Abstract
A new nematode species, Proparasitylenchus californicus n. sp., is described from the intertidal rove beetle Tarphiota geniculata (Mäklin) (Coleoptera: Staphylinidae) in California, USA. The new species differs from European representatives of the genus by possessing a cleft stylet in both sexes. The parasitic female is ovoviviparous and produces numerous juveniles that moult twice in the beetle host, then exit and moult twice to the adult stage in the environment. After mating, the free-living fertilised females enter a new host. Heavy infections sterilise the beetles. This is the first record of the genus Proparasitylenchus Wachek, 1955 in the New World and the first allantonematid parasite of a marine, intertidal beetle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nematodes in the family Allantonematidae Pereira, 1931 are cosmopolitan parasites of insects and arachnids and have a fossil record dating back some 40–50 mya (Siddiqi, 2000; Poinar, 2011). They most frequently occur in holometabolous insects, especially Diptera and Coleoptera. During ecological studies on intertidal invertebrates inhabiting the beaches in Santa Barbara, California (USA), a nematode was discovered infecting the intertidal rove beetle Tarphiota geniculata (Mäklin, 1852) (Coleoptera: Staphylinidae) (Figs. 1, 2). An examination of the nematodes revealed that they were a new species in the genus Proparsitylenchus Wachek, 1955 and a description follows.
Materials and methods
Host beetles were dissected in 1% saline solution and the juvenile parasitic nematodes were released into the solution. In two weeks, the nematodes had matured to the adult stage and mated. The living stages were examined and then the nematodes were killed in a heated 70°C saline solution, fixed in 5% formalin and mounted in 100% glycerin. Measurements and photographs were made with a Nikon stereoscopic microscope SMZ-10-R at a magnification of ×80 and a Nikon Optiphot microscope at ×1000. All measurements are in micrometers unless otherwise specified and are presented as the range followed by the mean in parentheses.
Order Tylenchida Thorne, 1949
Family Allantonematidae Pereira, 1937
Genus Proparasitylenchus Wachek, 1955
Proparasitylenchus californicus n. sp.
Type-host: Tarphiota geniculata Mäklin (Coleoptera: Staphylinidae).
Type-locality: Goleta Beach, California, USA.
Type-host: Tarphiota geniculata Mäklin (Coleoptera: Staphylinidae).
Type-material: Free-living female (USDANL T-688t) and paratype (free-living male USDANL T-6545p) deposited in the USDA Nematode Laboratory, Beltsville, Maryland, USA. Paratypes deposited in the Poinar collection.
Etymology: The specific epithet refers to the geographical location of the host.
Description (Figs. 3–12)
General. Nematodes bisexual, cycle homogonic (parasitic cycle only - no alternating free-living or plant-feeding cycle); lips not offset; stylet bases cleft; cuticle with fine annulations; gonads outstretched; tail of free-living adults pointed. Females with body straight when fixed; with well-developed dorsal and paired subventral pharyngeal glands; post-vulvar extension present; vulva posteriorly located. Males with body “banded” and the posterior end curved ventrally when fixed; dorsal and paired subventral pharyngeal glands weakly developed, bursa wide, lateral edge entire; spicules paired, slightly curved, shaft narrow but with wide head; gubernaculum slender, short; genital papillae absent.
Free-living infective stage female (n = 10) (Figs. 3–6). Length 594–832 (612); greatest body width 16–27 (22); stylet length 14–18 (16); head to nerve-ring 61–85 (78); head to excretory pore 72–115 (97); head to gland reservoirs 35–42 (39); tail length 24–38 (33), % vulva 77–90 (85); length of post-vulvar extension 8–32 (15).
Free-living male (n = 10) (Figs. 7–11). Length 515–872 (659); body width 16–30 (21); stylet length 8–13 (10); distance from head to nerve-ring 51–85(68); distance from head to excretory pore 77– 101 (93); length of spicules 16–21 (19); length of gubernaculum 3–6 (4), tail length 19–35 (28); bursa peloderan, 38–51 (44) long.
Parasitic female (n = 4) (Fig. 12). Body white, swollen, sausage-like with head and tail not constricted from rest of body; ovoviviparous; tail rounded; anus present; vulva subterminal; body length 740–900 (817); greatest width 63–90 (79). The bodies of the parasitic females are usually curved, sometimes very tightly and forming two coils.
