Abstract
A new nematode Parapharyngodon hugoi n. sp. (Oxyuroidea: Pharyngodonidae) is described parasitising the large intestine of the tree frog Trachycephalus typhonius (Linnaeus) (Anura: Hylidae) from the wetlands of Pantanal, State of Mato Grosso do Sul, Brazil. The new species exhibits a unique structure of the posterior cloacal lip in males, which is supported by a rigid V-shaped structure. Parapharyngodon hylidae parasitic in hylid frogs, including T. typhonius, from Mexico, is the most similar congener to P. hugoi n. sp. but is distinguished from the new species by the presence of a gubernaculum (vs absence), by the lateral alae in males ending far anterior to cloacal opening (vs near to it) and because in gravid females the ovaries encircle the oesophageal corpus. Additionally, the new species differs from its congeners as well as from species of Thelandros Wedl, 1862, a very closely related genus, by the combination of features such as spicule length, number of caudal papillae, morphology of the anterior cloacal lip, which is echinate, and position of ovaries. The geographical distribution of hosts seems to play an important role in the speciation process of Parapharyngodon spp.; however, due the lack of molecular data this issue along with the validity of both Thelandros and Parapharyngodon are still questions to be solved in the future, after improvement of the genetic database. A key to the species of Parapharyngodon parasitic in amphibians from the American continent is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Much has been discussed about the synonymy of Parapharyngodon Chatterji, 1933 with Thelandros Wedl, 1862 (e.g. Freitas, 1957; Garcia-Calvante, 1958; Adamson, 1981; Vicente et al., 1993; Anderson et al., 2009). In fact, the boundaries that separate both genera are fragile and still blurry. However, most of the recent studies deal with Parapharyngodon and Thelandros as separate genera (e.g. Bursey & Goldberg, 2015; Velarde-Aguilar et al., 2015; Garduño-Montes de Oca et al., 2016).
Currently, the genus Parapharyngodon in the Neotropical and Caribbean Regions (sensu Proches & Ramdhani, 2012) is composed by 20 species. Of those, five infect anurans, 14 lizards and one has been reported in both groups (Araujo Filho et al., 2015; Bursey & Goldberg, 2015; Velarde-Aguilar et al., 2015; Garduño-Montes de Oca et al., 2016; Ramallo et al., 2016).
During a survey of helminth parasites of amphibians from the Pantanal wetlands, State of Mato Grosso do Sul, Brazil, some nematodes were recovered from the large intestine of the tree frog Trachycephalus typhonius (Linnaeus) (Anura: Hylidae). Detailed morphological examination based on light and scanning electron microscopy (SEM) revealed that the specimens represent a new species of Parapharyngodon, which is described herein.
Materials and methods
One specimen of Trachycephalus typhonius was collected in the Pantanal Biome, municipality of Corumbá, State of Mato Grosso do Sul, Brazil (18°59′S, 56°39′W) and immediately dissected for analysis. Host nomenclature and classification follows Frost (2016). Nematodes were found alive, washed in saline solution (0.9% NaCl), fixed in hot 4% formalin and preserved in 70% ethanol. For measurements and drawings, specimens were cleared in glycerine and observed under a light microscope Olympus BX51 with an attached drawing tube. Measurements are given in micrometres, unless otherwise stated. Two males and two females were taken for scanning electron microscopy (SEM), dehydrated through a graded ethanol series, dried in hexamethyl disilazane, coated with gold and examined in a JEOL JSM-740 1F, at an accelerating voltage of 4 kV. Parasites were deposited in the Coleção Helmintológica do Instituto Oswaldo Cruz (acronym CHIOC) and the host in the Coleção Zoológica de Referência da Universidade Federal do Mato Grosso do Sul (acronym ZUFMS).
Family Pharyngodonidae Travassos, 1920
Genus Parapharyngodon Chatterji, 1933
Parapharyngodon hugoi n. sp.
Type-host: Tree frog Trachycephalus typhonius (Linnaeus) (Anura: Hylidae), 66 mm snout cloacal length (ZUFMS AMP3263).
Type-locality: Pantanal Biome, municipality of Miranda, State of Mato Grosso do Sul, Brazil, near research base of the UFMS (coordinates not available).
Site in host: Large intestine.
Intensity: 19 specimens found in a single frog examined.
