Abstract
Two species of the trematode genus Phyllodistomum Braun, 1899 (Gorgoderidae) are reported infecting teleost fishes from Moreton Bay, Queensland, Australia. Phyllodistomum hyporhamphi n. sp. is described from two species of garfishes (Hemiramphidae), Hyporhamphus regularis ardelio (Whitley) and H. australis (Steindachner). The new species differs from other marine species of Phyllodistomum in possessing a forebody length less than half that of the body, a body length to width ratio < 4:1, an oral sucker width to ventral sucker width ratio > 1:1 and < 2:1, 7–9 strong, marginal undulations on each side of the body and large, slightly lobed vitelline masses. Phyllodistomum pacificum Yamaguti, 1951 is reported, for the first time in Australian waters, from Pantolabus radiatus (MacLeay) (Carangidae). The new material agrees closely with the original description of P. pacificum, in Carangoides equula (Temminck & Schlegel) off Hamazima, Mie Prefecture, Japan, although the specimens from Moreton Bay are larger than those of the original description (4,575–5,338 × 1,111–1,328 vs 2,200–3,100 × 570–930 μm). Cetiotrema carangis (Manter, 1947) Manter, 1970 is found to be a synonym of Cetiotrema carangis (MacCallum, 1913) Williams & Bunkley-Williams, 1996 and the species is formally moved to Phyllodistomum as P. carangis (MacCallum, 1913) n. comb. Phylogenetic analyses of 28S rDNA data showed that the six marine species of Phyllodistomum for which molecular data are available form a strongly-supported clade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phyllodistomum Braun, 1899 is the most speciose gorgoderid genus, with species infecting marine and freshwater teleost fishes globally. The phylogenetic analysis of Cutmore et al. (2013) demonstrated that species of Phyllodistomum form several different clades, with those infecting marine fishes forming a clade distinct from freshwater species. As the type-species of Phyllodistomum was described from a freshwater fish, Cutmore et al. (2013) speculated that the species infecting marine fishes should ultimately be considered a distinct genus. Species of Phyllodistomum from marine fishes infect the urinary bladder and ureters, with 27 species reported from fishes of 17 teleost families (Ho et al., 2014). Reports incorporating molecular data have shown that marine species of this genus infect at least fishes of the Hemiramphidae, Labridae, Mullidae and Serranidae (Cutmore et al., 2013; Ho et al., 2014), and, although there are no molecular data for the majority of species, there is little evidence to doubt the validity of the other reported marine host families. The richness of this genus is likely to be far greater than presently known, because the infection location means species of this group are seldom sought specifically and are often only collected by chance. Here we report two Phyllodistomum species from Moreton Bay infecting fishes of two teleost families, the Carangidae and the Hemiramphidae.
Materials and methods
Specimen collection and morphological analysis Teleosts were collected by seine and tunnel netting in Moreton Bay, Queensland, Australia. Fish were euthanased and examined for trematodes as described by Cribb & Bray (2010). The urinary bladder was removed from the body cavity, placed in saline solution (0.85% NaCl solution) and opened. Host bodies were then soaked in saline solution for a minimum of 30 min, the supernatant decanted and the resulting sediment examined under a stereomicroscope. Trematodes were washed in vertebrate saline, fixed by pipetting into near-boiling saline, and preserved in 70% ethanol for parallel morphological and molecular characterisation. Some individual worms were processed for both morphological and molecular analysis (hologenophores sensu Pleijel et al., 2008).
Specimens for morphological analysis were washed in fresh water, stained in Mayer’s haematoxylin, destained in a solution of 1.0% HCl and neutralised in 1.0% ammonium hydroxide solution. Specimens were then dehydrated through a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Measurements were made using an Olympus SC50 digital camera mounted on an Olympus BX-53 compound microscope using cellSens Standard imaging software. Measurements are in micrometres (µm) and given as a range followed by the mean in parentheses. Where length is followed by breadth, the two measurements are separated by ‘×’. Drawings were made using an Olympus BX-53 compound microscope and drawing tube. Type- and voucher specimens are lodged in the Queensland Museum (QM), Brisbane. Specimens representing Phyllodistomum sp. 2 of Cutmore et al. (2013) were borrowed from the QM for morphological characterisation.
Molecular sequencing and phylogenetic analysis
Specimens for molecular analysis were processed according to the protocols used by Sun et al. (2014) and Wee et al. (2017). As recommended by Blasco-Costa et al. (2016), three genetic markers were sequenced in this study. The complete ITS2 rDNA region was amplified and sequenced using the primers 3S (Morgan & Blair, 1995) and ITS2.2 (Cribb et al., 1998), the partial D1-D3 28S rDNA region using LSU5 (Littlewood, 1994), 300F (Littlewood et al., 2000), ECD2 (Littlewood et al., 1997) and 1500R (Snyder & Tkach, 2001) and the partial cox1 region using Dig_cox1Fa (Wee et al., 2017) and Dig_cox1R (Wee et al., 2017). Geneious® version 10.2.3 (Kearse et al., 2012) was used to assemble and edit contiguous sequences and the start and end of the ITS2 rDNA region were determined by annotation through the ITS2 Database (Keller et al., 2009; Ankenbrand et al., 2015) using the ‘Metazoa’ model.
