Abstract
Prosthenhystera oonastica n. sp. is described as a cryptic species from the gall bladder of three ictalurid catfishes, Ictalurus punctatus (Rafinesque), Ictalurus furcatus (Valenciennes), and Pylodictis olivaris (Rafinesque), in rivers in the southeastern United States. The species was originally named by Wilmer A. Rogers in 1979 but never formally described. Material used for the description consists of two specimens of Roger’s original material and ten new specimens. We found no significant morphological features that are useful for discriminating between the new species and its closest relative Prosthenhystera obesa (Diesing, 1850) Travassos, 1922 that occurs in the gall bladders of freshwater characiform, perciform and siluriform fishes, ranging from South America to southern Mexico. However, we found substantial differences in the large subunit ribosomal DNA (partial 28S rRNA gene) between the two species justifying the naming of the new species. Prosthenhystera oonastica n. sp. is readily differentiated from Prosthenhystera caballeroi Jiménez-Guzmán, 1973 that occurs in the gall bladders of characid fishes in Central America and Mexico, by having a relatively straight or bent rather than highly convoluted oesophagus, a relatively smaller ovary, smaller and less coalesced vitelline follicles, narrower caeca and smaller eggs. Comparison of ribosomal DNA (partial ITS1, 5.8S, ITS2, and partial 28S gene) between P. oonastica n. sp. and P. caballeroi revealed large differences between the two species. Phylogenetic analysis based on partial 28S rRNA gene sequences from the three studied species of Prosthenhystera Travassos, 1922 and related digenean taxa revealed a closer relationship between P. oonastica n. sp. and P. obesa than either has had with P. caballeroi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prosthenhystera Travassos, 1922 is a genus in the family Callodistomidae Odhner, 1910 that contains three species occurring in the gall bladder of freshwater fishes in the Americas. Two of these species have been formally named and described in publications. Prosthenhystera was erected for Distomum obesum Diesing, 1850 creating the combination Prosthenhystera obesa (Diesing, 1850) Travassos, 1922 for a species that was originally found in the dorado, Salminus brasiliensis (Cuvier), from Brazil (Diesing, 1850; Kohn et al., 1997). Prosthenhystera obesa has since been reported from the same host in Argentina (Kohn et al., 1997, 2007), from a large variety of other fishes in Brazil and Colombia (Travassos et al., 1969; Thatcher, 1991; Kohn et al., 1997, 2007; Martins et al., 2012), and from the common snook Centropomus undecimalis (Bloch) in southern Mexico (Caballero y Caballero & Jiménez-Guzmán, 1969). In southern Mexico, mature specimens of P. obesa have been reported from characid fishes and the blue catfish Ictalurus furcatus (Valenciennes) (see Pérez-Ponce de León & Choudhury, 2005), and immature worms have also been reported from cichlids (Vidal-Martínez et al., 2001; Pérez-Ponce de León & Choudhury, 2002, 2005). These reports suggest that the parasite is a generalist with an enormous geographical range capable of maturing in the gall bladder of fishes in the orders Characiformes, Perciformes and Siluriformes. A detailed scrutiny of this species using molecular and morphological tools is obviously required to confirm its broad generalist nature.
A second species, Prosthenhystera caballeroi Jiménez-Guzmán, 1973, was described from the Mexican tetra Astyanax mexicanus (De Filippi) in Nuevo Leon, in the northern part of Mexico (Jiménez-Guzmán, 1973; Pérez-Ponce de León & Choudhury, 2005).
The third species was named Prosthenhystera oonastica by Wilmer A. Rogers who collected specimens from the channel catfish Ictalurus punctatus (Rafinesque) in southern Alabama, USA, in 1968. He later (1979) deposited some of the material in the United States National Parasite Collection (USNPC) but never formally described the species.
In the present study, we investigated identities of digeneans belonging in Prosthenhystera from various fishes in Peru, Nicaragua, Costa Rica and Mississippi, USA using morphological comparison combined with comparative analysis of ribosomal DNA. Our specimens from Peru are morphologically consistent with descriptions of P. obesa, those from Nicaragua and Costa Rica are morphologically consistent with P. caballeroi, and specimens from Mississippi are morphologically consistent with specimens from Alabama deposited in USNPC (Nos. 075499 and 075500) as P. oonastica. Herein P. oonastica is formally described based on the original specimens deposited in USNPC and newly-collected specimens from Mississippi. Morphological description of P. caballeroi from Costa Rica is also included and comparative measurements are provided from new material of P. obesa from Peru and museum specimens of P. obesa from elsewhere in South America. Partial sequences of the 28S rRNA gene are compared among the three species and results of the phylogenetic analysis based on Bayesian Inference (BI) are provided. Additionally, a sequence spanning the partial internal transcribed spacer 1 (ITS1) region, 5.8S gene, and complete ITS2 region is compared between P. caballeroi and P. oonastica. Unfortunately, DNA from P. obesa was not available for this study which prevented full length sequence comparison among all three species. Additionally, the partial ITS1 region from both P. caballeroi and the new species is compared with that of Prosthenhystera sp. from Panama available in the GenBank.
Materials and methods
Collections
Specimens for morphological examination were heat-killed with hot water and fixed in 10% formalin. Specimens for molecular study were fixed with cold 95% ethanol.