Type-material: Holotype and alotype CHIOC (38371a), 12 paratypes (6 males CHIOC and 6 females CHIOC 38371b).
ZooBank registration: To comply with the regulations set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Parapharyngodon hugoi n. sp. is urn:lsid:zoobank.org:act:B6AE93F7-C07C-4C33-81A5-60E217A0A273.
Etymology: The new species is named after Hugo Bisaggio Henriques, son of the first author.
Description (Figs. 1, 2)
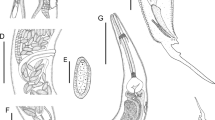
General. Small, whitish nematodes. Cuticle thin, delicate, brittle, with marked annulations, extending from cephalic end to tail region (Fig. 1E, J). Sexual dimorphism evident. Cephalic end of male with triangular mouth aperture internally outlined by cuticular projections, surrounded by 6 well-developed papillose lips: 2 dorsal, 2 ventral, each bearing poorly developed cephalic papillae, and 2 sublateral; sublateral lips bi-lobed, inferior lobe bearing large amphidial pore (Figs. 1C, F, 2A). Cephalic end of female with oval oral aperture internally outlined by cuticular projection, surroudend by 6 flattened lips: 2 dorsal, 2 ventral, each bearing small cephalic papillae, and 2 lateral; lateral lips bearing large amphidial pore (Figs. 1C, G, 2D). Oxyuroid oesophagus muscular, composed of long narrow corpus, followed by short isthmus, connected to well-developed bulb provided with non-sclerotised muscular valve; oesophageal bulb opens into intestine through muscular bi-lobed valve (Fig. 1A, B). Nerve-ring encircling oesophagus at first 1/3 of its length (Fig. 1A, B, E, J). Excretory pore posterior to oesophageal bulb, supported by internal rounded sclerotised plate (Fig. 1A, B, E, F). Lateral alae only in males (Fig. 1A).
Parapharyngodon hugoi n. sp. ex Trachycephalus typhonius . Holotype (male) and allotype (female). A, Anterior extremity of male, ventral view; B, Anterior extremity of female, lateral view; C, D, Cephalic end of male and female, respectively, apical views; E, Female specimen, ventral view; F, G, Cephalic end of male and female, dorsoventral and lateral view, respectively; H, Egg; I, Spicule; J, Male specimen, lateral view; K, L, Tail of male, ventral and lateral views, respectively; M, Vulva, lateral view; N, Tail of female, lateral view
Parapharyngodon hugoi n. sp. ex Trachycephalus typhonius. Scanning electron micrographs. A, Cephalic end of male, apical view (asterisks indicate damaged lateral lip); B, Tail of male, lateral view; C, Detail of double papillae and phasmid in caudal appendage of male; D, Cephalic end of female, apical view; E, Cloacal region, lateral view (arrowhead indicates V-shaped structure supporting the posterior cloacal lip); F, Anterior cloacal lip, subventral view (arrowheads indicate lip flanges); G, Tail of female, lateral view; H, Detail of phasmid in female tail. Abbreviations: a, amphid; l, lateral ala; p, phasmid
Male [Based on 9 adult specimens, measurements of holotype in parentheses.] Body length 2–2.6 (2.5) mm, width at level of excretory pore 154–200 (193). Cuticular annulations about 13 in holotype. Lateral alae beginning at 265–349 (337) from cephalic end, ending at 250–331 (331) from posterior extremity. Oesophageal corpus 395–441 (400) long, 29–36 (35) wide; isthmus 32–38 (35) long, 29–32 (32) wide; bulb 79–99 (98) long, 73–100 (92) wide. Entire length of oesophagus 478–528 (498), representing 19–24 (20)% of total body length. Nerve-ring and excretory pore at 148–171 (171) and 682–874 (855), respectively, from anterior extremity. Testis bents near level of excretory pore, never reaching oesophageal region (Fig. 1J). Caudal alae absent. Anterior cloacal lip echinate, posterior one supported by stark V-shaped structure (Figs. 1K, L, 2E, F). Caudal papillae 3 pairs: 2 pairs adcloacal, 1 subventral and 1 sublateral; third pair fused located ventrally on caudal appendage; pair of small papilliform phasmids slightly anterior to fused papillae (Figs. 1K, L, 2B, C, E, F). Spicules poorly sclerotised, translucent with proximal enlargement and distal sharp point (Fig 1I); 40–58 (51) long, representing 1.7–2.9 (2.0)% of total body length. Posterior extremity of body bearing ventrally directed caudal appendage, ending in thin tip, 86–121 (109) long (Figs. 1K, L, 2B).