ITS2 rDNA sequence data generated during this study were aligned with those of other adult marine species of Phyllodistomum (Table 1) using MEGA version 6 (Tamura et al., 2013) and pairwise differences estimated using the following conditions: “variance estimation method = none”, “model/method = no. of differences” and “substitutions to include = d: transitions + transversions”.
The partial 28S rDNA sequences generated during this study were aligned with sequences of related species from GenBank using MUSCLE version 3.7 (Edgar, 2004) run on the CIPRES portal (Miller et al., 2010), with ClustalW sequence weighting and UPGMA clustering for iterations 1 and 2. The resultant alignment was refined by eye using MESQUITE (Maddison & Maddison, 2017) and the ends of each fragment were trimmed to match the shortest sequence. Bayesian inference and maximum likelihood analyses of the 28S rDNA dataset were conducted to explore relationships among these taxa. Bayesian inference analysis was performed using MrBayes version 3.2.6 (Ronquist et al., 2012) and maximum likelihood analysis using RAxML version 8.2.10 (Stamatakis, 2014), both run on the CIPRES portal. The best nucleotide substitution model was estimated using jModelTest version 2.1.10 (Darriba et al., 2012). The Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) predicted the GTR+Γ and TPM3uf+Γ models as the best estimators, respectively; Bayesian inference and maximum likelihood analyses were conducted using the closest approximation to these models. Nodal support in the maximum likelihood analysis was estimated by performing 100 bootstrap pseudoreplicates. Bayesian inference analysis was run over 10,000,000 generations (ngen = 10,000,000) with two runs each containing four simultaneous Markov Chain Monte Carlo (MCMC) chains (nchains = 4) and every 1,000th tree saved. Bayesian inference analysis used the following parameters: nst = 6, rates = gamma, ngammacat = 4, and the priors parameters of the combined dataset were set to ratepr = variable. Samples of substitution model parameters, and tree and branch lengths were summarised using the parameters sump burnin = 3,000 and sumt burnin = 3,000. Species of Phyllodistomum and Gorgodera Looss, 1899 that infect freshwater fishes and amphibians were designated as functional outgroup taxa, sensu Cutmore et al. (2013).
Results
Gorgoderid trematodes were collected from three species of teleost fishes, Hyporhamphus australis (Steindachner), Hyporhamphus regularis ardelio (Whitley) and Pantolabus radiatus (MacLeay). Specimens from H. australis were previously characterised genetically by Cutmore et al. (2013) as part of a phylogenetic analysis of the family, in which they were reported as an undescribed species, Phyllodistomum sp. 2. These specimens, together with those from H. regularis ardelio, represent a species new to science which is formally described here. The specimens from Pantolabus radiatus are identified as Phyllodistomum pacificum Yamaguti, 1951. This is the first report of this species from Pantolabus radiatus and the first from Australian waters; novel molecular data were generated for this species and a description based on the new specimens is provided.
Family Gorgoderidae Looss, 1899
Genus Phyllodistomum Braun, 1899
Phyllodistomum hyporhamphi n. sp.
Syn. Phyllodistomum sp. 2 of Cutmore et al. (2013)
Type-host: Hyporhamphus regularis ardelio (Whitley), river garfish (Beloniformes: Hemiramphidae).
Other host: Hyporhamphus australis (Steindachner), eastern sea garfish (Beloniformes: Hemiramphidae).
Type-locality: Western Moreton Bay, Queensland, Australia (27°22′S, 153°13′E).
Other locality: Eastern Moreton Bay, Queensland, Australia (27°26′S, 153°24′E).
Site in host: Urinary bladder.
Type-material: Holotype (QM G236796) and 15 paratypes (QM G234204–10, G234212, G236797–03; one hologenophore QM G236800.
Representative DNA sequences: KF013190 (28S rDNA) and KF013150 (ITS2 rDNA) of Cutmore et al. (2013) ex H. australis; ITS2 rDNA, three replicates ex H. regularis ardelio (one submitted to GenBank: MG845600); cox1 mtDNA, one replicate ex H. regularis ardelio (submitted to GenBank: MG845602).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Phyllodistomum hyporhamphi n. sp. is urn:lsid:zoobank.org:act:AAE30852-A3DC-404F-9228-69DB40539AEC.
Etymology: This species is named after the genus of fishes it infects, Hyporhamphus.
