Nanowebs of palladium, platinum, and niobium consisting of nanowires 3–4 nm in diameter with average length of about 200 nm were produced by laser ablation of metallic targets in superfluid helium. When the nanowebs were tested in the oxidation of CO by oxygen at 573 K, an appreciable yield of CO 2 was observed for all the catalysts. According to TEM, palladium and platinum nanowires at this temperature in air disintegrate into chains consisting of individual nanoparticles; the nanowires of niobium remain intact. According to XPS contact between niobium wires and oxygen leads to oxidation of the niobium to Nb 2 O 5 , the palladium nanowires are partially oxidized, while the platinum nanowires remain in the metallic state. Despite the substantial differences in the morphology and structure the activity of the investigated metal nanowires in the oxidation of CO is fairly close.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Quasi-1D metals or fine unidimensional wires (NW) are attracting increasing interest in connection with their unique properties such as high surface/volume ratio, high surface curvature, mechanical elasticity, and conductivity [1–3]. These characteristics open up the possibility of new applications, including nanocatalysis [4–7]. The surface curvature of the active phase is an important parameter that determines the efficiency of nanocatalysts. Therefore, with other conditions (chemical composition, specific surface area, etc.) equal, nanowires of transition metals, like nanoparticles (NP) and nanofilms with nanodimensional folds, are active in catalysis [8].
Oxidation of CO is widely used as a test reaction for solving fundamental problems of heterogeneous catalysis associated with size-dependent characteristics of the nanoparticles, the effect of synthesis procedures, the methods of stabilization and structurization of their surface, and the role of metal–support interaction [9]. Finally, the specific catalytic activity (related to unit surface area) of NWs should not correspond fully to the activity of NPs of similar diameter. The degree of oxidation of the metal, the shape, the roughness, and even the electric charge of the nanostructure can differ for NWs and NPs, and these characteristics can have a considerable effect on the catalytic activity [10–12]. Study of fine wires (quasi-1D metals) produced by laser ablation in superfluid He [13] can be of particular interest for catalysis because their use makes it possible, in principle, to reduce to a minimum the effect of metal–support interaction on the catalytic process by isolating the reaction stage relating directly to the metal in the nanodimensional state.
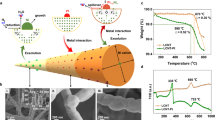
An important advantage of laser ablation in superfluid helium is the unique possibility of growing fine wires of any metals and alloys having a compact structure and regular form. Strictly speaking, the product of our method is a nanoweb representing a three-dimensional network of nanowires of the same (but different for the various metals) thickness linked to each other by electric contacts. The nanowires can subsequently be deposited on various surfaces, including the metal gauzes coated with carbon with apertures of micrometer size that are used in transmission electron microscopes. The web is stretched over these apertures, which in principle makes it possible to study its catalytic activity in the absence of any support. Here the total surface of such a web is large enough when it is used as catalyst to analyze the concentration of the reaction products by the traditional procedures of gas chromatography.
In the present work the oxidation of CO by oxygen with the reagents in the stoichiometric ratio (2% CO, 1% O2 in He) was chosen as a model catalytic reaction. This choice was based mainly on the fact that we had previously studied this reaction on nanowires and nanoparticles of gold and copper [7] with similar diameters. It was shown that considerable heating (to 550 and 600 K in the case of NW and NP respectively) was required when the stoichiometric amount of oxygen was used. These temperatures are of course significantly lower than the melting points T m of the metals usually employed as catalysts, but they are significantly higher than the temperature for unfreezing the mobility of the surface atoms (the Hüttig temperature), which is close to 0.3T m and amounts to approximately to 450 K for gold and copper. For very thin NWs (with diameter of less than 5 nm) this leads to disintegration into chains of individual nanoclusters [13–15] on account of Rayleigh instability.