Specimens consistent with P. oonastica of W. A. Rogers were collected from the gall bladders of catfishes (Ictaluridae) from Pearl River (30°19′37″N, 89°37′47″W) in Mississippi as follows: two mature worms from a single specimen of I. furcatus (21.x.2004); six mature worms from a single specimen of the flathead catfish Pylodictis olivaris (Rafinesque) (28.x.2004); one mature worm from a single specimen of I. punctatus (28.x.2004). Specimens consistent with P. oonastica of W. A. Rogers were also collected from the gall bladders of specimens of I. punctatus from Pascagoula River in Mississippi as follows: four mature worms from two I. punctatus (two worms per fish) from 30°38′00″N, 88°39′07″W (3.iii.2008); one mature worm from I. punctatus from 30°36′03″N, 88°37′42″W (27.ii.2009); one mature worm from I. punctatus from 30°36′03″N, 88°37′42″W (3.iii.2011); two mature and two immature worms from three specimens of I. punctatus from 30°31′59″N, 88°35′03″W (11.xi.2013).
Prosthenhystera caballeroi was collected from the gall bladder of the banded tetra Astyanax aeneus (Günther) (Characidae) in Quezalguaque River near Telica, Nicaragua (12°31′00″N, 86°51′54″W) (24.vii.2003). Prosthenhystera caballeroi was also collected from various fishes from in and around Santa Rosa National Park in Guanacaste, Costa Rica during 8-20 July 2007 as follows: four mature worms from four individuals of A. aeneus and one mature worm from Bryconamericus sclaroparius (Regan) (Characidae) in Tempisque River (10°47′20″N, 85°33′03″W); two mature worms from two individual specimens of the machaca Brycon guatemalensis Regan (Characidae) in Animas River (11°02′59″N, 85°35′11″W); six mature worms from individual specimens of A. aeneus in Tempisque River (10°47′20″N, 85°33′03″W).
Prosthenhystera obesa was collected from the gall bladders of various fishes from the area around Iquitos, Peru during 3–8 August 2001 as follows: one worm from the zamurito Calophysis micropterus (Lichtenstein) (Pimelodidiae) in Amazon River (near 3°53′55″S, 85°33′03″W); two worms from a specimen of the trahira Hoplias malabaricus (Bloch) (Erythrinidae), in Itaya River (4°13′31″S, 73°28′58″W); one immature worm from a second H. malabaricus and one immature worm from Moenkhausia sp. (Characidae) both from the same Itaya River collection; one mature worm from a third specimen of H. malabaricus from the same Itaya River collection. Voucher specimen from Calophysis micropterus was deposited in the Harold W. Manter Laboratory (HWML), Lincoln, Nebraska, USA under accession number HWML 75078. A single specimen from H. malabaricus collected from Itaya River served as the source for the 18S and 28S rRNA gene sequences (GenBank accessions AY222108 and AY222206) published by Olson et al. (2003). All DNA from that specimen was used for that amplification and sequencing and was not available for the present study.
Whole mounts were made using specimens preserved in 10% formaldehyde solution following the procedure described by Pulis et al. (2013). The description of the new species is based on measurements obtained from the two specimens from I. punctatus from the USNPC (Nos 075499 and 075500) plus ten newly-collected specimens from Mississippi (one from I. furcatus, three from P. olivaris, and six from I. punctatus). The description of P. caballeroi is based on seven specimens from Costa Rica (one from B. sclaroparius, two from B. guatemalensis, and four from A. aeneus). Additionally, measurements from nine specimens of P. obesa were taken for comparison [one from Erythrinus erythrinus (Bloch & Schneider) (Erythrinidae) from San Pablo, Ecuador (from the Geneva Museum of Natural History; GMNH No. EC-2930); one we collected from C. micropterus from Peru; two we collected from H. malabaricus from Peru; two from Acestrorhynchus altus Menezes (Acestrorhynchidae) from River Paraguay, Paraguay (GMNH PY-7397); and three from S. brasilliensis from Sao Paulo, Brazil (USNPC No. 32515)]. Measurements and digital images of specimens were obtained using an Olympus BX-61 microscope and iSolution Lite software Version 9.7 (Image & Microscope Technology Inc., Vancouver, British Columbia, Canada). All measurements are in micrometres. Illustrations were made with the aid of a drawing tube.
