Effective catalysts of CO2 hydrogenation towards methane were obtained by impregnation of aluminum oxide with cobalt and iron nitrates. The catalysts have been characterized by XRD, SEM-EDX, argon physisorption, and TPD with mass-spectroscopy analysis. It is shown that an increase in the amount of applied Co-Fe active mass in the composition of a nanocomposite catalyst leads to an increase in the CO2 conversion and CH4 yield.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Carbon dioxide is one of the greenhouse gases, the content of which is quite high in the atmosphere. The process of hydrogenation is one of the effective methods of reducing CO2 concentration that results in methane [1,2,3]. A development of metal catalysts with high activity and selectivity for methane is important from an economic and ecological point of view. Nowadays, many studies have been conducted to obtain bulk and supported metal catalysts with high CH4 yield in the CO2 hydrogenation reaction [4,5,6,7]. Transition 3d metals, namely Ni, Co, and Fe, which individually or in the form of bimetallic composites contribute to the selective formation of methane should be noted among the highly effective catalysts of the CO2 hydrogenation. Metallic nickel is the common catalyst used in the conversion of CO2 into methane (methanation). However, one of the disadvantages of this catalyst is its rapid deactivation caused by sintering and coking. Monometallic cobalt catalysts were widely used to obtain long-chain linear hydrocarbons from synthesis gas (CO and H2). However, cobalt begins to catalyze the methanation reaction when CO gas is replaced by CO2 in the reaction mixture [8]. The addition of Fe to a monometallic Co catalyst is one of the ways to increase methane yield and selectivity [9,10,11]. A study of the properties and catalytic activity of bulk Co-Fe catalysts shows that the addition of even a small amount of Fe to a cobalt catalyst facilitates the dissociation of CO2 and leads to effective methane formation due to the inhibition of CO formation [12].
Iron acts as a structural component in the bimetallic Co-Fe composition, which as a part of an active catalytic site [10, 13] promotes the dissociation of CO2 molecules due to the high affinity of Fe towards oxygen. One of the most effective methods of increasing the stability and activity of a catalyst is its deposition on an inert support. It is possible to increase the selectivity of the process for the target product, achieve a higher yield of products, or lower the temperature of their maximum yield. Aluminum oxide occupies a prominent place among the large number of supports used for the CO2 hydrogenation, due to its inertness and high dispersion [13, 14]. The addition of Fe to the Co/Al2O3 composition reduces the cobalt interaction with a support and the formation of aluminates. Instead, it promotes the formation of a Co-Fe alloy on a suport surface [15], which provides a higher yield of methane in comparison with a monometallic cobalt. It is necessary to determine the ratio between the active mass of a catalyst and a support, as well as to determine the properties of a support for the formation of catalytically active sites on its surface considering promising results in the study of bulk and supported Co-Fe catalysts.
EXPERIMENTAL
Sample preparation. An active catalyst mass containing 93 wt.% Co and 7 wt.%Fe was deposited on the surface of aluminum oxides. The results given in [11] show that bulk Co-Fe catalysts with an iron content of 3-10 wt.% most effectively contribute to the selective conversion of CO2 into methane under atmospheric pressure and in the temperature range of 300-350°C. The amount of deposited active mass was 5, 10, and 20 wt.% from the weight of a support. Aluminum oxide with different specific surface values was used as a support (Table 1).
The samples were prepared by impregnating the support with concentrated nitrate solutions of the corresponding metals. At first, the mixture with the calculated ratio of initial metals was dissolved in an excess of concentrated nitric acid under heating. The resulting solution cooled to room temperature was applied for impregnating the alumina sample weight. The wet substrate was air-dried at room temperature for 24 h, and then calcined at 250°C for 8 h. Therefore, oxides of the corresponding metals are formed on the surface of aluminum oxide. Metal nanocomposite CoFe/Al2O3 catalysts were prepared by reducing oxide precursors in a reducing atmosphere (50 vol.%H2 + 50 vol.% Ne, gas flow rate was 100 mL/min) at a temperature of 450°C and atmospheric pressure for 2 h. The reduction process was performed immediately before the catalytic tests to prevent the oxidation of the catalysts.