In [7], therefore, we were possibly dealing with NWs that had already disintegrated into chains of nanoparticles before the reaction began. In any case this investigation showed that Au-Cu(NW) deposited on aluminum oxide is more effective in the catalytic oxidation of CO than Au-Cu(NP) with similar diameter. In the course of successive heating–cooling cycles the Au-Cu(NW) nanowires quickly went on to a stationary regime characterized by an unusually low activation energy (about 20 kJ/mol).
In the present work we decided to compare both the thermal stability and the catalytic activity of palladium, platinum, and niobium produced by laser ablation in superfluid helium. These metals have melting points of 1828, 2041, and 2742 K respectively. The Hüttig temperature for Nb amounts to about 900 K, which gives hope of maintaining the initial morphology of the Nb NWs during catalysis at temperatures right up to 900 K. The Hüttig temperature for Pd and Pt is 550 and 612 K respectively. During the oxidation of CO at temperatures higher than 600 K, therefore, the nanowires from these metals will most likely disintegrate into short fragments (Pt) or even individual particles of the metal (Pd). Thus, the subjects that we selected for investigation should help to mark the differences in catalysis on individual particles of palladium, disconnected short fragments of nanoweb from platinum, and a whole nanoweb of niobium.
EXPERIMENTAL
The Pd, Pt, and Nb wires were produced in an optical liquid-helium cryostat (Institute of Problems of Chemical Physics, Russian Academy of Sciences) with vapor pumping to transfer liquid helium during cooling to the superfluid state. The detailed procedure was described earlier [1]. The nanowires were grown from atoms and small clusters of the metals placed in superfluid helium at 1.7 K by laser ablation from the surface of metal targets immersed in liquid helium as described in [17]. For ablation we used a solid-state waveguide Nd : LSB laser with diode pumping (wavelength 1.06 μm, pulse repetition frequency between 1 and 4 kHz, energy and duration of pulse 10−4 J and 0.4 ns respectively). Plates of Pd, Pt, and Nb (99.999% purity) were used as ablation targets. Quantized vortices were formed as a result of the perturbing action of the laser at its focal point, i.e., at the place and time required for them to be templates for coagulation of the metallic particles.
Standard carbon-coated copper grids (CCG) were used as supports during preparation of the samples for measurements by transmission electron microscopy (TEM). The morphology of the NW/CCG samples without any previous treatment was investigated on a JEOL JEM-2100/UHR transmission electron microscope with resolution of 0.1 nm. The structures of the metals were interpreted by energy-dispersive X-ray analysis using a JED-2300 X-ray spectrometer.
In order to create the NW catalysts the nanoweb was placed on the surface of a thin microporous glass filter 0.1 cm thick and 2.1 cm in diameter. The duration of laser ablation during the formation of the catalyst was about 30 min. In this time about 1·10–6 mol of the metal was deposited on the glass filter, forming a layer of nanoweb 20–30 nm thick. A typical TEM microphotograph of the nanoweb is presented in Fig. 1. The glass filters coated with the NW were used to analyze the degree of oxidation of the deposited metals by X-ray photoelectron spectroscopy (XPS) on an Axis Ultra DLD spectrometer (Kratos Analytical Ltd.) using AlK α radiation (1486.6 eV). The electron binding energy scales were calibrated using an external standard (gold foil, Au4f 7/2 83.96 eV).
In order to study the activity of the Pd, Pt, and Nb nanowires we used the pulsed microcatalytic method normally used for testing catalysts [7, 18, 19]. Oxidation of CO by molecular oxygen was conducted under pulsed mode in a tubular quartz reactor. In typical experiments the NW/glass filter sample was placed in the reactor on a Schott filter (S = 0.785 cm2). Helium was passed through the reactor constantly at atmospheric pressure at a rate of 60 mL/min. When the required temperature had been established in reactor a pulse of 1 mL of the gas mixture (CO : O2: He = 2 : 1 : 97 vol.%) was injected into the helium flow at atmospheric pressure. The composition of the gas at the outlet from the reactor was determined quantitatively by gas chromatography on an LKhM-80 chromatograph with a thermal conductivity detector and a column filled with Porapak Q (length 1 m, internal diameter 2 mm). The conversion of the CO was calculated from the areas of the CO and CO2 peaks by means of calibration curves. Between 10 and 30 reagent pulses were used to determine the steady-state conversion rate of CO at a given temperature (α). The temperature dependence of the conversion of CO, far from its limiting value, reflects the temperature dependence of the rate of the catalytic reaction.