DNA extraction, sequencing and phylogenetic analysis
Genomic DNA was extracted from three specimens consistent with P. oonastica of W. A. Rogers from Mississippi, one specimen of P. caballeroi from Nicaragua, and three specimens of P. caballeroi from Costa Rica following the protocol of Tkach & Pawlowski (1999). The targeted fragment of rRNA spanning almost complete internal transcribed spacer 1 (ITS1) region, complete 5.8S gene, complete ITS2 region, and 5′end of the 28S rRNA gene (including variable domains D1-D3) was amplified from genomic DNA from each of the individual worms. Amplifications were conducted using Polymerase Chain Reactions (PCR) on an Eppendorf EP Gradient thermal cycler using forward primer mitf1 (5′-CGT AAC AAG GTT TCC GTA G-3′) and 1500R (5′-GCT ATC CTG AGG GAA ACT TCG-3′). The PCR reactions were performed according to protocols described in Tkach et al. (2003). PCR Products were purified using Qiagen Qiaquick columns (Qiagen, Germantown, Maryland), cycle-sequenced using ABI BigDye kit 3.1 chemistry, alcohol-precipitated, and run on an ABI Prism 3100 or 3130 Genetic Analyzer (Life Technologies, Grand Island, New York). PCR primers and additional internal forward primer Digl2 (5′-AAG CAT ATC ACT AAG CGG-3′) and internal forward primers digl2r (5′-CCG CTT AGT GAT ATG CTT-3′), 300R (5′-CAA CTT TCC CTC ACG GTA CTT G-3′) and ECD2 (5′-CTT GGT CCG TGT TTC AAG ACG GG-3′) were used in sequencing reactions. Contiguous sequences were assembled and edited using Sequencher software (GeneCodes Corp., ver. 4.1.4; Ann Arbor, Michigan). The sequences were aligned using Clustal W software (Larkin et al., 2007), and further aligned manually and compared using BioEdit software version 7.0.5.3 (Hall, 1999). Boundaries between the 5.8S rRNA gene, ITS2 region, and 28S rRNA gene fragment were estimated using the Internal Transcribed Spacer 2 Ribosomal Database (Keller et al., 2009). The partial 28S rRNA gene fragments obtained from P. caballeroi and P. oonastica were aligned with complimentary sequences from eight other species of digeneans available from GenBank including P. obesa (Table 2). The alignment was trimmed at both ends to the length of the shortest sequence and positions that could not be aligned unambiguously were excluded from the analysis. Phylogenetic analysis was done using Bayesian inference as implemented in MrBayes 3.1.2 software (Huelsenbeck & Ronquist, 2001; Ronquist & Huelsenbeck, 2003). The best nucleotide substitution model was estimated using jModelTest Version 0.1.1 (Posada & Crandall, 1998; 2001; Guindon & Gascuel, 2003; Posada, 2008). The best fitting model was the general time reversible model including estimates of gamma distributed among site-rate variation and the proportion of invariant sites (GTR+G+I). The following model parameters were used in the MrBayes analysis: lset nst = 6, rates = invgamma, ngen = 1,000,000 and samplefreq = 100 imputed into MrBayes. Burn-in value was 2,500 estimated by plotting the log-probabilites against the generations and visualizing plateau in parameter values (sump burnin = 2,500), and nodal support was estimated by posterior probabilities (sumt) (Huelsenbeck & Ronquist, 2001), with all other settings left as default.
Prosthenhystera oonastica n. sp.
Type-host: Ictalurus punctatus (Rafinesque), channel catfish (Siluriformes: Ictaluridae).
Type-locality: Lake Eufaula, Barbour County, Alabama, USA (31°49′46″N, 85°09′23″W).
Other hosts: Ictalurus furcatus (Valenciennes), blue catfish; Pylodictis olivaris (Rafinesque), flathead catfish (both Siluriformes: Ictaluridae).
Other localities: Chaba River, Perry County, Alabama, USA; Tombigbee and Alabama Rivers, Clarke County, Alabama, USA; Pearl River, Hancock County, Mississippi (30°19′37″N, 89°37′47″W); Pascagoula River, Jackson County, Mississippi, USA (30°35′56″N, 88°37′41″W) (in I. punctatus); Pearl River, Hancock County, Mississippi, USA (30°19′37″N, 89°37′47″W) (in I. furcatus and P. olivaris).
Site in host: Gall bladder.
Type-material: Holotype (USNPC no. 75499) and one paratype (USNPC no. 75500) (deposited by Wilmer A. Rogers); eight paratypes (HWML no. 75082-75089) (newly-collected material).
Etymology: The species name oonastica was given by Wilmer A. Rogers in 1979 but the species was not formally described until now.
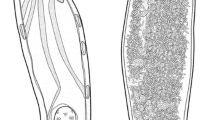
Description (Figs. 1A, B, 2A, B)
[Metrical data in Table 1.] Body very large, pyriform, plump in life, slightly dorso-ventrally flattened in mounted specimens. Ratio of body length to width 1.6–2.2:1. Tegument thick, spineless. Remnants of cercarial eye-spots present in anterior body region. Oral sucker subterminal, subglobular, with posterior margin nearly cuboidal, convex, or rounded. Prepharynx absent. Pharynx broadly fusiform. Ratio of body length to pharynx length 21–26:1. Oesophagus much longer than pharynx, usually nearly straight, sometimes bending but never convoluted, surrounded by small gland cells. Ventral sucker nearly circular. Ratio of oral sucker to ventral sucker width 1:0.9–1.0. Forebody represents 33–38% of body length. Caeca bifurcate near midpoint between pharynx and ventral sucker, terminate blindly near posterior extremity of body. Postcaecal space represents 12–17% of body length.
