL) or visible (Vis) cut-off filters. The distance between the lamp and the reactor was 20 cm. The analysis of samples taken before and after 360 min irradiation was performed by matrix-free laser desorption/ionization time-of-flight mass spectrometry (LDI MS) and high-performance liquid chromatography (HPLC).
LDI MS studies were performed using an Autoflex II mass spectrometer (Bruker Daltonics Inc., Germany) equipped with a nitrogen laser (337 nm). Samples were deposited onto a steel target and subjected to laser desorption/ionization in pulse mode. The duration of the laser pulse was 3 ns, the frequency was 20 Hz. Spectra were recorded in linear mode with an ion extraction delay of 10 ns and an accelerating voltage of 20 kV. The resulting spectra were the sum of 20 individual spectra obtained as a result of irradiating 25 pulses at each point of the target with the deposited sample. It should be noted that the ionization efficiency is higher in the form of alkali metal adduct ions for most organic molecules, in contrast to the form of protonated ions. Therefore, mass spectra obtained in the positive ion registration mode in matrix-free LDI conditions were interpreted considering the high probability of formation of sodium and potassium metal ion adducts, which are always present in samples due to contact with solvents, water, and glassware.
An Agilent 1100 chromatograph (Agilent Technologies, Germany) with a diode-matrix detector and a Zorbax C18 column 4.6 × 250 mm (a grain diameter of 5μm) were used for HPLC studies. The volume of the injected sample was 25 μl. Elution was performed according to the following procedure: 0-5 min – 100% A + 0% B at a flow rate of 1 mL/min, 10 min – 0% A + 100% B at a flow rate of 1 mL/min, 20 min – 0% A + 100% B with an acceleration of the flow rate to 1.5 mL/min, where A was aqueous solution of 0.05 M H3PO4) and B was methanol. The injection volume was 25 μl, and the column temperature was 20°C. The detection was performed at a wavelength of 206 nm with the preservation of spectra of the UV-visible range every 2 s.
The reaction with a stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) was used to evaluate the antioxidant activity of solutions containing lignin photocatalytic conversion products [12]. According to the standard DPPH test procedure, 1 mL of the test solution was added to 2 mL of 70% ethanol and 2 mL of a 0.15 mM DPPH solution in 70% ethanol. The concentration of stable radicals was determined at different time intervals after the beginning of the reaction by spectrophotometry observing the change in intensity at the maximum absorption of the DPPH solution at 520 nm. A solution with the same DPPH concentration, but without test solutions was used as a control.
Results and Discussion
TiO2, unmodified (TixFeyOz), and nitrogen-modified (N/TixFeyOz) iron titanate films are selected as catalysts to study the photocatalytic conversion of lignin [12, 13]. By X-ray diffraction analysis, it is determined that the TiO2 film is crystallized to anatase after treatment at 450°C, whereas the iron-containing films with the ratio of Fe: Ti = 1:1 contain mixed phases of pseudobrookite (Fe2TiO5) and landauite (Fe2Ti2O7) [13, 14]. The crystallite size of the formed semiconductors is 15 nm and 23-25 nm in the case of anatase and iron titanates.
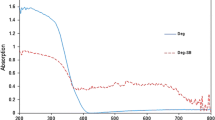
Changes in the optical absorption spectra, namely the intensity decrease/increase at λ = 355 nm and a shoulder in the region of 255-300 nm, indicate the photocatalytic conversion of lignin with the participation of the films. Absorption spectra of the lignin solution during light irradiation at λ ≥ 330 nm in the presence of TiO2 films (Fig. 1a) reveal a monotonous decrease pointing on polymer degradation. However, the photoconversion of lignin with the participation of iron titanate films under the action of light at λ ≥ 330 nm leads to an increase in the intensity of the band in the region of 250-300 nm and an alternating decrease/increase in the intensity of the main band (Fig. 1b). Such changes may be associated with the oxidation processes of lignin monomer units, which lead to the formation of aromatic compounds with higher extinction coefficients [12]. According to the literature data [13, 14], intermediate fragments are formed, which are transformed into oxybenzene derivatives. The authors do not exclude re-polymerization processes under certain conditions [16, 17]. It should be noted that a significant effect of visible light irradiation on the evolution of absorption spectra is not observed.