Female [Based on 9 adult ovigerous specimens, measurements of alotype in parentheses.] Body length 3.6–5.3 (4.2) mm, width at level of vulva 302–497 (369). Cuticular annulations about 15 in holotype. Oesophageal corpus 578–656 (400) long, 38–42 (38) wide; isthmus 31–35 (31) long, 35–38 (38) wide; bulb 108–135 (108) long, 117–158 (117) wide. Entire length of oesophagus 699–776 (707), representing 15–20 (17)% of total body length. Nerve-ring and excretory pore at 138–162 (162) and 1,000–1,400 (1,100), respectively, from anterior extremity. Vulval lips not elevated, followed by short vagina and muscular ovijector (Fig. 1M). Vulva in anterior half of body, 1.7–2.5 (1.9) mm from anterior end, at 44–48 (44)% of body length. Uterus amphidelphic. Ovaries never encircling oesophageal corpus (Fig. 1E). Eggs widely ellipsoid, thin shelled, non-embryonated, with subterminal single operculum (Fig. 1H), measuring 136–149 × 62–68. Tail conical, with long, narrow, pointed appendage, bearing small lateral phasmids located at its posterior half, visible only in SEM micrographs (Fig. 2G, H). Distance from anus to posterior extremity of body 361–593 (405).
Remarks
The present specimens were placed in Parapharyngodon exclusively because the eggs possess a single subterminal operculum and were non-embryonated in all females analysed. These are the main features differentiating Parapharyngodon from Thelandros according to the most recent publications (Bursey et al., 2013; Velarde-Aguilar et al., 2015).
Eighty-two nominal species have been assigned to Parapharyngodon, but according to recent publications only 51 are valid (Araujo Filho et al., 2015; Bursey & Goldberg, 2015; Velarde-Aguilar et al., 2015; Garduño-Montes de Oca et al., 2016; Ramallo et al., 2016). Thus, we compared our material with all of the 82 species emphasising those parasitic in amphibians as well as those from the American continent.
Besides the new species, other two congeners have been reported parasitising the tree frog T. typhonius namely, P. duniae Bursey & Brooks, 2004 in Costa Rica and P. hylidae Velarde-Aguilar, Mata-López, Guillén-Hernandez & León-Règagnon, 2015 in Mexico. Parapharyngodon hugoi n. sp. resembles P. hylidae biometrically and in many morphological aspects, but differs from the latter by the position of ovaries in large females (not encircling oesophageal corpus vs encircling it), by the posterior extension of lateral alae in males (ending near cloacal region vs ending far anterior to it), because the new species lacks a gubernaculum, and mainly based on differences in the structure of the postcloacal lip that is supported by a stark V-shaped pointed structure in P. hugoi n. sp. (vs bearing a pedunculate, cylindrical projection with apical ornamentations in P. hylidae) (Velarde-Aguilar et al., 2015). The new species also differs from P. duniae based on the position of the excretory pore in females that is well posterior to oesophagus end in P. hugoi n. sp. and anterior to the oesophageal bulb in P. duniae (see Bursey & Brooks, 2004).