A, B, Prosthenhystera oonastica n. sp. A, Ventral view of adult specimen with eggs omitted from uterus; B, Terminal genitalia; C, Prosthenhystera caballeroi, ventral view of adult specimen with eggs omitted from uterus. Scale-bars: A, 1 mm; B, C, 500 µm. Abbreviations: c, caecum; cs, cirrus sac; ex, excretory vesicle; gp, genital pore; m, metraterm; md, male duct; o, ovary; sv, seminal vesicle; t, testis; u, uterus; v, vitelline follicles; vd, vas deferens; ve, vasa efferentia; vs, ventral sucker
A, B, Photomicrographs of Prosthenhystera oonastica n. sp. A, Ventral view of adult; B, Close up of right side of adult specimen in region of terminal genitalia; C–E, Photomicrographs of Prosthenhystera caballeroi taken in the same scale. C, Dorsal view of anterior end of adult; D, Ventral view near ventral sucker showing groups of large vitelline follicles; E, Ventral view of posterior region of adult specimen. Scale-bars: A, B, 500 µm; C–E, 300 µm
Testes 2, subspherical, opposite in anterior region of hindbody, intercaecal or overlapping caeca in ventral hindbody. Vasa efferentia merge in forebody, forming very short vas deferens. Cirrus-sac claviform, usually with posterior end only slightly wider than anterior end, rounded on both ends, contains subspherical internal seminal vesicle at proximal end and straight male duct surrounded by prostatic cells; duct opening into dorsal aspect of small genital atrium. Genital atrium shallow, opens at median genital pore on ventral surface at or near level of caecal bifurcation.
Ovary subspherical, submedian, immediately adjacent to posterior region of ventral sucker on left or right side, at level with anterior region of both testes. Seminal receptacle subspherical, submedian, immediately adjacent to ovary. Mehlis’ gland immediately medial to ovarian complex. Uterus thin-walled, looping throughout body, with proximal region extending into left side of body, then extending into right hindbody, distal end with mature eggs ascending into right then left forebody prior to communicating with metraterm; metraterm approximately as long as cirrus-sac, ventral to cirrus-sac in forebody, communicating with posteroventral aspect of genital atrium. Vitellarium consists of 2 separate pre-testicular groups of small follicles; groups loosely arranged in bands surrounding caeca; bands extend anteriorly to level of caecal bifurcation. Eggs in distal half of uterus with well-developed miracidia with 2 prominent fused eye-spots.
Excretory vesicle I-shaped, extends to level of ovary. Excretory pore slightly subterminal on dorsal side.
Remarks
Prosthenhystera oonastica n. sp. from ictalurid fishes in North America is morphologically very similar to P. obesa from C. undecimalis in southern Mexico (see Caballero y Caballero & Jiménez-Guzmán, 1969) and from various fishes in South America (see Kohn et al., 1997). We could find no unambiguous morphological differences between the two species aside from differences in egg-size between specimens of each species that we measured. The average for each of the traits we measured for P. oonastica n. sp. (with the exceptions of egg-length and width) overlaps the range of measurements for the same trait in P. obesa (Table 1). Furthermore, various ratios associated with many of our observed measurements [with or without inclusion of some reported measurements from Caballero y Caballero & Jiménez-Guzmán (1969) and Kohn et al. (1997)] are likewise ambiguous for discriminating between the two species. It should be noted that the level of pressure applied during the fixation of these relatively large digeneans may substantially distort metric morphological characters; therefore it is important to use properly fixed, fully-mature specimens for identification whenever possible. Comparison of egg-size provides the most compelling evidence of morphological difference between the two species when only the present data is considered. Well-formed eggs from the distal uterus of P. oonastica n. sp. averaged 64 × 36 µm (range 57–69 × 30–39 µm), whereas they were smaller in specimens of P. obesa we measured (mean 54 × 30 µm; range 49–59 × 25–34 µm; Table 1). Kohn et al. (1997) reported egg-size range of 49–81 × 30–47 µm in 15 specimens of P. obesa from S. maxillosus, and of 47–87 × 23–48 µm in 34 specimens of P. obesa collected from the gall bladders of fishes other than S. maxillosus. Despite the clear size differences we observed between our specimens of P. oonastica n. sp. and P. obesa, the sizes of the eggs we measured from both species are all within the wide ranges reported for P. obesa by Kohn et al. (1997). Taking into account that eggs from the middle region of uterus in Prosthenhystera spp. are often larger than those at the distal end, it is possible that Kohn et al. (1997) included measurements of eggs from various uterine regions.
Only much larger samples of specimens representing both species, similarly fixed and for which overall body size is taken into account, will allow for sufficient comparison of sizes or ratios of the morphological features within Prosthenhystera. At present, we distinguish P. oonastica n. sp. from P. obesa by the former parasitising ictalurid fishes in North America and the latter parasitising various fishes in the orders Characiformes, Perciformes, and Siluriformes in South America and southern Mexico. Combination of the non-overlapping host taxa, different geographical ranges and considerable genetic divergence (see below) provides compelling evidence for these two forms being different species.
Prosthenhystera caballeroi Jiménez-Guzmán, 1973
Type-host: Astyanax mexicanus (De Filippi), Mexican tetra (Characiformes: Characidae).
Type-locality: Nuevo Leon, Mexico.
Other hosts: Astyanax aeneus (Günther), banded tetra; Bryconamericus sclaroparius (Regan), unnamed tetra; Brycon guatemalensis Regan, machaca (all Characiformes: Characidae).