Discussion
Species in four genera of allantonematids are known to parasitise staphylinid beetles, e.g. Proparasitylenchus Wachek, 1955, Parasitylenchoides Wachek, 1955, Allantonema Leuckart, 1884 and Metaparasitylenchus Wachek, 1955. Until the present, all infestations have been reported in Europe (Wachek, 1955; Lipkow, 1968; Poinar, 1975; Siddiqi, 2000). Species of Parasitylenchoides and Metaparasitylenchus have dark-yellow to brown parasitic females. The parasitic females of Allantonema are bean-shaped and the lips of the free-living males and females are offset. The above characters are absent in the present species.
Parasitic females of Proparasitylenchus are white to light yellow, the lips of the free-living adults are usually not offset and the stylets of the free-living stages have basal enlargements (Wachek, 1955; Poinar, 1975, 1977; Siddiqi, 2000). Wachek (1955) assigned seven species to the genus Proparasitylenchus, one of which, P. boopini (Wachek, 1955), Siddiqi (2000) transferred to the genus Metaparasitylenchus because the excretory pore is located anterior to the nerve-ring.
The remaining species of Proparasitylenchus can be separated from P. californicus n. sp. by the following characters: P. platystethi Wachek, 1955 has a leptoderan bursa, the head of the parasitic female of P. medonis Wachek, 1955 is narrow in comparison to the rest of the body, not sausage-shaped like in P. californicus n. sp. The head of the parasitic female of P. athetae Wachek, 1955 is sunk into the body, the tail is stumpy and the free-living stages have blunt tail tips and short stylets, which separates it from P. californicus n. sp. In P. oxyteli Wachek, 1955 the excretory pore is located only slightly posterior to the nerve-ring and the stylet is very short, which distinguishes it from P. californicus n. sp. The narrow bursa and spicule structure of P. trogophloei Wachek, 1955 separate it from P. californicus n. sp. The cleft trifid stylet base (Figs. 5, 6) is one character that separates P. californicus n. sp. from all Old World members of Proparasitylenchus, which have knobbed but not cleft stylets (Wachek, 1955).
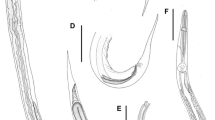
Proparasitylenchus californicus n. sp. 4, Ventral view of anterior portion of female showing subventral gland ampoules filled with secretions (arrows) and opening of excretory pore (E); 5, Head region of female showing cleft stylet base (S), dorsal gland duct (D), nerve-ring (N) and excretory pore (E). Scale-bars: 4, 20 µm; 5, 10 µm
Many characters on such small, delicate parasites are best observed on living individuals. In the present case, the glandular secretions that accumulate in the spherical ampoules of the free-living females (Fig. 4) are rarely evident in fixed material. Also the bands on the body of the males are much less evident in fixed material (Fig. 7). These “bands” appear to represent the remains of nutrient-lipid deposits that have accumulated along the walls of the intestinal cells. They were not noted on any of the European members of the genus (Wachek, 1955). Fine cuticular annules are also more easily seen on living than fixed specimens (Fig. 8).
The parasitic females are very difficult to locate in infected beetles as they are covered with host tissue and surrounded by newly-emerged juvenile nematodes. Growth of the parasitic females is mostly in width and their swollen body is quickly filled with eggs and developing juveniles (Fig. 12). The juveniles moult twice (first and second moult) in the body of the host and then twice again (third and fourth moult) after leaving the host.
Life-cycle, ecology and effect on host
The free-living juveniles and infective stages of P. californicus n. sp. occur in the damp microhabitat beneath the kelp wrack together with developing larvae and pupae of T. geniculata. The juveniles of P. californicus n. sp. moult twice before leaving the host and twice after leaving the host to become sexually mature (Fig. 11). The males die after mating in the environment and the females search for a host, using the secretions in their pharyngeal glands to penetrate the integument and enter the body cavity. Infection probably occurs in the pre-pupal or pupal stage of the beetle, as Wachek (1955) observed that infection of the European species of Proparasitylenchus occured in the pupal stage of staphylinid hosts. The infective stage females could either enter the anus and perforate the gut wall or penetrate directly through the body wall to reach the haemocoel. Development of eggs and juveniles occurs in the adult and possibly the pupal stage of the beetle. Exit of the mature juveniles probably occurs via the anus or body wall of the host.
Little is known about the effect of the parasite on host behavior or ecology, other than the nematodes cause partial or complete sterilisation of the host. The nematode was present in all populations of T. geniculata sampled in the area of Santa Barbara, California (USA) with the percentage of parasitism, which is highest during the summer, ranging from 0 to 11% (Table 1).