Phyllodistomum hyporhamphi n. sp., terminal genitalia (holotype, QM G236796). Abbreviations: AVC, antero-ventral chamber of seminal vesicle; CSD, common sperm duct; ED, ejaculatory duct; GP, genital pore; PDC, postero-dorsal chamber seminal vesicle; PP, pars prostatica; UCh, uterine chamber; UCo, uterine constriction; VS, ventral sucker. Scale-bar: 100 μm
[Based on 16 whole-mounted gravid specimens.] Body spatulate, widest at level of ovary, 1,830–5,080 × 874–2,928 (3,402 × 1,716). Forebody narrow and tapering, 662–1,912 (1,240) long, occupying 31–45 (37)% of body length. Hindbody with 7–9 strong marginal undulations on each side produced by distinct lateral muscular loops, most pronounced in largest specimens. Body length to width ratio 1.72–2.45:1 (2.05:1); ratio smallest in most gravid specimens. Oral sucker subspherical, opens subterminally, with consistent shallow depression anterior to aperture, 259–529 × 198–449 (410 × 346). Ventral sucker far smaller than oral sucker, 135–286 × 145–310 (224 × 235). Oral sucker width to ventral sucker width ratio 1.15–1.80:1 (1.51:1). Intestinal bifurcation midway between oral and ventral suckers. Oesophagus 116–481 (287) long. Caeca blind, slightly sinuous, terminating 344–799 (580) from posterior extremity.
Testes entire, smooth, oblique, in mid-hindbody; anterior testis 135–429 × 145–433 (229 × 264); posterior testis 161–535 × 149–435 (268 × 267). Seminal vesicle saccular, bipartite, with chambers of similar size; dorsal, postero-dorsal chamber dorsal to genital pore, constricting anteriorly to antero-ventral chamber; antero-ventral chamber anterior to genital pore. Cirrus-sac absent. Prostatic chamber and surrounding cells ventral and posterior to antero-ventral chamber of seminal vesicle. Ejaculatory duct short. Genital pore median, 65–283 (192) anterior to ventral sucker.
Ovary subspherical, almost entire but with slight irregular indentations, posterior to ventral sucker and lateral to anterior testis, dextral in 6 specimens and sinistral in 10, 140–328 × 13–440 (231 × 231). Oviduct expands to form distinct chamber prior to entering Mehlis’ gland. Vitelline masses two, entire with slightly irregular outline, immediately posterior to ventral sucker; left mass 100–281 × 129–290 (197 × 185); right mass 114–301 × 125–322 (198 × 194). Laurer’s canal opens dorsally at level of vitelline masses, opposite ovary. Canalicular seminal receptacle absent. Uterus extensive in hindbody, inter- and extracaecal; coils extending posteriorly beyond posterior testis or to just beyond intestinal caeca; uterine chamber distinct and prominent dorsal to ventral sucker and genital pore, filled with eggs in gravid specimens, constricted prior to genital pore, up to 736 in diameter in fully gravid specimens. Eggs develop in utero; largest eggs 33–49 × 17–26 (39 × 21).
Excretory vesicle tubular, extending anteriorly to level of ovary; main collecting ducts extending laterally between vitelline masses and anterior testis and ovary. Excretory pore dorsally subterminal, close to posterior end of body.
Phyllodistomum pacificum Yamaguti, 1951
Type-host: Carangoides equula (Temminck & Schlegel), whitefin trevally (Perciformes: Carangidae).
Type-locality: Off Hamazima, Japan.
New host: Pantolabus radiatus (MacLeay), Fringefin trevally (Perciformes: Carangidae).
New locality: Western Moreton Bay, Queensland, Australia (27°22′S, 153°13′E).
Site in host: Urinary bladder.
Voucher material: Five voucher specimens (QM G236804–8, including one hologenophore QM G236805).
Representative DNA sequences: 28S rDNA, one replicate (submitted to GenBank: MG845599); ITS2 rDNA, one replicate (submitted to GenBank: MG845601); cox1 mtDNA, one replicate (submitted to GenBank: MG845603).
Description (Fig. 1B)
[Based on 4 whole-mounted gravid specimens.] Body lanceolate, widest at level of anterior testis, tapering anteriorly and posteriorly, 4,575–5,338 ×1,111–1,328 (4,950 × 1,226). Forebody 1,443–1,960 (1,709) long, occupying 35–37 (36)% of body length. Hindbody with 4–5 weak marginal undulations on each side, produced by distinct lateral muscular loops. Body length to width ratio 3.44–4.52:1 (3.94:1). Oral sucker subspherical, opens subterminally, with consistent shallow depression anterior to aperture, 312–410 × 310–384 (356 × 337). Ventral sucker slightly smaller than oral sucker, 260–340 × 268–321 (293 × 288). Oral sucker width to ventral sucker width ratio 1.13–1.21:1 (1.17:1). Intestinal bifurcation between oral and ventral suckers, closer to oral than ventral. Oesophagus 301–525 (436) long. Caeca simple, blind, terminating 628–791 (713) from posterior extremity.