Other localities: Quezalguaque River near Telica, Nicaragua (12°31′00″N, 86°51′54″W), Tempisque River, Guanacaste, Costa Rica (10°47′20″N, 85°33′03″W) (A. aeneus and B. sclaroparius); Animas River, Guanacaste, Costa Rica (11°02′59″N, 85°35′11″W) (B. guatemalensis).
Site in host: Gall bladder.
Voucher material: Three mature specimens (two from A. aeneus from Tempisque River, one from B. guatamalensis from Animas River) (HWML nos 75079–75081).
Description (Figs. 1C; 2C–E)
[Metrical data in Table 1.] Body elongate-pyriform, plump in life, slightly dorso-ventrally flattened in mounted specimens. Ratio of body length to width 2.2–2.8:1. Tegument thick, spineless. Remnants of cercarial eye-spots present in anterior body region. Oral sucker subterminal, subglobular, with posterior margin rounded or nearly straight. Prepharynx absent. Pharynx broadly fusiform to cuboidal. Ratio of body length to pharynx length 17–25:1. Oesophagus much longer than pharynx, convoluted, surrounded by small gland-cells. Ventral sucker nearly round in outline. Ratio of oral sucker to ventral sucker width 1:0.6–0.8. Forebody represents 37–43% of body length. Caeca with broad lumen, bifurcate much closer to pharynx than ventral sucker, terminate blindly near posterior extremity of body. Postcaecal space represents 8–13% of body length.
Testes 2, subspherical, opposite in anterior region of hindbody, intercecal or overlapping caeca in ventral hindbody. Vas deferens not observed. Cirrus-sac claviform, with posterior end only slightly wider than anterior end, rounded on both ends, contains subspherical internal seminal vesicle at proximal end and straight male duct surrounded by prostatic cells; duct opening into dorsal aspect of small genital atrium. Genital atrium shallow, opens at median genital pore on ventral surface posterior to level of caecal bifurcation.
Ovary relatively large, subspherical, submedian, on left or right side in anterior quarter of hindbody, at level with anterior region of both testes. Seminal receptacle subspherical, submedian, immediately adjacent to ovary. Mehlis’ gland immediately medial to ovarian complex. Uterus thin-walled, looping throughout body, with proximal region extending into left side of body, then extending into right hindbody, distal end with mature eggs ascending into right then left forebody prior to communicating with metraterm; metraterm approximately as long as cirrus-sac, ventral to cirrus-sac in forebody, communicating with posteroventral aspect of genital atrium. Anterior extent of uterus at oral sucker. Vitellarium consists of 2 separate groups of relatively large, usually densely clumped follicles, clumping around caeca but largely ventral, extending from level of ovary anteriorly to middle of forebody. Eggs in distal half of uterus with well-developed miracidia with 2 prominent fused eye-spots.
Excretory vesicle I-shaped, extends to level of ovary. Excretory pore opens slightly subterminal on ventral surface.
Remarks
Prosthenhystera caballeroi was originally described on the basis of five specimens from the gall bladder of A. mexicanus from the northernmost part of Mexico and has rarely been reported since (Jiménez-Guzmán, 1973; Pérez-Ponce de León & Choudhury, 2005). Our specimens compare favourably with the description by Jiménez-Guzmán (1973) in most respects. The oesophagus is convoluted, the ovary is relatively large, and vitelline follicles are large and coalesced into two compact groups. Although the specimens in the original material were generally larger than our material from Costa Rica, the ratio of body length to width is essentially the same for both (ratio of body length to width 2.3–2.7:1 in Mexican material compared with 2.2–2.8:1 in Costa Rican material). Material from Mexico has a relatively shorter pharynx (ratio of body length to pharynx length 29–39:1 vs 17–25:1), and relatively larger eggs (53–63 × 25–35 vs 42–56 × 25–32 µm) (see Jiménez-Guzmán, 1973). We tentatively attribute this minor variation to small sample sizes and differences in fixation and levels of pressure applied during mounting. Prosthenhystera caballeroi is readily distinguished from both of its congeners by having a relatively narrower body (usually), pharynx longer relative to body length (usually), convoluted rather than straight or bending oesophagus, caeca bifurcating much closer to pharynx than ventral sucker (rather than at midpoint between them), shorter post-caecal space, smaller ventral sucker relative to oral sucker, relatively much larger ovary, larger vitelline follicles, and smaller eggs (Table 1). Furthermore, the extent of the vitelline follicles is restricted between the ovarian level and the middle of the forebody in P. caballeroi compared with from the testicular level to the caecal bifurcation in P. oonastica n. sp. and P. obesa, and occasionally extending anteriorly to the oesophageal region in P. obesa. In addition, the average egg-size in P. caballeroi (50 × 29 µm) is much smaller than that observed for the congeners (see Table 1).