The analysis of absorption spectra of the lignin solution during irradiation in the presence of film photocatalysts cannot allow to estimate the photoconversion degree and determine its products. Therefore, the photocatalytic process efficiency is estimated by the qualitative and quantitative characteristics of reaction products using LDI-MS and HPLC methods. The results of the identification of lignin photocatalytic conversion products by time-of-flight mass spectrometry with laser desorption/ionization in the positive ion mode are shown in Table 1. Determination of fragmented ions of the corresponding m/z signals of the studied compound before and after irradiation in the presence of film samples is performed taking into account the model structure of lignin (the ratio of phenylpropanoid monomer units depends on the origin of raw material) [18] and literature data.
Light absorption by lignin (photolysis) at λ ≥ 330 nm results in changes of the lignin polymer structure, which is confirmed by the presence of intense peaks in the m/z range of 84-200, attributed to the formation of ions with the hydrocarbon chain length of C5-C9. According to [19], the absorption of light by lignin leads to the formation of several radicals (R• + RO• + ROO•), which participate in its depolymerization to oligomers/monomers by opening β-O-4 bonds.
In comparison of the LDI-MS spectra of the initial solutions and solutions after photolysis with the ones after irradiation with SSL at λ ≥ 330 nm in the presence of TiO2, TixFeyOz, and N/TixFeyOz films, the disappearance of signals with m/z 429-465 (the m/z=449 peak is low intensive peak in the case of N/TixFeyOz) is observed indicating the conversion of monolignol oligomeric C21-C22 fragments occurs.
Redistribution of relative intensities of the signals is a sign of a conversion mechanism that differs from photolytic processes (Table 1). The appearance of the signal at m/z=145 with the highest absolute intensity as well as its increase at m/z=147 and its decrease at m/z 84, 104 and 207 are noted for all three samples after photocatalytic process. The presence of peaks with m/z 550-650 (C21-C34) in the mass spectra of the lignin solutions exposed SSL irradiation over TiO2 and TixFeyOz films indicates the formation of associates of low-molecular-weight species formed in result of photoconversion.
Effective conversion of lignin molecule under the action of visible light is fixed in the presence of TixFeyOz and N/TixFeyOz films, which are characterized by favorable energy positions of the conduction and valence bands as well as the certain energy of band gap providing the generation of reactive species and direct interaction of the target compound with the initially formed charge carriers [13]. It should be noted that the most intensive peak with m/z = 145 is registered for all samples after acting both sources of light, excluding the one after visible light irradiation in the presence of TiO2 film. Peaks with m/z at 450-610 are absent in the mass spectra (the signal with m/z 579 is low-intensive in the case of TixFeyOz). The intensity of peaks with lower m/z values (145, 147) is increased, while the signals with m/z 413, 429, and 449 are decreased/disappeared indicating the conversion of lignin molecules into lower-molecular-weight compounds.
The photocatalytic conversion of lignin with the participation of TiO2 film under the visible light is similar to the photolytic process. It apparently occurs according to the photosensitized decomposition mechanism, which involves the absorption of a quantum of light by a lignin molecule, followed by the transfer of an electron to the conduction band of a semiconductor. Such processes are accompanied by the destruction/decomposition of an organic molecule, which is confirmed by an increase in absolute intensity and the appearance of peaks with m/z values (145, 147), which are absent after photolytic conversion.