Seven other species of Parapharyngodon have been described in amphibians, P. garciai (Schmidt & Whittaker, 1975) parasitic in Eleutherodactylus portoricensis Schmidt from Porto Rico, P. japonicus Bursey & Goldberg, 1999 in Onychodactylus japonicus (Houttuyn) from Japan and P. silvoi have smooth-edge anterior cloacal lip in males (Schmidt & Whittaker, 1975; Bursey & Goldberg, 1999; Araujo Filho et al., 2015) different from the same structure in P. hugoi n. sp., which is clearly echinate. Furthermore, P. japonicus has the caudal appendage reduced in males and rounded tail ending in a stout spike in females (Bursey & Goldberg, 1999), different from P. hugoi n. sp. that has long thin appendages in both male and female tails. Parapharyngodon garciai and P. silvoi, along with P. chamelensis Velarde-Aguilar, Mata-López, Guillén-Hernandez & León-Règagnon, 2015 parasitic in Diaglena spatulata (Günther) from Mexico, P. grenadaensis Bursey, Drake, Cole, Sterner, Pinckney & Zieger, 2013 in Rhinella marina (Linnaeus) from Grenada and P. osteopili Adamson, 1981 in Osteopilus sptentrionalis (Duméril & Bibron) from Cuba have different number of caudal papillae than the new species (other than three pairs) (Schimidt & Whittaker, 1975; Adamson, 1981; Bursey at al., 2013; Araujo-Filho et al., 2015; Velarde-Aguilar et al., 2015). In females of P. grenadaensis and P. osteopili the ovaries are pre-bulbar, encircling the oesophageal corpus, males of P. chamelensis have a gubernaculum and those of P. osteopili lack the lateral alae (Adamson, 1981; Bursey at al., 2013; Velarde-Aguilar et al., 2015).
Parapharyngodon alvarengai Freitas, 1957 originally described as a parasite of the lizard Trachylepis atlantica (Schmidt), has been recorded once parasitising the toad Rhinella icterica (Spix) (Luque et al., 2005). This species differs from P. hugoi sp. in the morphology of anterior cloacal lip (smooth vs echinate), in the length of spicule (80–100 vs 40–58 µm), in the position of ovaries (anterior to oesophageal bulb vs posterior to it) and in the structure of tail in females (ending in a stout spike vs conical long thin appendage).
The remaining 17 species of Parapharyngodon in the American continent are all parasites of lizards; they differ from P. hugoi n. sp. as follows. Parapharyngodon ayotzinapensis Garduño-Montes de Oca, Mata-López, León-Règagnon, 2016, P. bainae Pereira, Sousa & Souza Lima, 2011, P. guerreroensis Bursey & Goldberg, 2015, P. grismeri Bursey & Goldberg, 2007, P. riojensis Ramallo, Bursey & Goldberg, 2002, P. sanjuanensis, P. senisfaciecaudus Freitas, 1957, P. sceleratus (Travassos, 1923) and P. tikuinii Garduño-Montes de Oca, Mata-López, León-Règagnon, 2016 have longer spicule than the new species (minimum 70 vs 40–58 µm). Parapharyngodon californiensis (Read & Amrein, 1952), P. largitor Alho & Rodrigues, 1963, P. maestro Jiménez, León-Règagnon & Pérez-Ramos, 2008, P. ocalaensis Bursey & Telford, 2002 and P. verrucosus Freitas & Dobbin, 1959 have males bearing the anterior cloacal lips with smooth edges. Females of Parapharyngodon colonensis Bursey, Goldberg & Telford, 2007, P. iguanae Telford, 1965 and P. lamothei Jiménez, León-Règagnon & Pérez-Ramos, 2008 have the ovaries encircling the oesophageal corpus. Furthermore, males of P. colonensis and P. lamothei have four and three pairs of caudal papillae, respectively, plus an unpaired one (vs only three pairs in the new species). Males of P. iguanae also have the lateral alae beginning at midlength of body (vs at level of oesophageal corpus in P. hugoi n. sp.).
Due to the taxonomic proximity and possible confusions when separating species within Parapharyngodon and Thelandros, the newly collected material was compared with the species assigned to the former genus. Currently, there are only three species of Thelandros parasitic in amphibians. Thelandros salamandrae (Schad, 1960), a parasite of salamanders from the genus Aneides Baird in New Mexico and California, differs from P. hugoi n. sp. because males lack lateral alae, the cloacal lip is smooth and they have four pairs of caudal papillae (Schad, 1960). The other two species namely, T. minutus Read & Amrein, 1952 and T. magnavulvaris (Rankin, 1937) were incompletely described, and the male of T. magnavulvaris is still unknown (Rankin, 1937; Read & Amrein, 1952). Thus, according to Bursey & Goldberg (2005) the referred species should be considered as species inquirendae.