RESULTS AND DISCUSSION
Morphology of Pd, Pt, and Nb Nanowires
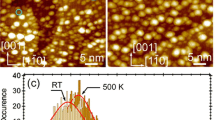
In order to understand how the structure of the NW varies at temperatures typical of those at which the catalytic reaction was conducted the morphology of the initial NW/CCG samples and of samples heated to 623 K in air and held at this temperature for 1 h was investigated. The results are presented in Fig. 2. The left part of Fig. 2 illustrates the morphology of the palladium, platinum, and niobium NWs immediately after their synthesis, and the right part shows the morphology of the same NWs after calcination.
As seen from Fig. 2a, the Pd NWs represent a three-dimensional web cross-linked from individual nanowires. According to the TEM measurements, the palladium wires have a diameter of 4 nm. The average length of the nanowires is about 200 nm. The sample also contains a small number of spherical particles with the size of 30–60 nm (about five particles on an area of 1000×1000 nm). The simultaneous formation of wires and particles is a common phenomenon in the laser ablation of metals [20]. Heating of the Pd NWs leads to disintegration of the metal wires and to aggregation of the disintegrated products to particles with the size of 13–25 nm (Fig. 2b). Here some of the particles are sintered into “enormous” agglomerates measuring 100–200 nm (Fig. 2b). The diameter of the initial platinum wires is 3 nm, and the size of the spherical particles in the NW amounts to 8–20 nm (Fig. 2c). When the Pt NWs are heated the wires 200 nm in length disintegrate into short fragments, and this is followed by aggregation of the individual fragments into clusters measuring 10–16 nm (Fig. 2d).
The niobium wires have a diameter of 3 nm, and the size of the spherical particles in the Nb NWs is 11–25 nm (Fig. 2e). In contrast to palladium and platinum, heating of nanowebs from the high-melting niobium does not lead to appreciable changes in its morphology. This conclusion follows from Fig. 2f. It is seen that heating does not lead either to disintegration of the nanowires or to their aggregation.
Thus, the morphology of the metal nanowires studied in the present work changes after heating in air. At 623 K, which as will be shown later is the characteristic temperature for catalytic oxidation of CO by oxygen, the palladium nanoweb disintegrates completely into chains of clusters, the platinum nanoweb disintegrates into chains of short isolated fragments, while the niobium nanoweb does not undergo any visible changes.
These results are fully understandable since the Hüttig temperature for Pd is lower in comparison with the characteristic reaction temperature of 623 K, for Pt it is only a little higher than 623 K, while for Nb it is almost 300 K higher than the characteristic temperature. It can therefore be expected that, unlike Pt and particularly Pd, disintegration should not occur for the Nb nanoweb. Indeed, the heat treatment of the sample for analysis on the electron microscope was conducted in air, and this differed significantly from the heat treatment in the catalytic tests conducted in a mixture containing only 1% of O2, which was consumed just as quickly in the oxidation of CO. In our opinion, however, these conditions may not be crucial.
Electronic Characteristics of Pd, Pt, and Nb Nanowires
At low temperatures in the absence of contact with oxygen nanowires of any of the metals have through electric conductivity [13]. In order to make sure that presence of the nanoweb in air does not destroy electric contact between the individual wires a nanoweb of platinum was grown between two gold-coated electrodes. After the synthesis the cryostat was heated up to room temperature, and all the gaseous helium in it was replaced with undried air. After holding under these conditions for three months the resistance of the platinum web to electric current remained practically unchanged. The niobium web was subjected to a similar test. As shown in Fig. 3, on heating to room temperature in helium the electric resistance of the niobium nanoweb was finite and small in value; replacement of the helium in the cryostat by air led at once to complete disappearance of conductivity, and this was clearly due to oxidation of the niobium.