Quantitative analysis of samples by HPLC shows that several low-molecular products are formed as a result of lignin decomposition under the action of light, in particular, phenol, vanillic acid, resorcin, and p-coumaryl alcohol. It should be noted that according to the identification of possible ions by the LDI MS method, [C6H6O+H]+, [C7H6O+K]+, and [C8H8O4+K]+ ions
(Table 1) correspond to chromatographically determined products, namely phenol, vanillic acid, and p-coumaryl alcohol. However, according to the established molecular formulas and the presence/absence of signals of a certain intensity, the formation of other fragmented structures/conversion products is not excluded. According to the HPLC data, unlike the photodecomposition of sodium lignosulfonate [12], a significant change in the concentration of phenol in the lignin solution during photolysis under the action of light at λ ≥ 330 nm is not observed. A slightly higher phenol yield is formed as a result of photolytic conversion and photosensitized conversion of lignin with the participation of a TiO2 film under the action of light at at λ ≥ 400 nm. The highest yield of vanillic acid (0.11 μg/mL), resorcin (0.04 μg/mL), and p-coumaryl alcohol (0.09 μg/mL), which are not detected in the lignin solution before irradiation (Fig. 2b and c), are observed in the photocatalytic conversion reaction in the presence of TiO2 films (Fig. 2d). N/TixFeyOz and TixFeyOz films are promising for the synthesis of vanillic acid (0.075 mg/g/mL) and p-coumaryl alcohol (0.055 μg/mL), respectively, under the action of light at λ ≥ 330 nm. It should be noted that although the highest content of all products is fixed with the participation of the TiO2 film under the action of various irradiations, however, according to the results of LDI MS, more effective conversion of the polymer structure of the original compound is observed in the presence of the N/TixFeyOz film, which is confirmed by the absence of m/z 457-607 and 313-381 signals accompanied by a significant decrease in the relative intensity of peaks (except for 145, 147) when compared to the solution before irradiation and after photolysis.
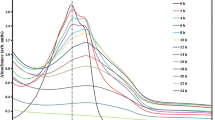
The antioxidant capacity of lignin photocatalytic conversion products is determined in the test reaction of inhibition of DPPH radicals. As can see from Fig. 3, the initial lignin exhibits low activity in the reaction with DPPH radicals (curve 1). Sample, obtained by photolysis of a lignin solution at pH 2 without a photocatalyst under the action of simulated sunlight, is characterized by slightly higher activity, reducing about 10% of radicals for 30 min (curve 2). Even higher reactivity is observed for the solutions obtained with the use of TiO2 (curve 3) and TixFeyOz (curve 4) photocatalysts (a decrease in the concentration of radicals ~20% within 30-60 min). The use of the N/TixFeyOz catalyst under the action of SSL does not lead to the formation of a significant amount of antioxidants (curve 5), while the solution obtained in the presence of the same catalyst under visible light shows the best antiradical properties, reducing ~50% of DPPH radicals within 30-60 min (curve 6), which may be associated with the appearance of antioxidants, such as phenol, resorcin, vanillic acid, and p-coumaryl alcohol, as well as their synergistic action [20] with a complex of other compounds that can be formed as a result of lignin decomposition.
Reduction of 2,2-diphenyl-1-picrylhydrazyl radicals at pH 2 upon contact with the initial lignin (1), products of its photolysis (2), and products of photocatalytic conversion in the presence of TiO2 (3), TixFeyOz (4), and TixFeyOz (5) films under the action of SSL and N/TixFeyOz (6) under the action of light at λ ≥ 400nm .
Thus, the photocatalytic conversion of the polymer structure of lignin over TiO2 films and iron titanates is an approach that allows to synthesize phenolic compounds under conditions that do not require their separation from the solid state of the photocatalyst. The TiO2 film is the most active photocatalyst for the synthesis of a mixture of hydroxybenzenes during the photocatalytic conversion of lignin. Vanillic acid is formed mainly during photocatalytic processes with the participation of the N/TixFeyOz film under the action of light at λ ≥ 330 nm (p-coumaryl alcohol is formed in the presence of TixFeyOz), while it is inverse at at λ ≥ 400 nm. It is determined that the course of the process, the composition, and the ratio of products are mostly affected by such factors as the nature of a semiconductor and the irradiation wavelength.