Testes slightly lobed, oblique, in mid-hindbody; anterior testis 320–489 × 247–423 (396 × 307); posterior testis 418–598 × 272–487 (501 × 353). Seminal vesicle saccular, bipartite; postero-dorsal chamber large, dorsal to genital pore, constricting anteriorly to antero-ventral chamber; antero-ventral chamber anterior to genital pore. Prostatic chamber and surrounding cells ventral to postero-dorsal chamber of seminal vesicle. Genital pore median, 196–301 (256) anterior to ventral sucker.
Ovary subspherical, entire, posterior to ventral sucker and slightly anterior to anterior testes, dextral in 2 specimens and sinistral in 2, 163–308 × 122–246 (209 × 167). Oviduct expands to form distinct chamber prior to entering Mehlis’ gland. Vitelline masses two, entire, immediately posterior to ventral sucker; left mass 95–174 × 116–186 (126 × 140); right mass 87–160 × 126–192 (113 × 146). Laurer’s canal not detected. Uterus almost entirely intercaecal in hindbody, with extensive coils extending posteriorly to just beyond ends of intestinal caeca; uterine chamber distinct and prominent dorsal to ventral sucker and genital pore, filled with eggs in gravid specimens, constricted prior to genital pore, up to 457 in diameter in fully gravid specimens. Eggs develop in utero; largest eggs 32–51 × 21–32 (42 × 26).
Excretory vesicle tubular, extends anteriorly to level with anterior testis. Excretory pore dorsally subterminal, close to posterior end of body.
Phyllodistomum carangis (MacCallum, 1913) n. comb.
Syns Distomum carangis MacCallum, 1913; Gorgoderina carangis (MacCallum, 1913) Yamaguti, 1971; Cetiotrema carangis (MacCallum, 1913) Williams & Bunkley-Williams, 1996; Phyllodistomum carangis Manter, 1947; Cetiotrema carangis (Manter, 1947) Manter, 1970
Type-host: Caranx crysos (Mitchill), Blue runner (Perciformes: Carangidae).
Type-locality: Off Woods Hole, Massachusetts, USA.
Remarks
There has been some confusion relating to gorgoderids described with the species name ‘carangis’. MacCallum (1913) described Distomum carangis MacCallum, 1913 infecting Caranx crysos (Mitchill) off Woods Hole, Massachusetts, USA. This species was later transferred to a genus of frog-infecting gorgoderids, Gorgoderina Looss, 1902, by Yamaguti (1971). Phyllodistomum carangis Manter, 1947 was described by Manter (1947) in Caranx ruber (Bloch) from the Dry Tortugas, off Florida. There have been several subsequent reports of specimens identified as this species (Bravo-Hollis & Manter, 1957; Winter, 1958; Parukhin, 1976; Violante-González et al., 2016). It was transferred to Cetiotrema Manter, 1970 by Manter (1970) as the second species of that genus. The issue of the two species was considered briefly by Williams & Bunkley-Williams (1996), who considered the two forms to be synonyms. These authors recognised C. carangis (MacCallum, 1913); in our view the full authority for this combination should be C. carangis (MacCallum, 1913) Williams & Bunkley-Williams, 1996.
We agree with Williams & Bunkley-Williams (1996) in considering the specimens reported by Manter (1947) to be synonymous with those reported by MacCallum (1913), however consider the assignment to Cetiotrema erroneous. Manter (1970) argued that the possession of broadly rounded posterior and anterior ends, tri-lobed vitelline masses, narrow, intercaecal uterine coils and a seminal vesicle anterior to the genital pore placed this species in the new genus. However, many of these features are shared by other marine species of Phyllodistomum, and do not justify a position in Cetiotrema. The broadly rounded posterior and anterior ends that are apparent in the type-species, Cetiotrema crassum Manter, 1970, are not homologous with the body shape seen in the illustration of C. carangis, and the uterine coils appear to be restricted to the hindbody in P. carangis, whereas they are well developed in the forebody in C. crassum; the possession of uterine coils in the forebody was noted as one of the key features distinguishing Cetiotrema by Campbell (2008). Furthermore, the forebody is exceptionally short in C. crassum, whereas in P. carangis it resembles the longer forebody seen in all other marine species of Phyllodistomum. Thus, we consider this species as a member of Phyllodistomum [Group C sensu Ho et al. (2014)] and consider the two named species to be subjective synonyms. As a result, we propose the new combination Phyllodistomum carangis (MacCallum, 1913) n. comb.
Phyllodistomum carangis appears to be restricted to the Atlantic and the eastern Pacific (Manter, 1947; Bravo-Hollis & Manter, 1957; Winter, 1958; Violante-González et al., 2016), with just a single report from the Tropical Indo-west Pacific (Parukhin, 1976). Parukhin (1976) reported this species from Scomberoides lysan (Forsskål) [as Chorinemus lysan (Forsskål)] in the South China Sea and the Gulf of Mannar. However, based on the limited data reported by Parukhin (1976) it is impossible to discern whether this record represents P. carangis or the morphologically similar species P. pacificum. Given the locations of the report being in the west Pacific and Indian Ocean, and the type-locality of P. pacificum being off Hamazima, Japan, it seems likely this report actually represents P. pacificum.
