We show that the effect of treatment of supported Mn+-ZrO2 catalysts is different in oxygen and hydrogen plasma. The activating effect of an oxygen plasma is connected with a decrease in the activation energy for dehydrogenation and with a change in the oxidation state of modifier ions. We obtain linear correlations of the catalytic characteristics with the reduction potential and radius of M in different oxidation states.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Today low-temperature plasma is widely used to solve various research and industrial problems [1,2].
The change in chemical composition and structure of the surface layer of the sample after plasma chemical treatment is due to the simultaneous effect of exposure to the plasma emission and the chemically active species formed in the plasma: ions, atoms, radicals, and vibrationally excited molecules. In this case, the penetration depths into the material for all the active species are not greater than a few micrometers. Thus plasma treatment allows us in fact to obtain a new material, retaining the properties of the original material but with substantially different surface characteristics. This fact is used in activation of catalysts and adsorbents [3–5]. On exposure to a plasma, not only does the elemental composition of the surface layer change but also the oxidation state of the atoms and accordingly the oxidation–reduction properties of the treated material can change.
The aim of this work was to study the effect of plasma chemical treatments in an oxygen and hydrogen glow discharge for Mn+/ZrO2 systems with M = Ag+, Cu2+, Au3+ on their catalytic activity in the reaction of dehydrogenation of 2-butanol.
Experimental
The Mn+/ZrO2 samples studied in this work, where M= Ag+, Cu2+, Au3+ at a concentration of 1 wt.%, were obtained by soaking zirconium dioxide in solutions of the corresponding salts Cu(NO3)2, AgNO3, and HAuCl4 followed by heat treatment in a muffle furnace at temperatures of 373 K, 573 K, and 773 K for 3 h. After calcination, oxide phases of the metals are formed on the surface, and a certain color of the samples corresponds to each phase: black (CuO), dark brown (Au2O3), and brownish black (Ag2O).
Plasma chemical treatment was carried out in the burning zone of an oxygen and hydrogen glow discharge in a vacuum flow-through ac (50 Hz) electric discharge apparatus. For this purpose, we used a reactor letting us treat solid samples in the burning zone of the discharge with temperature measurement in parallel. The hollow cavities inside the electrodes were water-cooled during burning of the discharge. Samples were placed on a quartz paddle ina1mmlayer. Flow-through operating conditions were provided by withdrawing gaseous products followed by concentrating them in a low-temperature trap cooled by liquid nitrogen. The sample was pumped out down to a residual pressure of 10–4 torr; the discharge current was 150 mA, and the interelectrode voltage was 1.4 kV. Treatment was carried out for 15 min at 433 K, then the sample was cooled down to room temperature while pumping.
For studying the catalytic properties of Mn+/ZrO2, we used a flow-through apparatus with chromatographic analysis of the products (helium as the carrier gas, flame-ionization detector) after contact of a bubbling 1 : 1 mixture of helium and 2-butanol with 30-mg samples in the temperature range 473–673 K, for partial pressure of the alcohol equal to 1.87 kPa and total flow rate 1.1 L/h. From the temperature dependences of the yields of the alcohol conversion products, we determined the apparent activation energies of the dehydrogenation reaction and the values of the pre-exponential factor in the Arrhenius equation.
The surface acidity of the samples was tested using the adsorption of pyridine (Py) from a solution in octane with initial concentration C 0 Py = 0.3 mmol/L, using the spectrometric method. The Gibbs adsorption was calculated from the formula \( \varGamma =\frac{{({C_0}-C)V}}{m}=\frac{{({A_0}-A)V}}{{m\upvarepsilon l}} \), where the volume of the solution was V = 4 mL, the sample weight was m= 0.01 g, cuvet thickness l = 1 cm, extinction ε = 2∙106 L/mol∙g, A 0 and A are the absorption of the Py solution at the maximum before and after adsorption. The absorption band (λmax = 253 nm) was not shifted in the presence of the adsorbent.
Results and discussion
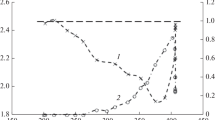
In all the studied samples in the temperature range 490–670 K, only the dehydrogenation reaction occurs, in which methyl ethyl ketone (MEK) is formed. The Mn+/ZrO2 samples are more active than ZrO2, where the activity increases in the series Au < Ag < Cu (Fig. 1).
Since dehydrogenation of alcohol is a redox reaction, it seemed advisable to analyze the effect of Mn+ on the acid (electron-acceptor) properties of the surface from the pyridine adsorption data. From Table 1 and Fig. 2a, we see that the equilibrium Gibbs adsorption of the test material on the original surface ofMn+/ZrO2 increases linearly in the series Au < Ag < Cu, which is consistent with the decrease in the standard reduction potentials \( E_r^0 \) in volts [6] in the series 1.401 (Au3+/Au+) > 0.799 (Ag+/Ag0) > 0.153 (Cu2+/Cu+). The catalytic activity in dehydrogenation of alcohol increases in the same series (Fig. 1, Table 1, Fig. 2a). We note that for gold and copper, the best linear relationship between the rate of MEK formation and the reduction potential for Mn+ is observed for semi-reduced singly-charged forms. Consequently, the value of \( E_r^0 \) can be the criterion for the activity of Mn+/ZrO2, taking into account the different degrees of reduction of Mn+ as the active site for the reaction of dehydrogenation of alcohol, the limiting steps for which are reduction of Mn+ and subsequent re-oxidation. In the case of gold, this involves the steps
The more difficult it is for the active site to be reversibly re-oxidized, the lower is the alcohol dehydrogenation rate.