Four additional species of Thelandros have been reported in the American continent, T. capacyupanquii (Freitas, Vicente & Ibañez, 1968) parasite of the lizard Dicrodon holmbergi Schmidt in Bazil (Freitas et al., 1968), differs from P. hugoi n. sp. based upon many features such as males with four pairs of caudal papillae (vs three pairs), longer spicule (120–130 vs 40–58 µm) and precloacal lip of smooth edge (vs echinate edge), and females with tail ending in short filament (vs long thin conical filament) (Freitas et al., 1968). The three remaining species have been reported as parasites of lizards from North America, i.e. T. bicaudatus Read & Amrein, 1952, T. pseudothaparius Lucker, 1951 and T. xantusi Lucker, 1951, all clearly differing from the new species by having a longer spicule (minimum 125 µm long vs 40–58 µm) (Read & Amrein, 1952; Telford, 1965). Moreover, females of T. bicaudatus and T. xantusi have the tail ending in short filament and males of T. pseudothaparius have anterior cloacal lip with smooth edge as in T. capacyupanquii (see Read & Amrein, 1952; Telford, 1965).
Considering all the differences discussed above, we strongly believe that P. hugoi n. sp. undoubtedly represents a new taxon. A taxonomic key to the species of Parapharyngodon parasitising amphibians from the American continent is provided.
Key to the species of Parapharyngodon parasitic in amphibians from the American continent
-
1a
Anterior cloacal lip in males with smooth edge ……………………………………………. 2
-
1b
Anterior cloacal lip in males with echinate edge …………………………………………… 4
-
2a
Males with more than three pairs of caudal papillae ……………………………………… 3
-
2b
Males with three pairs of caudal papillae …………………………………… P. alvarengai
-
3a
Males with four pairs of caudal papillae …………………………………………. P. garciai
-
3b
Males with four pairs of caudal papillae plus unpaired one …………………. P. silvoi
-
4a
Males with three pair of caudal papillae ………………………………………………………. 5
-
4b
Males with more than three pairs of caudal papillae ……………………………………… 6
-
5a
Lateral alae in males ending far anterior to cloacal region ………………… P. hylidae
-
5b
Lateral alae in males ending in the level of cloacal opening ……….. P. hugoi n. sp.
-
6a
Males with three pairs of caudal papillae plus unpaired one …………………………… 7
-
6b
Males with four pairs of caudal papillae ………………………………………………………. 8
-
7a
Gubernaculum present ………………………………………………………….. P. chamelensis
-
7b
Gubernaculum absent …………………………………………………………………… P. duniae
-
8a
Females with vulva conspicuously prominent ………………………….. P. sanjuanensis
-
8b
Females without prominent vulva ……………………………………………………………….. 9
-
9a
Males with lateral alae ………………………………………………………… P. grenadaensis
-
9b
Males without lateral alae …………………………………………………………… P. osteopili
Discussion
It is quite challenging to deal with the taxonomy of Parapharyngodon and Thelandros due their great morphological proximity, unclear boundaries and considerable number of poorly described species. From all the features that have been used to separate both genera, e.g. presence/absence of lateral alae in males and structure of the tail in both sexes, the most reliable seem to be the position of the operculum in eggs (i.e. terminal or subterminal) as well as the stage of embryonic development during posture (see Bursey et al., 2013). Molecular approaches may represent an important tool for the resolution of the real relationships between Parapharyngodon and Thelandros. However, the current data are scarce and the phylogenetic approaches have been superficial, inconclusive and with dubious morphological identification of some species (see Chaudary et al., 2013 as an example).
Species of Parapharyngodon seem to exhibit low host specificity, since a single species may parasitise several different hosts from different families and, conversely more than one parasite of the genus may occur a single host (Vellarde-Aguilar et al., 2015; Garduño-Montes de Oca et al., 2016). In this sense, the host range of P. alvarengai represents an interesting finding because this is the only species originally described parasitising a lizard (Freitas, 1957) that was also reported in an amphibian (Luque et al., 2005); however, it cannot be discarded that Luque et al. (2005) misidentified the nematode since their study was focused on ecology and not on taxonomy.
Parapharyngodon hylidae, the congener most closely related to the new species, was also reported as a parasite of the tree frog T. typhonius, but in Mexico (Velarde-Aguilar et al., 2015). According to Chabaud & Brygoo (1962), the specificity of nematodes parasitic in reptiles is generally low, and the geographical distribution of their hosts plays an important role in the speciation process. Our results seem to support this assertion, even though further genetic evaluation is highly recommended.