Phylogenetic analysis
A preliminary analysis incorporating all available gorgoderid taxa (not shown) identified that all marine species of Phyllodistomum formed a strongly-supported clade to the exclusion of freshwater species of Phyllodistomum, as previously recognised by Cutmore et al. (2013), Pérez-Ponce de León et al. (2015) and Urabe et al. (2015). Consequently, we show here a reduced analysis of the marine Phyllodistomum clade relative to selected freshwater gorgoderid outgroup taxa.
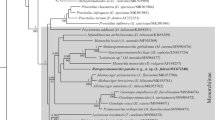
Alignment of the 28S rDNA dataset (Table 1) yielded 1,143 characters for analysis (including indels). Bayesian inference and maximum likelihood analyses resulted in phylograms with almost identical topologies (Fig. 4). In both analyses all marine species of Phyllodistomum formed a strongly-supported clade to the exclusion of the freshwater gorgoderid taxa. In the phylogram resulting from the maximum likelihood analysis, Phyllodistomum sp. 4 and Phyllodistomum sp. 5 formed a clade with P. hoggettae Ho, Bray, Cutmore, Ward & Cribb, 2014 and the asexual stages taken from an infection in the bivalve Lioconcha castrensis (Linnaeus), whereas in the phylogram from the Bayesian inference analysis they formed a clade with P. hyporhamphi, P. pacificum and P. vaili Ho, Bray, Cutmore, Ward & Cribb, 2014. In both analyses, P. pacificum formed a strongly-supported clade with P. vaili, which was sister to P. hyporhamphi. Phyllodistomum pacificum and P. vaili appear to be closely related, differing by just seven bases in the D1-D3 28S analysis; however, these two species differed by 13 bases in the ITS2 region, a level consistent with species-level distinction for trematodes (e.g. Diaz et al., 2015; Cutmore et al., 2016).
Relationships between marine species of Phyllodistomum based on maximum likelihood analysis of the partial 28S rDNA dataset. Maximum likelihood bootstrap support values are shown above the nodes. Bayesian inference posterior probabilities are shown below for relationships also found in the Bayesian inference analysis. Support values < 80 not shown
Discussion
Morphology
Given the genetic distance between clades of marine and freshwater species of Phyllodistomum found by Cutmore et al. (2013), marine species are likely to represent a distinct genus. Therefore, morphological comparisons here are made relative only to marine representatives of the genus. Ho et al. (2014) considered 28 species of Phyllodistomum infecting marine fishes to be valid. They also included P. centropomi Mendoza-Garfias & Pérez-Ponce de León, 2005 because it infects a host known from freshwater, estuarine and marine systems; however, Pérez-Ponce de León et al. (2015) used genetic data to show that this species belongs to one of the freshwater clades, and we thus do not consider the species as part of the marine clade.
Phyllodistomum hyporhamphi n. sp. belongs to Phyllodistomum Group C sensu Ho et al. (2014) in that it possesses a forebody length of less than half of the body length (31–45%) and a body length to width ratio < 4:1 (1.72–2.45:1). The new species is smaller than P. acceptum Looss, 1901, P. carangis, P. lancea Mamaev, 1968, P. marinae Bravo-Hollis & Manter, 1957, P. parukhini Yamaguti, 1971 and P. thalassomum Soheir & Ahmed, 2000 (1,830–5,080 vs 7,200, 13,000, 12,000, 7,060–7,400, 7,500–13,000 and 6,400–10,800 μm, respectively) and has a smaller body length to width ratio than P. carangis, P. hoggettae, P. leilae Nagaty, 1956, P. marinae, P. pacificum, P. thalassomum and P. vaili (1.72–2.45:1 vs 3.25, 2.39–3.60, 3.44, 3.00, 3.33, 3.08 and 2.85–3.85, respectively). The new species has a larger oral sucker width to ventral sucker width ratio than P. acceptum, P. borisbychowskyi Caballero y Caballero, 1969, P. crenilabri Dolgikh & Naidenova, 1968, P. lancea, P. leilae, P. mamaevi Parukhin, 1971, P. pacificum, P. pomacanthi Nahhas & Cable, 1964, P. scrippsi Brooks & Mayes, 1975, P. sobolevi Parukhin, 1979 and P. unicum Odhner, 1902 (1.15–1.80:1 vs 0.95, 0.82, 1.03, 0.87, 0.88, 0.91, 0.95, 0.71, 0.80, 0.89 and 0.95:1, respectively) and a smaller oral sucker width to ventral sucker width ratio than P. mirandai Lamothe-Argumedo, 1969 (1.15–1.80:1 vs 2.6–2.8:1). The new form differs from P. tongaatense Bray, 1985 and P. trinectes Corkum, 1961 in the possession of strong marginal undulations (versus absent or indistinct), and the possession of entire vitelline masses with a slightly irregular outline differentiates it from P. lewisi Srivastava, 1938, which has strongly lobed vitelline masses. Phyllodistomum hyporhamphi differs from the five other species for which molecular data are available by 41–68 bases in the ITS2 rDNA alignment.