The experimental activation energy for the butanol dehydrogenation reaction (E a) on the original surface of Mn+/ZrO2 catalysts correlates with the values of the radii (r i ) for the ions Ag+, Cu2+, and Au3+, obtained for crystals in [7], which indicates the importance of the geometric factor especially for adsorption of the substrate on the active site Mn+. From the data in Table 1 and Fig. 2b, it follows that with an increase in r i , we observe a linear decrease in E a.
After plasma chemical treatments, the characteristics of the Mn+/ZrO2 catalysts change, which is connected with the effect of plasma chemical treatment on the oxidation state of the introduced ions. Treatment in an oxygen glow discharge increased the activity of all the samples: by a factor of 2.4 (Au), by a factor of 1.8 (Cu), and by a factor of 1.1 (Ag), while the activity of the support decreased. The greatest increase in activity after O2 plasma chemical treatment was observed for the Au-containing sample, and the series changed to Ag < Au < Cu. The reaction rate and the surface acidity of the activated samples increase linearly with a decrease in the standard reduction potentials for the Mn+ site in the following series: 1.802 (Ag2O2/Ag0) > 1.401 (Au3+/Au+) > 0.521 (Cu+/Cu0). The charge state of the oxidized form of copper changed from Cu2+ to Cu+ after O2 plasma chemical treatment, while silver oxide is represented by the phase Ag2O2. The oxygen plasma has a reducing effect; this is apparent to a greater extent on the copper-containing catalysts [8]. In the case of the low-activity Ag+/ZrO2, under the catalysis conditions strongly adsorbed hydrogen ions accumulate on the surface, and the reduction reaction Ag2O2 + 4H+ + e↔2Ag0 + 2H2O is characterized by a high potential (\( E_r^0 \) = 1.802 V), which hinders re-oxidation of Ag0. Taking into account the changed oxidation state of the catalytically active ions, the activation energy of the dehydrogenation reaction correlates with the ionic radii for only the singly charged ions (Fig. 2b).
In contrast to the oxygen plasma, plasma chemical treatment in hydrogen does not have a substantial activating effect on the deposited phases. The acidity does not depend much on the nature of Mn+. The gold-containing sample has the lowest activity. A linear E a vs. r correlation is observed for the metallic form of the catalytic siteM0. The reduced state of the modifier has low activity in catalysis. We note that treatment in an H2 plasma leads to substantial activation of the support: the yield of MEK increases several-fold as a result of the decrease in the activation energy of the reaction and the increase in the pre-exponential factor (the number of active sites). However, adsorption data does not show an increase in the number of acid (electron-acceptor) sites. A preliminary explanation of this result is formation of new catalysis sites in the form of oxygen vacancies and Zr+3 ions, stabilized by H+.
Thus the experimental data obtained from study of the catalytic activity of Mn+/ZrO2 after plasma chemical treatments, with analysis of the charge state of M, show: 1) the effect of the plasma-forming gas; 2) a linear correlation between the butanol dehydrogenation rate (E a) and the reduction potential of the active site Mn+ for different oxidation states; 3) a linear correlation between E a and the radius of Min oxidized form (original sample), in semi-reduced form (after O2 plasma chemical treatment), and in reduced form (H2 plasma chemical treatment); 4) a many-fold increase in the catalytic activity of the support after H2 plasma chemical treatment.
References
R. P. Oullette, M. M. Barbier, and P. N. Cheremisinoff, Low-Temperature Plasma Technology Applications [Russian translation], Energoizdat, Moscow (1983).
M. G. Berdichevskii and V. V. Marusin, Deposition of Coatings, Etching, and Modification of Polymers Using a Low-Enthalpy Nonequilibrium Plasma [in Russian], Inst. Teplofiziki RAN, Novosibirsk (1993).
E. A. Dadasheva, T. V. Yagodovskaya, L. A. Beilin, et al., Kinet. Katal., 34, 939–941 (1993).
E. A. Dadasheva, T. V. Yagodovskaya, L. A. Beilin, et al., Kinet. Katal., 34, 746–749 (1993).
A. I. Pylinina, I. I. Mikhalenko, A. K. Ivanov-Shits, et al., Zh. Fiz. Khim., 80, 1011–1014 (2006).
V. A. Mikhailov, O. V. Sorokina, E. V. Savinkina, and M. N. Davydova, Chemical Equilibrium [in Russian], Binom, Moscow (2008).
L. T. Bugaenko, S. M. Ryabykh, and A. L. Bugaenko, Vestn. Mosk. Univ. Khim., 49, 363–384 (2008).
A. I. Pylinina, E. P. Dobrova, I. I. Mikhalenko, and T. V. Yagodovskaya, Zh. Fiz. Khim., 79, 650–655 (2005).
We would like to thank T. V. Yagodovskaya (Candidate of Chemical Sciences) from the Laboratory of Catalysis and Gas-Phase Electrochemistry, Chemistry Department of Moscow State University for the plasma chemical treatments of the catalysts. This work was done with the financial support of the Russian Foundation for Basic Research (grant No. 12-03-21168).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Teoreticheskaya i Éksperimental’naya Khimiya, Vol. 49, No. 1, pp. 60–63, January-February, 2013.
Rights and permissions
About this article
Cite this article
Pylinina, A.I., Mikhalenko, I.I. Activation of Cu-, Ag-, Au/ZrO2 Catalysts for Dehydrogenation of Alcohols by Low-Temperature Oxygen and Hydrogen Plasma. Theor Exp Chem 49, 65–69 (2013). https://doi.org/10.1007/s11237-013-9296-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11237-013-9296-8