Catalytic evaluation. The conversion of CO2 into CH4 over CoFe/Al2O3 catalysts (sample bulk volume of 1 cm3, that corresponds to 0.6-0.7 g) was studied in a flow reactor with a diameter of 8 mm in the temperature range of 30-450°C at a pressure of 0.1 MPa. The composition of the reaction mixture: 2 vol.% CO2 + 55 vol.% H2 + 43 vol.% He, the gas flow rate was 0.1 L/min. The high level of hydrogen (55 vol.%) in the reaction mixture prevents the formation of C2-C5 hydrocarbons and/or alcohol. The gas mixture from reactor outlet was analyzed for the content of CO2, CO, and CH4 using a Shimatzu GS-2014 gas chromatograph equipped with a thermal conductivity detector and a separation column filled with 5A molecular sieves. The efficiency of the catalyst was evaluated by the temperature of maximum conversion of CO2 into CH4. Catalytic activity was studied using two consecutive temperature raises. In the process of the first raise, the catalyst pre-development occurs involving the preliminary formation of catalytically active sites on its surface. The degree of conversion of CO2 and the amount of the formed product was directly registered during the second temperature raise.
Carbon dioxide conversion (\( {X}_{{\mathrm{CO}}_2} \)) and methane yield (\( {Y}_{{\mathrm{CH}}_4} \)) were calculated using the following equations:
where [CO2], [CH4], and [CO] are the chromatographically recorded amount of the gas mixture components (vol.%) at the reactor outlet.
Methods. X-ray diffraction patterns were recorded in the range of 2θ = 10-70° using a DRON-4-07 diffractometer (Burevestnyk, St. Petersburg) with CuKα radiation (λ = 0.15418 nm) and a Ni filter.
Scanning electron microscopy (SEM) using a Mira 3 Tescan device was performed to study the surface morphology of nanocomposite catalysts. Chemical analysis of the studied samples was conducted on an energy dispersive X-ray (EDX) spectrometer by Oxford INCA mounted on the microscope.
The specific surface area (Ssp) was measured chromatographically using a thermal conductivity detector by studying the adsorption of argon in a gas mixture of 5 vol.% Ar + 95 vol.% of He. Measurements were performed at a temperature close to the boiling point of liquid nitrogen [10]. The samples were analyzed under conditions identical to the standard (SiO2 and Silochrome C-80) with a stable specific surface value (80 m2/g).
Thermal desorption (TD) spectra were recorded after a full cycle of the catalytic process with the reaching of maximum CO2 conversion and subsequent slow cooling of the catalyst. A small amount of the catalyst was transferred from the reactor into a quartz ampoule and connected to a mass spectrometer under the reaction mixture stream. The ampoule with the sample was vacuumed to 10–4 Pa before analysis, then heated at a constant rate of 14°C/min from room temperature to 800°C with simultaneous analysis of desorbed products. Gradual heating promotes the separation of desorbed particles with different values of desorption energy (Ed). An MX 7304A quadrupole monopolar mass spectrometer (Selma, Ukraine) with electron impact ionization of molecules was used to study and register particles desorbed from the surface. Results of the studies of temperature-programmed desorption with mass spectrometric analysis (TPD MS) allow to calculate the activation energy of desorption (Ed) from the surface of nanocomposite catalysts according to the equation
where ν is the pre-exponential factor; n is the kinetic order of desorption (n = 1 or 2 for thermal desorption peaks of asymmetric and symmetric form, respectively); Tm is the temperature of the desorption peak maximum determined from the TPD profile; θm is the degree of surface coverage at Tm, β is the heating rate in TPD MS analysis [16, 17].
RESULTS AND DISCUSSION
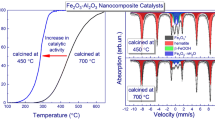
Figure 1 shows the results of the study of the catalytic performance of CoFe/Al2O3 nanocomposites (the composition of the nanocomposites is given in Table 2) in the CO2 methanation reaction in the form of temperature curves of CO2 conversion (Fig. 1a) and methane yield (Fig. 1b). According to the gas chromatography data, the complete conversion of carbon dioxide over 20%wt.Co(93)Fe(07)/A1 nanocomposite occurs in the temperature range of 375-450°C with a methane yield of 73-79% . Methane yield of 83-85% and 100% CO2 conversion over 20wt.%Co(93)Fe(07)/A2 catalyst was observed in the same temperature range. Reduction from 20 wt.% up to 10 wt.% of the active mass deposited on the surface of the A1 and A2 supports leads to a decrease in the methane yield (69-76%) in the temperature range of 350-400°C. For catalysts with 5 wt.% of deposited active mass the CO2 conversion slowly increases throughout the entire temperature range of the measurements and does not reach 100%. Only 10% of CO2 is converted in the case of 5wt.%Co(93)Fe(07)/A3 catalyst (Fig. 1a, curve 1). The chromatographic data show the formation of a by-product (carbon monoxide) simultaneously with the main product (methane) for 5wt.%Co(93)Fe(07)/A1 and 5wt.%Co(93)Fe(07)/A3 catalysts (Fig. 1b, curves 1* and 2*). Therefore, it can be supposed that the active mass of 5wt.%Co(93)Fe(07) deposited to all aluminum oxide types studied in the work is an insufficient amount for the formation of active catalytic sites. In addition, the use of A1 and A3 supports also does not further the effective course of methanation reaction over nanocomposite Co-Fe catalysts formed on their basis. The highly developed surface of A1 and A3 supports does not contribute to the formation of a critical mass of catalytically active bimetallic sites due to the high degree of their dispersion on the surface of a support. Co and Fe aluminates are likely to be formed under such conditions, which are inactive in the catalytic process of CO2 hydrogenation [15].