The new specimens of P. pacificum agree with the original description of Yamaguti (1951) in all morphological features, especially in the possession of similar-sized oral and ventral suckers, lobed testes, weak marginal undulations and a bipartite seminal vesicle that is antero-dorsal to the genital pore. The specimens from Pantolabus radiatus differ from those infecting Carangoides equula (Temminck & Schlegel) only in overall size, with the new specimens being larger than those described by Yamaguti (1951) (body length 4,575–5,338 × 1,111–1,328 vs 2,200–3,100 × 570–930 μm), and having a larger oral (312–410 × 310–384 vs 160–210 × 170–250 μm) and ventral sucker (260–340 × 268–321 vs 150–200 × 150–230 μm); however, the size of the testes (320–598 × 247–487 vs 260–450 × 170–380 μm), ovary (163–308 × 122–246 vs 150–270 × 100–195 μm) and eggs (32–51 × 21–32 vs 39–51 × 24–39 μm) were similar to those described by Yamaguti (1951).
Phyllodistomum pacificum is morphologically similar to P. carangis, with both species infecting carangids, but the two species differ dramatically in overall size. The type-specimen of P. carangis (from MacCallum, 1913) is much larger than all specimens of P. pacificum (13,000 vs 2,200–5,338 μm), and possesses a larger oral sucker (1,500 × 800 vs 312–410 × 310–384 μm), ventral sucker (900 × 700 vs 260–340 × 268–321 μm), testes (450–850 × 550–570 vs 320–598 × 247–487 μm), ovary (340 × 350 vs 163–308 × 122–246 μm) and vitelline masses (470–520 × 270–370 vs 87–174 × 116–192 μm). Specimens of P. carangis reported by Winter (1958) from the Pacific coast of Mexico were also much larger than any specimens of P. pacificum (total length 10,013–12,665 μm), as is the specimen reported by Manter (1947) (8,061 μm), despite appearing to be not yet fully gravid (having smaller gonads, a less extensive uterus and a uterine chamber not filled with eggs).
Host range of Phyllodistomum spp.
An interesting aspect of Phyllodistomum is the remarkable breadth of teleost families infected. Marine species of Phyllodistomum have been reported from 18 teleost families belonging to multiple feeding guilds. Although comparably wide host distributions have been reported for some other trematode genera, recent genetic studies have shown that some of these patterns are erroneously inflated groups of morphologically similar species (e.g. Neolebouria Gibson, 1976, Prosorhynchoides Dollfus, 1929, Podocotyloides Yamaguti, 1934 and Rhipidocotyle Diesing, 1858) rather than distinct monophyletic clades of related species (see Nolan et al., 2015; Martin et al., 2017, 2018). Interestingly, marine species of Phyllodistomum show no pattern of being linked by their ecology or diet; those that have been shown to be definitively part of the same clade by genetic analysis infect the Carangidae (pelagic piscivores), Hemiramphidae (epipelagic omnivores), Labridae and Mullidae (benthic invertivores) and Serranidae (benthic piscivores).
We speculate that the nature of the urinary bladder as a habitat explains the diverse range of hosts reported for species of Phyllodistomum. Compared with infection locations of other trematode families (i.e. intestinal tract or circulatory system), the urinary bladder is less variable between host families and orders, and we predict that this homogeneity has enabled extensive host switching. Cutmore et al. (2010) also showed that species of another gorgoderid genus have low specificity, reporting Staphylorchis cymatodes (Johnston, 1913) Travassos, 1922 from four families and three orders of elasmobranchs. This is again most likely due to the infection location (i.e. body cavity) being relatively homogenous across elasmobranch lineages.
References
Ankenbrand, M. J., Keller, A., Wolf, M., Schultz, J., & Förster, F. (2015). ITS2 Database V: Twice as much. Molecular Biology and Evolution, 32, 3030–3032.
Blasco-Costa, I., Cutmore, S. C., Miller, T. L., & Nolan, M. J. (2016). Molecular approaches to trematode systematics: ‘best practice’ and implications for future study. Systematic Parasitology, 93, 295–306.
Bravo-Hollis, M., & Manter, H. W. (1957). Trematodes of marine fishes of Mexican waters. X. Thirteen Digenea including nine new species and two new genera from Pacific coast. Proceedings of the Helminthological Society of Washington, 24, 35–48.
Campbell, R. A. (2008). Family Gorgoderidae Looss, 1899. In: Bray, R. A., Gibson, D. I. & Jones, A. (Eds) Keys to the Trematoda, Volume 3. Wallingford: CAB International, pp. 191–213.
Cribb, T. H., Anderson, G. R., Adlard, R. D., & Bray, R. A. (1998). A DNA-based demonstration of a three-host life-cycle for the Bivesiculidae (Platyhelminthes: Digenea). International Journal for Parasitology, 28, 1791–1795.