Despite exhaustive dissections of other staphylinid species whose larvae co-exist with T. geniculata, e.g. Bledius fenyesi (Bernhauer & Schubert), Aleochara sulcicollis (Mannerheim), Cafius seminitens (Horn) and Cafius canescens (Mäklin), P. californicus n. sp. was only found parasitising T. geniculata. Nematophagous uropodid mites (Rigby, 1996) probably prey on the free-living stages of the nematode and the predators of adult T. geniculata listed below would also consume nematodes in parasitised hosts through concomitant predation (Hechinger et al., 2011; Dunne et al., 2013).
Ecology of the host
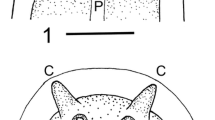
The marine staphylinid beetle Tarphiota geniculata (Mäklin) [syns Homalota geniculata (Mäklin), Tarphiota hirsutula (Casey), Tarphiota iota (Casey), Tarphiota insolita (Casey), Tarphiota litorina (Casey) and Tarphiota seditiosa (Casey)], occurs on sandy beaches along the West coast of North America from Alaska through Baja California Sur (Ahn, 1996; Klimaszewski et al., 2006). It is one of four species of the genus Tarphiota found along the Pacific coast of North America (Ahn, 1996, 1998; Klimaszewski et al., 2006). In Santa Barbara, T. geniculata is sympatric with other staphylinids, including its slightly larger congener T. fucicola, which is much less abundant. Adult T. geniculata are completely black and range between 2.0–2.6 mm in length (Ahn, 1996) (Fig. 1).
Tarphiota geniculata is the most abundant staphylinid associated with kelp wrack in the upper intertidal and lower supra-littoral zones. It is generally not found under fresh or completely desiccated kelp, but prefers to colonise wrack a few days old (Lavoie, 1985). The adults recover almost immediately after immersion in seawater. At night, the beetles forage on the surface of the strand but during the day, they remain hidden under kelp. They are present in the spring, summer and fall, but disappear almost entirely during the stormy winter months.
Its small size probably protects T. geniculata from predation by birds and nocturnal mammals that forage in the kelp wrack. However, laboratory experiments show that adult T. geniculata are predated by a number of other adult arthropods, including the beetles Akephorus marinus (LeConte) (Carabidae), Aleochara sulcicollis (Mannerheim) (Staphylinidae), Bledius fenyesi Bernhauer & Schubert (Staphylinidae), Cafius canescens (Mäklin) (Staphylinidae), Cafius seminitens (Horn) (Staphylinidae), Cafius luteipennis (Horn) (Staphylinidae), Omalium algarum (Casey) (Staphylinidae), Pontomalota opaca (LeConte) (Staphylinidae) as well as the spider Terralonus californicus Peckham & Peckham (Salticidae) and the pseudoscorpion Garypus californicus Banks (Garypidae). The large predatory staphylinids such as Thinopinus pictus (LeConte) and Hadrotes crassus (Mannerheim) ignore T. geniculata.
The prey of T. geniculata include larvae of kelp flies of the genera Coelopa (Coelopidae) and Fucellia (Anthomyiidae), larval and adult Leptocera (Sphaeroceridae) flies and the predatory mite Neomolgus littoralis (Linnaeus) (Bdellidae). Tarphiota geniculata has also been observed scavenging amphipod and kelp fly carcasses and it probably preys on uropodid mites that colonise the kelp wrack. Little is known about the developmental stages of T. geniculata (see Ahn, 1996). The larvae are generally encountered in the moist sand below decaying kelp in the upper intertidal zone and likely forage on the larvae of small flies.
Proparasitylenchus californicus n. sp. is the first description of an extant allantonematid parasite of a staphylinid beetle in the New World. It is also the first allantonematid parasite of a beetle living in a marine habitat. The only other allantonematid known to attack an intertidal insect is Halophilanema prolata Poinar, 2012 a parasite of the hemipteran Saldula laticollis Reuter (Hemiptera: Saldidae) along the Oregon coast (Poinar, 2012).