Finally, we agree with Velarde-Aguilar et al. (2015) that the description of cephalic structures (i.e. cephalic papillae, amphids, lips) in Parapharyngodon is still poorly detailed, and several species lack studies using SEM of these structures. The cephalic end in Parapharyngodon bears important intraspecific morphological features and usually shows sexual dimorphism; thus an accurate description of this region is highly recommended (Velarde-Aguilar et al., 2015).
Parapharyngodon hugoi n. sp. represents the first species in the genus with postcloacal lip supported by a V-shaped structure, and the first reported parasitising an amphibian in the Pantanal wetlands, Brazil.
References
Adamson, M. L. (1981). Parapharyngodon osteopili n. sp. (Pharyngodonidae: Oxyuroidea) and a revision of Parapharyngodon and Thelabdros. Systematic Parasitology, 3, 105–117.
Anderson, R. C., Chabaud, A. G., & Willmott, S. (2009). Keys to the nematode parasites of vertebrates: Archival volume. Wallingford: CABI Publishing. 480 pp.
Araujo-Filho, J. A., Brito, S. V., Almeida, W. O., Morais, D. H., & Ávila, L. W. (2015). A new species of Parapharyngodon (Nematoda: Pharyngodonidae) infecting Dermatonotus muelleri (Anura: Microhylidae) from Caatingta, Northeastern Brazil. Zootaxa, 4012, 386–390.
Bursey, C. R., & Brooks, D. R. (2004). Parapharyngodon duniae n. sp. (Nematoda: Pharyngodonidae) in Phrynohylas venulosa (Anura: Hylidae) from the Area de Conservación Guanacaste, Guanacaste, Costa Rica. Journal of Parasitology, 90, 137–139.
Bursey, C. R., Drake, M., Cole, R., Sterner, M., Pinckney, R., & Zieger, U. (2013). New species of Parapharyngodon (Nematoda: Pharyngodonidae) in Rhinella marina (Anura: Bufonidae) from Grenada, West Indies. Journal of Parasitology, 99, 475–479.
Bursey, C. R., & Goldberg, S. R. (1999). Parapharyngodon japonicas sp. n. (Nematoda: Pharyngodonidae) from the Japanese Clawed Salamander, Onychidactylus japonicas (Caudata: Hynobiidae), from Japan. Journal of the Helminthological Society of Washington, 66, 180–186.
Bursey, C. R., & Goldberg, S. R. (2005). Two new species of Pharyngodonidae (Nematoda: Oxyuroidea) and other nematodes in Agama caudospina (Squamata: Agamidae) from Kenya, Africa. Journal of Parasitology, 91, 591–599.
Bursey, C. R., & Goldberg, S. R. (2015). Description of a new species of Parapharyngodon (Nematoda: Pharyngodonidae) from Mexico, with a list of current species and key to the species from the Panamanian Region. Journal of Parasitology, 101, 374–381.
Chabaud, A. G., & Brygoo, E. R. (1962). Nématodes parasites de caméléons malgaches. Deuxième note. Annales de Parasitologie Humaine et Comparée, 37, 569–602.
Chaudary, A., Kansal, G., Singh, N., & Singh, H. S. (2013). New molecular data for parasites Hammerschmidtiela indicus and Thelandros sceleratus (Nematoda: Oxyurida) to infer phylogenetic position. Turkish Journal of Zoology, 38, 1311–1318.
Freitas, J. F. T. (1957). Sôbre os gêneros Thelandros Wedl, 1862 e Parapharyngodon Chatterji, 1933, com descrição de Parapharyngodon alvarengai sp. n. (Nematoda, Oxyuroidea). Memórias do Instituto Oswaldo Cruz, 55, 21–45.
Freitas, J. F. T., Vicente, J. J., & Ibañez, H. N. (1968). Fauna helmintológica do Perú: Parathelandros capacyupanquii sp. n., parasito de Dicrodon holmergi Schmidt, 1957 (Nematoda, Oxyuroidea). Atas da Sociedade de Biologia do Rio de Janeiro, 11, 217–219.
Frost, D. R. (2016). Amphibian species of the world. World Wide Web electronic publication. http://research.amnh.org/herpetology/amphibia/index.html, version 6.0.
Garcia-Calvante, I. (1958). Revisión del gênero Parapharyngodon y descripción de nuevas espécies. Revista Ibérica de Parasitologia, 8, 367–410.