The obvious difference in the balance between the quantities of products and reagents (Fig. 1) can be explained by the fact that part of CO2 contained in the reaction mixture is adsorbed on the free surface (without metal particles) of the support and does not participate in the mechanism of methane formation under the given reaction conditions, remaining strongly chemisorbed on the surface of aluminum oxide.
The specific surface values of the initial supports and Co-Fe/Al2O3 nanocomposites after studying their catalytic activity is given in Table 1. According to the results of the measurements, the Ssp value of nanocomposites is almost reduced by half when compared to the corresponding initial supports. In addition, the specific surface area of nanocomposite Co(93)Fe(07)/A1 catalyst has a tendency to decrease when the amount of deposited bimetallic phase is increased. The amount of deposited CoFe phase does not affect the Ssp value of the obtained nanocomposites when using the A2 support.
SEM images of 5wt.%Co(93)Fe(07)/A1 and 5wt.%Co(93)Fe(07)/A2 catalysts do not reveal a significant difference in the surface morphology of the initial supports. A smooth surface with fissures is observed for both supports A1 and A2. It can be seen that small fragments of the active phase are randomly scattered on the surface of the support (Fig. 2a,b).
The deposited Co-Fe active mass occurs on the surface of the support as agglomerates with a size from 100 nm to 1 мm (Fig. 2c). The image of 20wt.%Co(93)Fe(07)/A2 nanocomposite shows an uneven distribution of deposited Co-Fe active compounds on the surface of the support. The amount of Co+Fe in the analyzed regions 1 and 2 significantly exceed the amount specified in the synthesis, and amount of the metal found in the region 3 almost equal to defined with synthesis (Table 2). The EDX data show an uneven distribution of the active mass on the surface of the support, while the Co/Fe ratio in the active mass differs from that specified in the synthesis.
EDX analysis shows an increase in the amount of Fe to 20-25% against Co, for a catalyst with 5 wt.% of the active mass, that leads to a decrease in catalytic activity. In contrast, the amount of Fe in the Co-Fe ratio is approximately 3 wt.% for the 20wt.%Co(93)Fe(07)/A2 catalyst; and, according to [11] is sufficient for effective CO2 hydrogenation.
Figure 3 shows the phase composition of catalysts after the methanation and the corresponding initial supports. The X-ray diffraction patterns of the aluminum oxide of A1 and A2 types show the difference in their crystal structure. γ-Al2O3 reflections are typical for type A1 aluminum oxide (Fig. 3, curve 2), while A2 aluminum oxide has a mixed structure; where admixtures of corundum -phase crystals are present on the background of fine-crystalline γ-phase (Fig. 3, curve 4). The deposited particles of the active Co-Fe bimetallic mass were determined only by a low-intensity signal of cobalt(II) oxide for both studied samples (Fig. 3, curves 2 and 4). We believe that the fine-crystalline CoO phase appears after the passivation of the samples in the air [9, 11]. Since the content of iron in the composition of a nanocomposite is very small, its identification is beyond the sensitivity of the device.
Thermal desorption studies were performed for the samples that contained the largest amount of active mass (20 wt.%). They showed the maximum activity in the methanation reaction among all studied catalysts in the given research. The state of the surface of these samples reflects best the processes occurring during the reaction (Fig. 4).
The TD profiles with m/z 16, 18, 28, 29, and 44 corresponding to the desorbed groups \( {\mathrm{CH}}_4^{\ast } \), H2O*, CO*, HCO*, and \( {\mathrm{CO}}_2^{\ast } \) were registered on the TPD spectra. The TD profile with m/z 18 (Fig. 4a, insert) of the 20 wt.%Co(93)Fe(07)/A2 sample has two high-intensity peaks, namely, the first one with a temperature maximum (Tmax) at 80°C refers to a physisorbed water; while the second one, which is a broad and smooth peak in the temperature range of 250-600°C, refers to a water formed in the process of CO2 hydrogenation.