Molecular differentiation
The targeted fragment encompassing most of the ITS1 region, 5.8S gene, complete ITS2 region, and partial 5′ end of the 28S rRNA gene region measured 2,274 bp in P. oonastica n. sp and 2,271 bp in P. caballeroi. No intraspecific variability was observed among the three specimens of P. oonastica n. sp. from Mississippi and similarly, no differences were observed between the single specimen of P. caballeroi from Nicaragua and the three specimens from Costa Rica. The two species differed from each other by 110 bp (4.8% of sites were variable) across the entire fragment. The partial ITS1 fragment measured 474 bp in P. oonastica n. sp. and 470 bp in P. caballeroi and differed at 48 sites (10.1%) between species. The complete 5.8S gene measured 156 bp in both species and differed at two sites (1.3%) between species. The complete ITS2 region measured 278 bp in P. oonastica n. sp. and 279 bp in P. caballeroi and differed at 29 sites (10.4%) between species. The partial fragment of 28S rRNA gene was 1,366 bp long in both species and demonstrated much lower variability than the ITS region with only 31 variable sites (2.3%). Only partial 28S rDNA sequence of P. obesa previously deposited in the GenBank was available for comparison. This sequence covered 1,252 bp at the 5′ end of the 28S gene. Its alignment with sequences of P. oonasitca n. sp. and P. caballeroi required no gaps. This sequence fragment of P. obesa differed at 23 sites (1.8% of the sequence length) from that of P. oonasitca n. sp. and in 25 sites (2.0%) from that of P. caballeroi. The whole alignment had 39 variable sites when all three species were compared, indicating that the majority of mutations were in the same variable positions across species.
Phylogenetic analysis
The BI analysis of a dataset containing nine ingroup taxa (represented by the families, Allocreadiidae Looss, 1902, Callodistomidae, and Gorgoderidae Looss, 1899) and rooted by the dicrocoeliid Dicrocoelium dendriticum (Rudolphi, 1819) Odhner, 1910 produced a phylogram with very strong nodal support of all topologies that suggests that the Allocreadiidae and Callodistomidae are more closely related to each other than either is to the Gorgoderidae (Fig. 3). The topology exhibited in the phylogram is consistent with the results from previously published phylogenetic analyses of digeneans containing relevant taxa (Olson et al, 2003; Curran et al., 2006, 2007, 2011, 2012; Choudhury et al., 2007; Rosas-Valdez et al., 2011; Cutmore et al., 2013; Martínez-Aquino et al., 2013; Razo-Mendivil et al., 2014a, b). The present phylogeny mirrors morphological data and suggests that P. oonastica n. sp. and P. obesa are more closely related to each other than either is to P. caballeroi.
Phylogram resulting from Bayesian Inference analysis (1,000,000 generations) of partial 28S rRNA gene sequences from some digeneans. The analysis is rooted by Dicrocoelium dendriticum (Dicrocoeliidae) and estimates the phylogenetic relationship among species of Prosthenhystera (Callodistomidae), and the position of the Callodistomidae relative to the Gorgoderidae represented by Gorgodera cygnoides and Nagmia floridensis and the Allocreadiidae represented by Allocreadium lobatum, Auriculostoma astyanace, Creptotrematina aquirrepequenoi, and Crepidostomum illinoiense. Branch support values indicate posterior probabilities
Discussion
The family Callodistomidae is a small family of digeneans whose life histories are entirely unknown. Since phylogenetic analyses demonstrate that callodistomids are most closely related to the Allocreadiidae and the Gorgoderidae, families that use bivalves as first intermediate hosts, it can be hypothesised that callodistomids might also cycle through bivalves. Presently the family comprises four genera, two in Africa and two in the Americas (Bray, 2002). Such a geographical distribution for a family parasitic in freshwater fishes seems untenable, but molecular data from Africa are presently lacking.
The present study substantially extends the known range of P. caballeroi to include Nicaragua and Costa Rica. Both morphological and molecular analyses confirmed uniformity of this species across all our collection sites.
Pairwise sequence comparisons of different fragments of nuclear ribosomal DNA of P. caballeroi and P. oonastica n. sp. demonstrated very similar levels of interspecific divergence in the ITS1 and ITS2 regions (10.1% and 10.4%, respectively), but much lower differences in the partial 28S gene sequences (only 2.3%).
Our analysis (Fig. 3) indicates a closer phylogenetic relationship between P. obesa and P. oonastica n. sp. than either species has with P. caballeroi. After the present study the distribution range of P. caballeroi spans at least from Costa Rica in the south to northern Mexico. Prosthenhystera caballeroi is probably confined to characid fishes and therefore its northern extent should be limited to the Rivers Pecos and Grand in New Mexico and Texas, USA that represent the northern boundary for characid fishes. Its presence south of Costa Rica needs to be investigated. If P. oonastica n. sp. is confined to ictalurid catfishes, then its southern boundary should coincide with that of I. furcatus in southern Mexico and northern Guatemala (Lee et al., 1980). There is some possibility that Prosthenhystera oonastica n. sp. and P. obesa, which are extremely similar morphologically, may potentially overlap geographically in southern Mexico (see Vidal-Martínez et al., 2001; Pérez-Ponce de León & Choudhury, 2002, 2005). Consequently, the identification of specimens reported as P. obesa in southern Mexico should be re-evaluated using molecular tools. The partial fragment of 28S rRNA gene sequence (GenBank accession EF032690) identified as P. obesa from I. punctatus from the USA used by Curran et al. (2006, 2007, 2011, 2012) is conspecific with P. oonastica n. sp. The partial fragment of 28S rRNA gene sequence (GenBank accession HQ325029) identified as P. obesa from A. aeneus from Costa Rica published by Rosas-Valdez et al. (2011) is shorter (882 bp) but identical to our sequences of P. caballeroi. Martínez-Aquino et al. (2013) published two sequences of ITS1 rDNA (GenBank KC899973, KC899974) from Prosthenhystera sp. from a species of characid fish from Panama. When trimmed to 464 bp and aligned with the ITS1 rDNA sequences from P. oonastica n. sp. and P. caballeroi, the two Panamanian sequences differed from each other at 9 sites (1.9%), and sequences KC899973 and KC899974 differred from that of P. caballeroi at 6 sites (1.3%) and 7 sites (1.5%) respectively. Both Panamanian sequences (KC899973, KC899974) differ from that of P. oonastica n. sp. at 52 sites (11.2%). Thus, the Panamanian sequences may potentially represent two species that are very similar to P. caballeroi. More genetic and morphological data are required to verify this suggestion.