Strictly speaking, the catalytic activity can depend on the degree of oxidation of the metal deposited on the surface of the catalyst [8–11]. We note that after heating to room temperature and removal from the cryostat for analysis and/or further use as catalysts the nanowires were in contact with air for a considerable time. Moreover, under the conditions of the catalytic experiments the reactor was heated to fairly high temperatures, and the reaction mixture contained oxygen. The degree of oxidation of the metal in the initial wires and in the wires having contact with oxygen could therefore differ. In order to check this hypothesis the nanowires of the metals were studied by XPS after a long time spent in air at room temperature and after realization of the catalytic reaction.
The XPS spectra of the samples of Pd, Pt, and Nb after contact with air at room temperature are presented in Fig. 4a, b, and c respectively. Note, that the most stable oxides of palladium are PdO and PdO2. Thus, three forms of palladium (PdO, PdO2, and Pd0) can exist in the Pd nanowires that were taken out into the air. According to [21–23], the binding energy (E b) of Pd3d 5/2 electrons is 335.1-335.4 eV for Pd0, 336.8-337.2 eV for PdO, and 337.8-339.3 eV for PdO2. The E b value for Pd3d 5/2 of the Pd nanowires is 335.7 eV (Fig. 4a), which most likely indicates that Pd0 and the oxide PdO are present in the Pd nanowires. According to [23–25], E b of Pt4f 7/2 is 71.2-71.5 eV for Pt, 72.3-73.9 eV for PtO, and 74.1-74.9 eV for PtO2. The E b value of Pt4f 7/2 for Pt nanowires was 71.2 eV (Fig. 4b), and there is therefore every reason to suppose that the Pt nanowires contain zerovalent platinum. The E b value of Nb3d 5/2 for Nb nanowires is 207.1 eV (Fig. 4c), which is close to the value of 207.2 eV for Nb2O5 [23]. It is particularly important to note that niobium is not oxidized to NbO, which has metallic conductivity (and even superconductivity at low temperatures). Instead Nb2O5, which is an insulator, is formed during oxidation.
To our surprise the XPS spectra of the Pd, Pt, and Nb samples after catalysis were identical with the XPS spectra that were recorded for the samples before catalysis (Fig. 4). In our opinion this result indicates that the redox processes occurring during contact between theNWand the reagents (O2 and CO) are reversible. In this case, the electronic state of the metals in the NW returns to the initial state when the NWs are removed from the reactor to record the XPS spectra.
Catalytic Oxidation of CO in the Presence of Pd, Pt, and Nb Nanowires
Figure 5 shows the temperature dependence of the conversion of CO in the presence of palladium, platinum, and niobium NWs on the number of pulses introduced into the reaction mixture. Figure 5a, c, e corresponds to successive increase of the reactor temperature, while Fig. 5b, d, f shows the results obtained with successive decrease of temperature.
For all the metals it is seen that appreciable catalytic activity of their nanowires only appears after heating to 523 K. In the region of 573–623 K at fixed temperature the conversion of CO in the case of palladium increases from pulse to pulse, reaching 95% ± 5% after 7–15 pulses (Fig. 5a). A similar situation arises in the nanowires from Pt and Nb (Fig. 5c, e). More detailed investigations are needed in order to understand such unusual behavior. At present we can only list a few of the possible reasons. According to the TEM data for Pd and Pt NWs their running-in during heating may arise from disintegration of the initialNWat 623 K (Fig. 2a-d). However, as follows already from Fig. 2e, f, the running-in of niobium wires resistant to disintegration cannot be explained by this hypothesis. From [8–10, 21] it is known that the activity of catalysts for the oxidation of CO is strongly affected by the M(0)/M(+n) ratio. Accordingly, the reason for the running-in of the NWs could be the different rates for oxidation of Pd, Pt, and Nb by oxygen and reduction of their oxides by CO. It is not possible also to rule out the possibility that the surface characteristics of the various NWs that determine the strength of adsorption and activation of the reaction substrates and, consequently, the catalytic characteristics of the NW may be modified during contact with the molecules of the reagents and products. Effects of such a type were discussed in [8–10].