The TD profiles of \( {\mathrm{CO}}_2^{\ast } \), CO*, HCO*, and \( {\mathrm{CH}}_4^{\ast } \) groups show similar forms, but with different intensities. These features indicate that all particles participate in the same process [17]. The presence of a TD profile with m/z 29 indicates that the hydrogenation process over 20wt.%Co(93)Fe(07)/Al2O3 catalysts is caused by the formation of an intermediate HCO* group. The profile has a low intensity and its temperature maxima coincide with those for CO* and \( {\mathrm{CO}}_2^{\ast } \). The recorded HCO* groups can be formed on the surface due to functional OH groups that exist on the surface of aluminum oxide [6, 7, 18].
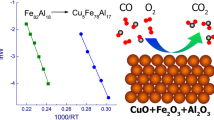
The process of methane formation over the Co-Fe catalyst deposited on aluminum oxide can be described by the participation of functional OH groups on the surface of aluminum oxide. The first stage of the hydrogenation process over CoFe/Al2O3 catalysts should involve the CO2 and H2 adsorption followed by their dissociation on active bimetallic sites; at the same time, the presence of Fe, which contributes to the O=C=O bond cleavage, plays an important role. Part of the dissociated CO*, O*, and H* particles can diffuse from the active sites of a catalyst onto a support and be involved in further stages of CO2 hydrogenation. Chemosorbed CO molecules formed on active sites can interact with OH groups on the support surface to form bridged and linear forms [6, 7]. The formation of HCO* involves the joining of hydrogen atoms to adsorbed CO* as a result of dissociative or associative processes. Hydrogen atoms participate in the cleavage of C–O bonds in the dissociative process [19]. After diffusion of CO2 dissociation products from the active site of a catalyst to the surface of a support, this active site becomes available for CO2 molecules from a gas mixture that participate in the methanation.
The complex relief of TD profiles indicates the presence of a large number of adsorption sites with different energies of desorption on the surface of nanocomposite CoFe/A12O3 catalysts. The maxima at 700°C are observed on the TD profiles of CO2 for both samples (Fig. 4a, b), which correspond to strongly chemisorbed CO2, which does not participate in the hydrogenation in the temperature range of methane yield (200-450°C). The analysis of the TD profile of CO2 shows that the ratio of intensities of CO2 peaks, which directly participates in the methanation reaction (temperature range of up to 500°C) to intensities of CO2 peaks, which is strongly chemisorbed on the surface of the support and does not participate in the catalytic process (temperature range of above 600°C) is 6:1. Therefore, the majority of CO2 participates in the methanation in the temperature range of 200-450°C; while only approximately 1/7 of CO2 from the gas phase can be chemisorbed on the surface of the support.
Desorption of physisorbed groups with m/z 28 and 44 occurs in the temperature range of 50-200°C for both samples (Fig. 4). The high-temperature range of TD profiles is not similar for 20wt.%Co(93)Fe(07)/A2 (Fig. 4a) and 20wt.%Co(93)Fe(07)/A1 (Fig. 4b) samples. Broad peaks with Tmax = 250 and 380°C are shown in the TD profile of the catalyst with higher catalytic activity (20wt.%Co(93)Fe(07)/A2). These peaks correspond to groups with a high value of activation energy of desorption (Ed = 148 and 186 kJ·mol–1). In our opinion, these groups are desorbed from active sites on the surface of nanocatalysts and participate in the process of methane formation. The TD profiles of the \( {\mathrm{CO}}_2^{\ast } \) and CO* groups for the catalyst with lower activity (20wt.%CoFe/A1) in the temperature range of 300-400°C differ from the previous one. The desorption peaks of chemisorbed particles shift to higher temperatures with Tmax = 480°C and Ed = 216 kJ·mol-1. This leads to a decrease in the catalytic activity of this nanocatalyst in the methanation reaction in the temperature range of 300-400°C.
Therefore, the results of the research indicate that the methane yield increases at 400°C over the studied nanocomposite catalysts for the CO2 hydrogenation reaction in the following order: 5wt.%CoFe/A3 (1%) < 5wt.%CoFe/A1 (53%) < 5wt.%CoFe/A2 (72%) < 10wt.%CoFe/A1 (76%) < 20wt.%CoFe/A1 (76%) < 10wt.%CoFe/A2 (77%) < 20wt.%CoFe/A2 (84%). The catalyst, which consists of 20 wt.% of the active mass of the Co(93)Fe(07) composition, deposited on aluminum oxide with a specific surface of 70 m2/g, is optimal for highly efficient hydrogenation of CO2 to methane. Highly dispersed A12O3 with Ssp = 190-310 m2/g is not an effective support for Co-Fe catalysts. It can lead to an increase in the degree of dispersion of metal particles on the support surface with subsequent deactivation caused by the formation of Co and Fe aluminates.