References
Bray, R. A. (2002). Family Callodistomidae Odhner, 1910. In: Gibson, D. I., Jones, A. & Bray, R. A. (Eds) Keys to the Trematoda. Vol. 1. Wallingford, UK: CABI Publishing and The Natural History Museum, pp. 603–619.
Caballero y Caballero, E., & Jiménez-Guzmán, F. (1969). Presencia de Prosthenhystera obesa (Diesing, 1856) Travassos, 1920 (Trematoda: Digenea) en peces comestibles de aqua dulce de Mexico. Revista de Biología Tropical, 15, 283–287.
Choudhury, A., Rosas-Valdez, R., Johnson, R. C., Hoffmann, B., & Pérez-Ponce de León, G. (2007). The phylogenetic position of Allocreadiidae (Trematoda: Digenea) from partial sequences of the 18S and 28S ribosomal RNA genes. Journal of Parasitology, 93, 192–196.
Curran, S. S., Overstreet, R. M., & Tkach, V. V. (2007). Phylogenetic affinities of Plagiocirrus Van Vleave and Mueller, 1932 with description of a new species from the Pascagoula River, Mississippi. Journal of Parasitology, 93, 1452–1458.
Curran, S. S., Pulis, E. E., Hugg, D. O., Brown, J. P., Manuel, L. C., & Overstreet, R. M. (2012). Phylogenetic position of Creptotrema funduli in the Allocreadiidae based on partial 28S rDNA sequences. Journal of Parasitology, 98, 873–875.
Curran, S. S., Tkach, V. V., & Overstreet, R. M. (2006). A review of Polylekithum Arnold, 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitologica, 51, 238–248.
Curran, S. S., Tkach, V. V., & Overstreet, R. M. (2011). Phylogenetic affinities of Auriculostoma (Digenea: Allocreadiidae), with descriptions of two new species from Peru. Journal of Parasitology, 97, 661–670.
Cutmore, S. C., Miller, T. L., Curran, S. S., Bennett, M. B., & Cribb, T. H. (2013). Phylogenetic relationships of the Gorgoderidae (Platyhelminthes: Trematoda), including the proposal of a new subfamily (Degeneriinae n. subfam.). Parasitology Research, 112, 3063–3074.
Diesing, K. M. (1850). Systema helminthum (Vol. 1, p. 679). Vindobonae: Wilhelmum Braumüller.
Guindon, S., & Gascuel, O. (2003). A simple, fast and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52, 696–704.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95–98.
Huelsenbeck, J. P., & Ronquist, F. (2001). MrBayes: Bayesian inference of phylogeny. Bioinformatics, 17, 754–755.
Jiménez-Guzmán, F. (1973). Trematodos digeneos de peces dulceacuicolas de Nuevo Leon, Mexico I. Dos nuevas species y un registro Nuevo en el carácido Astyanax fasciatus mexicanus (Filippi). Cuadernos del Instituto de Investigaciones Cientificas Universidad Autonoma de Nuevo Leon, 17, 1–19.
Keller, A., Schleicher, T., Schultz, J., Müller, T., Dandekar, T., & Wolf, M. (2009). 5.8S - 28S rDNA interaction and HMM-based ITS2 annotation. Gene, 430, 50–57.
Kohn, A., Fernandes, B. M. M., & Baptista-Farias, M. (1997). Redescription of Prosthenhystera obesa (Diesing, 1850) (Callodistomidae, Digenea) with new host records and data on morphological variability. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro, 92, 171–179.
Kohn, A., Fernandes, B. M. M., & Cohen, S. C. (2007). South American trematodes parasites of fishes. Rio de Janeiro: Oswaldo Cruz Institute, 318 pp.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., McGeltigan, P. A., Williams, H., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics, 23, 2947–2948.
Lee, D. S., Carter, C. R., Hocutt, C. H., Jenkins, R. E., McAllister, D. E., & Stauffer, J. R. (1980). Atlas of North American Freshwater Fishes. Publication 1980–12 North Carolina Biological Survey. Raleigh, North Carolina: North Carolina State Museum of Natural History, 854 pp.