Garduño-Montes de Oca, E. U., Mata-López, R., & León-Règagnon, V. (2016). Two new species of Parapharyngodon parasites of Sceloporus pyrocephalus, with a key to the species found in Mexico (Nematoda, Pharyngodonidae). ZooKeys, 559, 1–16.
ICZN (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Zootaxa, 3450, 1–7.
Luque, J. L., Martins, A. N., & Tavares, L. E. R. (2005). Community structure of metazoan parasites of the yellow Cururu toad, Bufo ictericus (Anura, Bufonidae) from Rio de Janeiro, Brazil. Acta Parasitologica, 50, 215–220.
Proches, S., & Ramdhani, S. (2012). The world’s zoogeographical regions confirmed by cross-taxon analyses. BioScience, 62, 260–270.
Ramallo, G., Bursey, C., Castillo, G., & Acosta, J. C. (2016). New species of Parapharyngodon (Nematoda: Pharyngodonidae) in Phymaturus spp. (Iguania: Liolaemidae) from Argentina. Acta Parasitologica, 61, 461–465.
Rankin, J. S. (1937). New helminths from North Carolina salamanders. Journal of Parasitology, 23, 29–42.
Read, C. P., & Amrein, Y. U. (1952). Some new oxyurid nematodes from southern California. Journal of Parasitology, 38, 379–384.
Schad, G. A. (1960). The genus Thelandros (Nematoda: Oxyuroidea) in North American salamanders, including a description of Thelandros salamandrae n. sp. Canadian Journal of Zoology, 38, 115–120.
Schmidt, G. D., & Whittaker, F. H. (1975). Nematode parasites of Puerto Rico tree frogs, Eleutherodactylus spp.: two new species and proposal of Poekilostrongylus gen. nov. (Trichostrongylidae). Parasitology, 70, 287–294.
Telford, J. S. R. (1965). Some Thelandros (Nematoda: Oxyuridae) from Southern California lizards. Japanese Journal of Experimental Medicine, 35, 463–472.
Velarde-Aguilar, M. G., Mata-López, R., Guillén-Hernández, S., & León-Règagnon, V. (2015). Parapharyngodon n. spp. (Nematoda: Pharyngodonidae) parasites of hylid frogs from Mexico and review of species included in the genus. Journal of Parasitology, 101, 212–230.
Vicente, J. J., Rodrigues, H. O., Gomes, D. C., & Pinto, R. M. (1993). Nematóides do Brasil. Parte III: Nematóides de répteis. Revista Brasileira de Zoologia, 10, 19–168.
Acknowledgements
The authors are thankful to Dr Tomáš Scholz from the Institute of Parasitology, Biology Centre of the Czech Academy of Sciences (IPCAS) and the Staff of the Laboratory of Electron Microscopy from the same institute (IPCAS; RVO: 60077344) and the Czech Science Foundation (project No. P505/12/G112) for the support and for covering costs of SEM observations at the IPCAS.
Funding
Felipe B. Pereira was supported by a post-doctoral fellowship PNPD-CAPES (Programa Nacional de Pós-Doutorado-Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-CAPES, Brazil), José L. Luque (Nos. 474077/2011-0, 304254/2011-8, 402665/2012-0) and Luiz E. R. Tavares (No 311567/2013-4) were supported by a Research fellowship from CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico do Brasil).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed, in which the procedures were according to the rules of the Ethical Committee of the Universidade Federal do Mato Grosso do Sul (acronym CEUA/UFMS; protocol 435/2012).
Additional information
This article was registered in the Official Register of Zoological Nomenclature (ZooBank) as 3BD1DC0F-9502-46EF-8050-9997615DB798. This article was published as an Online First article on the online publication date shown on this page. The article should be cited by using the doi number. This is the Version of Record.
This article is part of the Topical Collection Nematoda
Rights and permissions
About this article
Cite this article
Pereira, F.B., Campião, K.M., Luque, J.L. et al. Parapharyngodon hugoi n. sp., a new nematode (Oxyuroidea: Pharyngodonidae) of the tree frog Trachycephalus typhonius (Linnaeus) from the Brazilian Pantanal, including a key to the congeners from amphibians of the American continent. Syst Parasitol 94, 599–607 (2017). https://doi.org/10.1007/s11230-017-9725-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-017-9725-5