Steady-state CO conversion rate of 95% ± 5%after the first pulse is realized for each of the investigated NWs that were heated above 623 K. Essentially it corresponds to a state of thermodynamic equilibrium and, as seen from Fig. 5a, c, e and Fig. 6a, does not depend either on the nature of the metal or on further heating of the catalysts to 723 K. If the temperature is reduced from 723 to 623 K the steady-state conversion of CO is reached likewise after the first addition of the reagents (Fig. 5b, d, f). Further reduction of temperature from 673 to 498 K leads to a decrease of the steady-state CO conversion rate (Fig. 6b). It is worth mentioning that the catalytic activity in the low-temperature region increases when the nanowebs of all the metals are cooled (Fig. 6a, b). Thus, during the cooling regime the CO conversion rate at the Pd, Pt, and Nb nanowires at 523 K is 15%, 15%, and 23% respectively, while the conversion rate during the heating regime is 5%, 6%, and 13% respectively. Further investigations are needed to explain such unusual behavior.
The most important practical result of the present work is the fact that the amount of nanowebs produced by the exotic low-temperature method is sufficient for them to be tested as nanocatalysts under standard conditions by the usual methods of analytical chemistry. The main and unexpected result from the scientific standpoint is the fact that the catalytic activity of Pd, Pt, and Nb nanowires in the oxidation of CO by oxygen is similar and appeared in an identical temperature range. It is necessary to mention that the metals selected as subjects for investigation in the present work are interesting in that their work function differs significantly, amounting to 4.37, 4.98, and 5.42 eV for Nb, Pd, and Pt respectively. However, we did not detect a large difference in the catalytic activity of metallic Pt and Pd. Moreover, Nb cannot be considered in this series because according to the data from XPS niobium is fully oxidized to Nb2O5 under the reaction conditions. Consequently, the mechanism of oxidation of CO at Pt and Pd must differ from the mechanism for Nb2O5 [26].
If we take note of the considerations presented here it seems surprising that the behavior of the NWs in the oxidation of CO is so close both for chains of nanoparticles on the surface of the glass filter, formed during disintegration of the palladium wire, and chains of individual fragments of the fibers (the products from disintegration of the platinum wire) and for the stable long wires of niobium oxide. Such behavior was so unexpected that we repeated blank tests time and again, and they showed that oxidation of CO to CO2 in the reactor in the absence of the nanoweb on the filter did not occur over the whole accessible temperature range. Thus, the catalytic nonspecificity found in the present work is not an artifact. It may be that fundamental causes that we have yet to discover in future investigations are responsible for it.
Nevertheless, there are certain important conclusions that can be made even now about the nature of the high catalytic activity in the nanostructured metals. In particular, the proximity effect, discussed in [27], cannot play a significant role in the efficiency of nanocatalysts. In fact, in contrast to the stochastic distribution of individual nanoparticles immobilized on the support, in the case of the disintegrating nanowires there are always two very close neighbors containing the catalyst. Nevertheless, the oxidation characteristics of stochastically arranged nanoparticles and ordered chains of nanoparticles (the products from disintegration of the nanowires) hardly differ at all.