Martínez-Aquino, A., Ceccarelli, F. S., & Pérez-Ponce de León, G. (2013). Molecular phylogeny of the genus Margotrema (Digenea: Allocreadiidae), parasitic flatworms of goodeid freshwater fishes across central Mexico: species boundaries, host-specificity, and geographical congruence. Zoological Journal of the Linnean Society, 168, 1–16.
Martins, A. N., Sabas, C. S. S., & Brasil-Sato, M. C. (2012). Prosthenhystera obesa (Diesing, 1850) (Digenea, Callodistomidae) in the São Francisco River basin, Brazil: new host records and their ecological parameters. Neotropical Helminthology, 6, 31–41.
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33, 733–755.
Pérez-Ponce de León, G., & Choudhury, A. (2002). Adult endohelminth parasites of ictalurid fishes (Osteichthyes: Ictaluridae) in Mexico: empirical evidence for biogeographical patterns. Comparative Parasitology, 69, 10–19.
Pérez-Ponce de León, G., & Choudhury, A. (2005). Biogeography of helminth parasites of freshwater fishes in Mexico: the search for patterns and processes. Journal of Biogeography, 32, 645–659.
Posada, D. (2008). jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution, 25, 1253–1256.
Posada, D., & Crandall, K. A. (1998). Modeltest: Testing the model of DNA substitution. Bioinformatics, 14, 817–818.
Posada, D., & Crandall, K. A. (2001). Selecting the best-fit model of nucleotide substitution. Systematic Biology, 50, 580–601.
Pulis, E. E., Fayton, T. J., Curran, S. S., & Overstreet, R. M. (2013). A new species of Intromugil (Digenea: Haploporidae) and redescription of Intromugil mugilicolus. Journal of Parasitology, 99, 501–508.
Razo-Mendivil, U., Mendoza-Garfias, B., Pérez-Ponce de León, G., & Rubio-Godoy, M. (2014a). A new species of Auriculostoma (Digenea: Allocreadiidae) in the Mexican tetra Astyanax mexicanus (Actinopterygii, Characidae) from central Veracruz, Mexico, described using morphological and molecular data. Journal of Parasitology, 100, 331–337.
Razo-Mendivil, U., Pérez-Ponce de León, G., & Rubio-Godoy, M. (2014b). Testing the systematic position and relationships of Paracreptotrema heterandriae within the Allocreadiidae through partial 28S rRNA gene sequences. Journal of Parasitology, 100, 537–541.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Rosas-Valdez, R., Choudhury, A., & Pérez-Ponce de León, G. (2011). Molecular prospecting for cryptic species in Phyllodistomum lacustri (Platyhelminthes, Gorgoderidae). Zoologica Scripta, 40, 296–305.
Thatcher, V. E. (1991). Amazon fish parasites. Amazoniana, 11, 263–571.
Tkach, V. V., Curran, S. S., Bell, J. A., & Overstreet, R. M. (2013). A new species of Crepidostomum (Digenea: Allocreadiidae) from Hiodon tergisus in Mississippi, and molecular comparison with three congeners. Journal of Parasitology, 99, 1114–1121.
Tkach, V. V., Littlewood, D. T. J., Olson, P. D., Kinsella, J. M., & Swiderski, Z. (2003). Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Systematic Parasitology, 56, 1–15.
Tkach, V. V., & Pawlowski, J. (1999). A new method of DNA extraction from the ethanol-fixed parasitic worms. Acta Parasitologica, 44, 147–148.
Tkach, V., Pawlowski, J., & Mariaux, J. (2000). Phylogenetic analysis of the suborder Plagiorchiata (Platyhelminthes, Digenea) based on partial lsrDNA sequences. International Journal for Parasitology, 30, 83–93.
Travassos, L., Freitas, J. F. T., & Kohn, A. (1969). Trematódeos do Brasil. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro, 67, 1–886.
Vidal-Martínez, V. M., Aguirre-Macedo, M. L., Scholz, T., González-Solís, D., & Mendoza-Franco, E. F. (2001). Atlas of the helminth parsites of cichlid fish of Mexico. Prague: Academia, 165 pp.
Acknowledgements
We are thankful to Dr. Wilmer A. Rogers (Auburn University) for his encouragement regarding the present description of a species he named. We also appreciate the help from many people who facilitated collections of specimens used in this study, especially: Michael W. Littmann (Department of Zoology, Field Museum of Natural History), Mark H. Sabaj (Academy of Natural Sciences), Jose Bernardo Ortega Gonzales and Lesber Salezar (Universidad Internacional de la Integración de América Latina, Chinandega, Nicaragua), Daniel R. Brooks (HWML) and Eric E. Pulis and Thomas J. Fayton (The University of Southern Mississippi). We are indebted to museum curators for their assistance with specimens: Patricia A. Pilitt and Eric P. Hoberg (USNPC), Alain de Chambrier (formerly of GMNH), and Scott L. Gardner (HWML). We also thank two anonymous reviewers for useful corrections and suggestions regarding our manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tkach, V.V., Curran, S.S. Prosthenystera oonastica n. sp. (Digenea: Callodistomidae) from ictalurid catfishes in southeastern United States and molecular evidence differentiating species in the genus across Americas. Syst Parasitol 90, 39–51 (2015). https://doi.org/10.1007/s11230-014-9531-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-014-9531-2