The antioxidant properties of lignin photocatalytic conversion products have been studied for the first time. It is established that the most effective lignin conversion (as for antioxidant properties of the product) occurs with the participation of the N/TixFeyOz film under the action of light at λ ≥ 400 nm. It is shown that the solution obtained under these conditions reduces ~50% of DPPH radicals in 30 min.
References
T. Saito, R.H. Brown, M.A. Hunt, et al., Green Chem., 14, 3295-3303 (2012).
R. Al’en, E. Kuoppala, and P. Oesch, J. Anal. Appl. Pyrolysis, 36, No. 2, 137-148 (1996).
N. Busse, D. Wagner, M. P. Kraume, et al., Am. J. Biochem. Biotechnol., 9, No. 4, 365-394 (2013).
J. Zakzeski, P. C. Bruijnincx, A. L. Jongerius, et al., Chem. Rev., 110, No. 6, 3552-3599 (2010).
H. Paananen, E. Eronen, M. Makinen, et al., Ind. Crop. Prod., 152, 112473 (2020).
R. Pan, M. Tian, Z.H. Jiang, et. al., Electrochim. Acta., 60, 147-153 (2012).
L. I. Granone, F. Sieland, N. Zheng, et al., Green Chem., 20, 1169-1192 (2018).
L. Xiaoqing, D. Xiaoguang, W. Wei, et al., Green Chem., 21, 4266-4289 (2019).
Y. Miyata, K. Miyazaki, M. Miura, et al., J. Polym. Environ., 21, No. 1, 115-121 (2012).
A. Shende, R. Jaswal, D. Harder-Heinz, et al., Cleantech., 120-123 (2012).
M. Ksibi, S.B. Amor, S. Cherif, et al., J. Photochem. Photobiol. A., 154, 211-218 (2003).
A. Kramar, V. Anishchenko, P. Kuzema, et. al., Appl. Nanosci., [12], 2345-2355 (2022), https://doi.org/10.1007/s13204-022-02492-9.
O. Linnik, N. Chorna, and N. Smirnova, Nanoscale Res. Lett., 12, 249-258 (2017).
N. Chorna, N. Smirnova, V. Vorobets, et. al., Appl. Surf. Sci., 473, 343-351 (2019).
W. Brand-Williams, M. E. Cuvelier, and C. Berset, LWT - Food Science and Technology, 28, 25-30 (1995).
J. Banoub, Jr. G.-H. Delmas, N. Joly, et al., J. Mass Spectrom., 50, 5-48 (2015).
R. J. A. Gosselink, Lignin As A Renewable Aromatic Resource For The Chemical Industry, PhD Thesis, Wageningen University, Wageningen, NL (2011).
D. Kun and B. Pukanszky, Eur. Polym. J., 93, 618-641 (2017).
J. A. Schmidt and C. Scown, J. Wood Chem. Technol., 15, 223-245 (1995), https://doi.org/10.1080/02773819508009509.
C. H. Lau, S. Gan, H. L. N. Lau, L. Y. Lee, et. al., Sustain. Energy Technol. Assessments, 52, 102296 (2022).
Acknowledgements
The work was performed with the financial support of the National Research Fund of Ukraine (project No. 2020.01/0136 “Effective Use of Renewable Plant Resources and Biomass Photocatalytic Conversion as Ecologically Innovative Approaches to the Environment Preservation and Human Biosafety”).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 59, No. 3, pp. 154-160, May-June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kramar, A.S., Malysheva, M.L., Anishchenko, V.M. et al. Photocatalytic Conversion of Lignin in the Presence of Titania and Iron Titanate Films. Theor Exp Chem 59, 178–185 (2023). https://doi.org/10.1007/s11237-023-09776-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-023-09776-3