Parasitism of rove beetles by allantonematid nematodes is an ancient association that was already established millions of years ago in both the Old and New Worlds. A fossil in 40–50 mya Baltic amber shows over 90 specimens of the allantonematid, Palaeoallantonema baltica Poinar, adjacent to an adult rove beetle (Poinar, 2011). Another fossil in 20–30 mya Dominican amber revealed some 44 juveniles of the allantonematid Palaeoallantonema dominicana Poinar that had emerged from an adult Neoxantholinus sp. rove beetle (Poinar & Brodzinsky, 1986) (Fig. 13).
12, Juveniles within the body of the parasitic female of Proparasitylenchus californicus n. sp.; 13, Juvenile allantonematids of Palaeoallantonema dominicana Poinar, 2011 that emerged from an adult Neoxantholinus sp. (Coleoptera: Staphylinidae) in 20–30 mya Dominican amber. Scale-bars: 12, 34 µm; 13, 418 µm
References
Ahn, K.-J. (1996). Revision of the intertidal Aleocharine genus Tarphiota (Coleoptera: Staphylinidae). Entomological News, 107, 177–185.
Ahn, K.-J. (1998). Tarphiota densus (Moore), a new combination and key to the species of the Genus Tarphiota Casey (Coleoptera: Staphylinidae: Aleocharinae). Journal of the Kansas Entomological Society, 71, 191–193.
Dunne, J. A., Lafferty, K. D., Dobson, A. P., et al. (2013). Parasites affect food web structure primarily through increased diversity and complexity. PLoS Biology, 11, e1001579.
Hechinger, R. F., Lafferty, K. D., McLaughlin, J. P., Fredensborg, B. L., et al. (2011). Food webs including parasites, biomass, body sizes, and life stages for three California/Baja California estuaries. Ecology, 92, 791–794.
Klimaszewski, J., Majka, C. J., & Langor, D. (2006). Review of the North American Tarphiota Casey, with a description of a new seashore-inhabiting Atheta species exhibiting convergent characterisitics (Coleoptera: Staphylinidae: Aleocharinae). Entomological Science, 9, 67–78.
Lavoie, D. R. (1985). Population dynamics and ecology of beach wrack macroinvertebrates of the central California coast. Bulletin of the Southern California Academy of Sciences, 84, 1–22.
Lipkow, E. (1968). Hymenopteren und Nematoden als Parasiten von Tachyporus-Arten (Col., Staphylinidae), unter besondere Berücksichtigung. Archiv für Mikroskopische Anatomie, 66, 355–366.
Poinar, G. O., Jr. (1975). Entomogenous nematodes. A manual and host lost of insect- nematode associations. Leiden: E. J. Brill, 317 pp.
Poinar, G. O., Jr. (1977). CIH Key to the Groups and Genera of Nematode Parasites of Invertebrates. Farnham Royal, Bucks, UK: Commonwealth Agricultural Bureaux, 43 pp.
Poinar, G. O., Jr. (2011). The Evolutionary history of nematodes. Leiden: E.J. Brill, 429 pp.
Poinar, G. O., Jr. (2012). Halophilanema prolata n. gen., n. sp. (Nematoda: Allantonematidae), a parasite of the intertidal bug, Saldula laticollis (Reuter) (Hemiptera: Saldidae) on the Oregon coast. Parasites & Vectors, 5, 24.
Poinar, G. O, Jr., & Brodzinsky, J. (1986). Fossil evidence of nematode (Tylenchida) parasitism in Staphylinidae (Coleoptera). Nematologia, 31, 353–355.
Rigby, M. C. (1996). The epibionts of beach hoppers (Crustacea: Talitridae) of the North American Pacific Coast. Journal of Natural History, 30, 1329–1336.
Siddiqi, M. R. (2000). Tylenchida parasites of plants and insects. (2nd ed.). Wallingford, UK: CABI Publishing, 833 pp.
Wachek, F. (1955). Die entoparasitischen Tylenchiden. Parasitologische Schriftenreihe, 3, 1–119.
Acknowledgements
The authors thank Armand M. Kuris for helpful discussions.
Funding
The UC Santa Barbara Coastal Fund (Grant # FALL10-03) supported this project.
Conflict of interest
The authors declare that they have no conflict of interest.
Compliance with ethical standards
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Poinar, G., Datlen, N., Espinoza, M. et al. Proparasitylenchus californicus n. sp. (Tylenchida: Allantonematidae), parasitic in the intertidal rove beetle Tarphiota geniculata (Mäklin) (Coleoptera: Staphylinidae) in California, USA. Syst Parasitol 92, 57–64 (2015). https://doi.org/10.1007/s11230-015-9573-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-015-9573-0