Cribb, T. H., & Bray, R. A. (2010). Gut wash, body soak, blender and heat-fixation: approaches to the effective collection, fixation and preservation of trematodes of fishes. Systematic Parasitology, 76, 1–7.
Curran, S. S., Tkach, V. V., & Overstreet, R. M. (2006). A review of Polylekithum Arnold, 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitologica, 51, 238–248.
Cutmore, S. C., Bennett, M. B., & Cribb, T. H. (2010). Staphylorchis cymatodes (Gorgoderidae: Anaporrhutinae) from carcharhiniform, orectolobiform and myliobatiform elasmobranchs of Australasia: Low host specificity, wide distribution and morphological plasticity. Parasitology International, 59, 579–586.
Cutmore, S. C., Diggles, B. K., & Cribb, T. H. (2016). Transversotrema Witenberg, 1944 (Trematoda: Transversotrematidae) from inshore fishes of Australia: description of a new species and significant range extensions for three congeners. Systematic Parasitology, 93, 639–652.
Cutmore, S. C., Miller, T. L., Curran, S. S., Bennett, M. B., & Cribb, T. H. (2013). Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n. subfam.). Parasitology Research, 112, 3063–3074.
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9, 772.
Diaz, P. E., Bray, R. A., Cutmore, S. C., Ward, S., & Cribb, T. H. (2015). A complex of species related to Paradiscogaster glebulae (Digenea: Faustulidae) in chaetodontid fishes (Teleostei: Perciformes) of the Great Barrier Reef. Parasitology International, 64, 421–428.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32, 1792–1797.
Ho, H. W., Bray, R. A., Cutmore, S. C., Ward, S., & Cribb, T. H. (2014). Two new species of Phyllodistomum Braun, 1899 (Trematoda: Gorgoderidae Looss, 1899) from Great Barrier Reef fishes. Zootaxa, 3779, 551–562.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161–169.
Kearse, M., Moir, R., Wilson, A., Stones-Havas, S., Cheung, M., Sturrock, S., et al. (2012). Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649.
Keller, A., Schleicher, T., Schultz, J., Müller, T., Dandekar, T., & Wolf, M. (2009). 5.8S-28S rRNA interaction and HMM-based ITS2 annotation. Gene, 430, 50–57.
Littlewood, D. T. J. (1994). Molecular phylogenetics of cupped oysters based on partial 28S rRNA gene sequences. Molecular Phylogenetics and Evolution, 3, 221–229.
Littlewood, D. T. J., Curini-Galletti, M., & Herniou, E. A. (2000). The interrelationships of Proseriata (Platyhelminthes: Seriata) tested with molecules and morphology. Molecular Phylogenetics and Evolution, 16, 449–466.
Littlewood, D. T. J., Rohde, K., & Clough, K. A. (1997). Parasite speciation within or between host species? - Phylogenetic evidence from site-specific polystome monogeneans. International Journal for Parasitology, 27, 1289–1297.
MacCallum, G. A. (1913). Notes on four trematodes parasites of marine fishes. Zentralblatt für Bakteriologie, Parasitenkunde und Infektionskrankheiten, 70, 407–416.
Maddison, W. P., & Maddison, D. R. (2017). Mesquite: a modular system for evolutionary analysis. Version 3.01 http://mesquiteproject.org.
Manter, H. W. (1947). The digenetic trematodes of marine fishes of Tortugas, Florida. American Midland Naturalist, 38, 257–416.
Manter, H. W. (1970). A new genus of trematode (Digenea; Gorgoderidae) from the ureter of tuna fish (Thunnus thynnus maccoyii) in Australia. Transactions of the Royal Society of South Australia, 94, 147–150.
Martin, S. B., Cutmore, S. C., & Cribb, T. H. (2017). Revision of Neolebouria Gibson, 1976 (Digenea: Opecoelidae), with Trilobovarium n. g., for species infecting tropical and subtropical shallow-water fishes. Systematic Parasitology, 94, 307–338.
Martin, S. B., Cutmore, S. C., & Cribb, T. H. (2018). Revision of Podocotyloides Yamaguti, 1934 (Digenea: Opecoelidae), resurrection of Pedunculacetabulum Yamaguti, 1934, and the naming of a cryptic opecoelid species. Systematic Parasitology, 95, 1–31.
Miller, M. A., Pfeiler, E., & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA, pp. 1–8.
Morgan, J. A., & Blair, D. (1995). Nuclear rDNA ITS sequence variation in the trematode genus Echinostoma: An aid to establishing relationships within the 37-collar-spine group. Parasitology, 111, 609–615.
Nolan, M. J., Curran, S. S., Miller, T. L., Cutmore, S. C., Cantacessi, C., & Cribb, T. H. (2015). Dollfustrema durum n. sp. and Heterobucephalopsis perardua n. sp. (Digenea: Bucephalidae) from the giant moray eel, Gymnothorax javanicus (Bleeker) (Anguilliformes: Muraenidae), and proposal of the Heterobucephalopsinae n. subfam. Parasitology International, 64, 559–570.