A most interesting further application of NWs in catalysis is the possibility of controlling the catalytic process in an external electric field by using electrically connected nanowires in place of individual nanoparticles. The influence of the electric field effect may be significant in so far as redox processes contain stages involving exchange of electrons between catalyst and reagents. Theoretical grounds for this effect were presented in [28]. However, we did not find papers in which such an effect would be detected experimentally. The nearest theoretical work was devoted to ab initio calculations of the effect of an electric field on the catalytic oxidation of CO on gold nanoparticles immobilized on graphene [29]. In particular, it was demonstrated in this work that an electric field has effects differing in sign on the adsorption of the reagents and desorption of the products. This means that for a given catalytic reaction and a given transition metal there must exist a value and sign of electric field corresponding to the maximum rate for the process as a whole.
We note in any case that in order to influence the reaction rate the electric field must be too large for use in ordinary chemical reactors. Only in nanocatalysis, on account of the small size of the nanoparticles, can a large value for the force of the electric field be created close to a surface with moderate applied voltage U. Already in the first experiments [7] it had been shown that the nanowebs produced by our method make it possible in principle to create large electric fields during the realization of the catalytic reaction. Actually, as a result of its through conductivity, confirmed by direct measurements [2, 30], an electric potential applied to the edges of the nanoweb must appear at its center even in the absence of a conducting base. This is important since a conducting support weakens the field close to the surface of a web lying on the support by an order of magnitude. Moreover, by using a freely suspended web as cathode, we observed significant field emission of electrons during application of a potential of only 30 V to the anode at a distance of 4 mm [2].
Thus, laser ablation of metallic targets in superfluid helium at 1.7 K gave nanowebs consisting of electrically connected nanowires of Pd, Pt, and Nb. The TEM data show that the diameter of all the nanowires is equal to 3–4 nm, while the average length of the individual nanowires in the nanowebs here amounts to about 200 nm. The webs produced from Pd and Pt disintegrate when heated to 623 K in air with the formation of chains of nanoparticles or fragments of threads 10–40 nm long. The structure of the Nb web remains unchanged when heated from 1.7 to 573 K. According to XPS data the Pd nanowires consist of metal coated with a thin layer of oxide. The Pt nanowires consist entirely of metallic platinum. When the niobium nanowires are heated the metal is oxidized completely to Nb2O5. (During the catalytic reaction partial reduction can occur on account of the low partial pressure of oxygen.)
The catalysts based on Pd, Pt, and Nb exhibit appreciable activity in the oxidation of CO, beginning at 523 K. In the heating regime the running-in of all three catalysts is observed, depending on the number of pulses of the CO + O2 mixture. Thus, in the region of 573–623 K at fixed temperature the conversion of CO increases from pulse to pulse, reaching a steady-state value of 95% ± 5%. Further heating to 723 K does not lead to decrease in the steady-state conversion of CO, which is realized immediately after the first injection of reagents. In the subsequent cooling regime all the catalysts acquire enhanced activity at low temperatures.
An important aim of the work was to determine the direction of future investigations. As found, the main obstacle to the use of nanowebs of transition metals in catalysis is their insufficient thermal stability. Promising, therefore, is the use of nanowires of platinum but not at very high temperatures in the catalytic oxidation ofCOwith air for example. In this case, as shown in [5], the activity of short platinum wires produced by the usual methods is high even at room temperature. An alternative path involves the use of nanowires made from very high-melting metals such as rhenium or rhenium–tungsten alloy [17].
References
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., J. Exp. Theor. Phys., 112, 1061–1070 (2010).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., Appl. Phys. Lett., 101, 052605-1-052605-5 (2012).
A. Loubat, L.-M. Lacroix, A. Robert, et al., J. Phys. Chem. C, 119, 4422–4430 (2015).
S. M. Alia, K. Duong, T. Liu, et al., ChemSusChem, 5, 1619–1624 (2012).
H. Zhu, Z. Wu, D. Su, et al., J. Am. Chem. Soc., 137, 10156–10159 (2015).
Sh. Guo, D. Li, H. Zhu, et al., Angew. Chem. Int. Ed., 52, 3465–3468 (2013).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., Gold Bull., 48, 119–125 (2015).