SEM-EDX studies revealed an uneven distribution of the Co-Fe active mass on the surface of the support. In particular, large agglomerates of deposited Co-Fe metals are observed in the 20wt.%Co(93)Fe(07)/A2 sample. This may result in the high activity of this catalyst in the process of CO2 conversion to methane. Therefore, further research is required for deposited catalysts with a higher content of active mass. The SEM-EDX data also showed that the ratio of the active mass of Co/Fe in the surface layer of the catalysts does not correspond to that specified in the synthesis. The increase in the Fe amount in the ratio of metals in the surface layer of the catalyst with 5 wt.% of the active mass decreases the catalytic activity. In contrast, the amount of Fe in the Co-Fe ratio is approximately 3 wt.% for the 20wt.%CoFe/A2 catalyst, and is sufficient for the effective CO2 hydrogenation. The results of the TPD MS studies of the catalyst surface after the CO2 hydrogenation process showed the formation of intermediate HCO* groups, which were not detected for the bulk catalyst with a similar Co/Fe ratio. This fact indicates that the methanation process over catalysts deposited on Al2O3 occurs by a different mechanism than on bulk catalysts, due to the presence of functional OH groups on the support surface.
References
C. Vogt, M.Monai, G. J. Kramer, and B. M. Weckhuysen, Nat. Catal., 2, 188-197 (2019).
S. Ronsch, J. Schneider, S. Matthischke, et al., Fuel, 166, 276-296 (2016).
A. Malara, P. Frontera, P. Antonucci, and A. Macario, Curr. Opin. Green Sustain. Chem., 26, 100376 (2020).
D. Beierlein, D. Haussermann, M. Pfeifer, et al., Appl. Catal. B., 247, 200-219 (2019).
O. V. Ishchenko, A. G. Dyachenko, A. V. Yatsymyrskiy, et al., E3S Web Conf., 154, 02001 (2020).
M. A. A. Aziz, A. A. Jalil, S. Triwahyono, and A. Ahmad, Green Chem., 17, 2647-2663 (2015).
A. G. Dyachenko, O. V. Ischenko, O. V. Goncharuk, et al., Appl. Nanosci., 12, No. 3, 349-359 (2022).
W. Chaitree, S. Jiemsirilers, and O. Mekasuwandumrong, Catal. Today, 164, No. 1, 302-307 (2011).
T. Das and G. Deo, J. Phys. Chem. C., 116, No. 39, 20812-20819 (2012).
O. V. Ischenko, A. G. Dyachenko, I. Saldan, et al., Int. J. Hydrog. Energy, 46, 37860-37871 (2021).
M. Zhludenko, A. Dyachenko, O. Bieda, et al., Acta Phys. Pol. A., 133, 1084-1087 (2018).
A. S. Sandupatla, A. Banerjee, and G. Deo, Appl. Surf. Sci., 485, 441-449 (2019).
S. Hwang, U. G. Hong, J. Lee, et al., Catal. Lett., 142, 860-868 (2012).
Y. Zhan, Y. Wang, D. Gu, et al., Appl. Surf. Sci., 459, 74-79 (2018).
N. Srisawad, W. Chaitree, and O. Mekasuwandumrong, React. Kinet. Mech. Catal., 107, No. 1, 179-188 (2012).
R. J. Cvetanovic and Y. A. Amenomiya, Catal. Rev., 6, No. 1, 21-48 (1972).
M. W. Roberts and C. S. McKee, Chemistry of the Metal-Gas Interface, Oxford, Oxford University Press (1979).
A. G. Dyachenko, O. V. Ischenko, M. G. Zhludenko, et al., Bul. Chem. Com., 52, No. 3, 342-347 (2020).
B. Miao, S. Ma, X. Wang, et al., Catal. Sci. Technol., 6, 4048-4058 (2016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoretychna ta Eksperymentalna Khimiya, Vol. 58, No. 2, pp. 119-125, March-April, 2022.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dyachenko, A.G., Ischenko, O.V., Borysenko, M.V. et al. Co-Fe/Al2O3 Nanocomposite Catalysts of the Process of CO2 Hydrogenation. Theor Exp Chem 58, 134–142 (2022). https://doi.org/10.1007/s11237-022-09731-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-022-09731-8