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755.
Parukhin, A. M. (1976). [Parasitic Worms of Food Fishes in the Southern Seas]. Kiev, USSR: Naukova Dumka, 184 pp (In Russian).
Pérez-Ponce de León, G., Pinacho-Pinacho, C. D., Mendoza-Garfias, M., & García-Varela, M. (2015). Phyllodistomum spinopapillatum sp. nov. (Digenea: Gorgoderidae), from the Oaxaca killifish Profundulus balsanus (Osteichthyes: Profundulidae) in Mexico, with new host and locality records of P. inecoli: Morphology, ultrastructure and molecular evidence. Acta Parasitologica, 60, 298–307.
Pleijel, F., Jondelius, U., Norlinder, E., Nygren, A., Oxelman, B., Schander, C., et al. (2008). Phylogenies without roots? A plea for the use of vouchers in molecular phylogenetic studies. Molecular Phylogenetics and Evolution, 48, 369–371.
Razo-Mendivil, U., Pérez Ponce de León, G., & Rubio-Godoy, M. (2013). Integrative taxonomy identifies a new species of Phyllodistomum (Digenea: Gorgoderidae) from the twospot livebearer, Heterandria bimaculata (Teleostei: Poeciliidae), in Central Veracruz, Mexico. Parasitology Research, 112, 4137–4150.
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539–542.
Snyder, S. D., & Tkach, V. V. (2001). Phylogenetic and biogeographical relationships among some Holarctic frog lung flukes (Digenea: Haematoloechidae). Journal of Parasitology, 87, 1433–1440.
Stamatakis, A. (2014). RAxML Version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30, 1312–1313.
Sun, D., Bray, R. A., Yong, R. Q-Y., Cutmore, S. C., & Cribb, T. H. (2014). Pseudobacciger cheneyae n. sp. (Digenea: Gymnophalloidea) from Weber’s chromis (Chromis weberi Fowler & Bean) (Perciformes: Pomacentridae) at Lizard Island, Great Barrier Reef, Australia. Systematic Parasitology, 88, 141–152.
Tamura, K., Stecher, G., Peterson, D., Filipski, A., & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 30, 2725–2729.
Urabe, M., Ishibashi, R., & Uehara, K. (2015). The life cycle and molecular phylogeny of a gorgoderid trematode recorded from the mussel Nodularia douglasiae in the Yodo River, Japan. Parasitology International, 64, 26–32.
Violante-González, J., Gallegos-Navarro, Y., Monks, S., García-Ibáñez, S., Rojas-Herrera, A. A., Pulido-Flores, G., et al. (2016). Parasites of the green jack Caranx caballus (Pisces: Carangidae) in three locations from Pacific coasts of Mexico, and their utility as biological tags. Revista Mexicana de Biodiversidad, 87, 1015–1022.
Wee, N. Q-X., Cribb, T. H., Bray, R. A., & Cutmore, S. C. (2017). Two known and one new species of Proctoeces from Australian teleosts: Variable host-specificity for closely related species identified through multi-locus molecular data. Parasitology International, 66, 16–26.
Williams, E. H., & Bunkley-Williams, L. (1996). Parasites of Offshore Big Game Fishes of Puerto Rico and the Western Atlantic. San Juan: Department of Biology, University of Puerto Rico, 384 pp.
Winter, H. A. (1958). Trematodos de peces marinos de aquas Mexicanas. XIII. Cuatro digeneos de peces del Oceano Pacifico dos de ellos nuevas especies de la familia Cryptogonomidae Ciurea, 1933. Anales del Instituto de Biología. Universidad de México, 28, 175–194.
Yamaguti, S. (1951). Studies on the helminth fauna of Japan. Part 44. Trematodes of fishes, IX. Arbeiten aus der Medizinischen Fakultät zu Okayama, 7, 247–282.
Yamaguti, S. (1971). Synopsis of Digenetic Trematodes of Vertebrates, Volume 1. Tokyo: Keigaku Publishing Company, 1042 pp.
Acknowledgements
We thank John Page and Dave Thompson for their assistance in the collection of fishes in Moreton Bay, and Dan Huston for assistance with dissections.
Funding
THC and SCC acknowledge the Australian Biological Resources Study (ABRS) for their ongoing support. This study was funded by the ABRS National Taxonomy Research Grant RF215-40.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as urn:lsid:zoobank.org:pub:2F7688C3-2E39-461A-BD22-7C5B4A89DB95. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Digenea.
Rights and permissions
About this article
Cite this article
Cutmore, S.C., Cribb, T.H. Two species of Phyllodistomum Braun, 1899 (Trematoda: Gorgoderidae) from Moreton Bay, Australia. Syst Parasitol 95, 325–336 (2018). https://doi.org/10.1007/s11230-018-9784-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-018-9784-2