M. S. Chen and D. W. Goodman, Science, 306, 252–255 (2004).
H.-J. Freund, G. Meijer, M. Scheffler, et al., Angew. Chem. Int. Ed., 50, 10064–10094 (2011).
B. R. Cuenya, Thin Solid Films, 518, 3127–3150 (2010).
O. G. Ellert, M. V. Tsodikov, S. A. Nikolaev, and V. M. Novotortsev, Russ. Chem. Rev., 83, 718–732 (2014).
T. N. Rostovshchikova, E. S. Lokteva, N. E. Kavalerskaya, et al., Teor. Éksp. Khim., 49, No. 1, 37–42 (2013). [Theor. Exp. Chem., 49, No. 1, 40–45 (2013) (English translation).]
E. B. Gordon, A. V. Karabulin, A. A. Morozov, et al., J. Phys. Chem. Lett., 5, 1072–1076 (2014).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., Phys. Chem. Chem. Phys., 16, 25229–25233 (2014).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., J. Phys. Chem. A, 119, 2490–2501 (2015).
A. Volk, D. Knez, P. Thaler, et al., Phys. Chem. Chem. Phys., 17, 24570–24575 (2015).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., Laser Phys. Lett., 12, 096002-1-096002-7 (2015).
S. A. Nikolaev, E. V. Golubina, L. M. Kustov, et al., Kinet. Catal., 55, 311–318 (2014).
S. A. Nikolaev, E. V. Golubina, I. N. Krotova, et al., Appl. Catal. B, 168/169, 303–312 (2015).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., High Energ. Chem., 48, 206–212 (2014).
A. S. Ivanova, E. M. Slavinskaya, R. V. Gulyaev, et al., Appl. Catal. B, 97, 57–71 (2010).
M. Brun, A. Berthet, J. C. Bertolini, J. Electron. Spectrosc. Relat. Phenom., 104, 55–60 (1999).
J. F. Moulder, W. F. Stickle, and P. E. Sobol (eds.), Handbook of X-Ray Photoelectron Spectroscopy, Perkin-Elmer Corporation, Physical Electronics Division, Eden Prairie, MN (1992).
C. R. Parkinson, M. Walker, and C. F. McConville, Surface Sci., 545, 19–33 (2003).
L. K. Ono, J. R. Croy, H. Heinrich, and B. R. Cuenya, J. Phys. Chem. C, 115, 16856–16866 (2011).
S. Royer and D. Duprez, ChemCatChem, 3, 24–65 (2011).
M. Nesselberger, M. Roefzaad, R. F. Hamou, et al., Nat. Mater., 12, 919–924 (2013).
A. Wieckowski, E. R. Savinova, and C. G. Vayenas (eds.), Catalysis and Electrocatalysis at Nanoparticle Surfaces, Marcel Dekker, New York (2003), DOI: 10.1201/9780203912713.
Y. H. Lu and Y. P. Feng, Sci. China. Phys. Mech. Astron., 54, 804–808 (2011).
E. B. Gordon, A. V. Karabulin, V. I. Matyushenko, et al., Low Temp. Phys., 36, 590–595 (2010).
The work was supported by the Russian Science Foundation (project No. 14-13-00574). The catalytic investigations were carried out on apparatus at Moscow State University (Program of MSU development). The authors thank K. I. Maslakov and A. V. Egorov for the XPS and TEM analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 52, No. 2, pp. 75–83, March-April, 2016.
Rights and permissions
About this article
Cite this article
Gordon, E.B., Karabulin, A.V., Matyushenko, V.I. et al. Quasi-1D Metals (Pd, Pt, Nb) as Catalysts for Oxidation of CO. Theor Exp Chem 52, 75–84 (2016). https://doi.org/10.1007/s11237-016-9453-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-016-9453-y