Abstract
In a study on the order Trypanorhyncha Diesing, 1863, a total of 35 specimens belonging to nine species of elasmobranch in the Gulf of Oman, was examined. The following trypanorhynch species were identified: Pterobothrium lesteri Campbell & Beveridge, 1996, Otobothrium carcharidis (Shipley & Hornell, 1906), Eutetrarhynchus platycephali Palm, 2004, Parachristianella indonesiensis Palm, 2004, Pa. monomegacantha Kruse, 1959 and Prochristianella mooreae Beveridge, 1990. Prochristianella garshaspi n. sp. is described from Pastinachus sephen (Forsskål) and Rhinoptera sp. The new species is allocated to the genus Prochristianella Dollfus, 1946 on the basis of the presence of two bothria, prebulbar organs, and a heteroacanthous typical tentacular armature with relatively few hooks in each principal row, hollow hooks increasing in size from antibothrial and then decreasing towards the bothrial surface of the tentacle, hooks 1 and 1′ being separated, and a basal swelling with characteristic billhooks increasing in size towards the bothrial surface. The lack of microscopically visible microtriches on the scolex distinguishes the new species from P. hispida (Linton, 1890), P. clarkeae Beveridge, 1990, P. thalassia (Kovaks & Schmidt, 1980), P. multidum Friggens & Duszynski, 2005 and P. cairae Schaeffner & Beveridge, 2012. Prochristianella garshaspi n. sp. can be distinguished from the remaining species within the genus by a combination of the following morphological features: the presence of numerous gland-cells within the tentacular bulbs, the number of rows on the basal swelling, the number of hooks per half spiral row, the size of the principal hooks, the number of the testes and the presence of an external seminal vesicle.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The order Trypanorhyncha Diesing, 1863 is a group of cestodes which predominantly occur in the marine realm, with elasmobranchs serving as their final hosts (Campbell & Beveridge, 1994; Palm, 2004). Many members of this group have wide geographic distributions (Palm, 2004), which is thought to be related to their relatively low host specificity (Palm & Caira, 2008). One of the regions for which low host specificity of trypanorhynch species has been assessed is the Persian Gulf (see Haseli et al., 2010, 2011), connected to the Indian Ocean by the Gulf of Oman. These water bodies are known as a branch of the north-western part of the Indian Ocean. Both regions harbour rich elasmobranch fauna (Randall, 1995; Assadi & Dehghani, 1997; Moore, 2011) and thus offer many potential hosts for trypanorhynchs. However, despite the relatively comprehensive knowledge of the trypanorhynch fauna of elasmobranchs in the Persian Gulf (see Haseli et al., 2010, 2011) the information from the Gulf of Oman is limited (El Naffar et al., 1992; Palm, 2004). To date, there are no reports of adult trypanorhynchs from this region. The present study from the Iranian waters of the Gulf of Oman reports on the trypanorhynch fauna of dominant elasmobranch species, and describes a new species of Prochristianella Dollfus, 1946.
Materials and methods
A total of 35 specimens belonging to nine species of elasmobranchs, caught by local fishermen from the northern Gulf of Oman (Iran; 25°12′N–25°17′N, 60°8′E–60°38′E) in May 2011, was examined. The elasmobranch species included Rhizoprionodon acutus (Rüppell) (3 males and 2 females; body weight 0.9–4.0 kg), Carcharhinus macloti (Müller & Henle) (4 males and 1 female; body weight 1.5–2.2 kg), Iago omanensis (Norman) (5 females; body weight 1.0–1.5 kg), Gymnura poecilura (Shaw) (2 males and 3 females; body weight 0.5–6.0 kg), Torpedo sinuspersici Olfers (1 male and 2 females; body weight 1.8–2.7 kg), Himantura imbricata (Bloch & Schneider) (3 males; body weight 0.6–1.1 kg), Himantura sp. (3 males and 1 female; body weight 2.2–5.0 kg), Pastinachus sephen (Forsskål) (4 females; body weight 8.0–16.0 kg), and Rhinoptera sp. (1 female; body weight 11.0 kg). Host identification was carried out using published keys for the Gulf of Oman (Compagno, 1984; Randall, 1995; Assadi & Dehghani, 1997). In order to aid future specific identification photographic records have been made of the hosts which were not identified to the species level.
Trypanorhynch cestodes were isolated from the spiral valves of the hosts and fixed in 70% ethanol. Worms were stained with acetic carmine or re-hydrated and then stained with haematoxylin, dehydrated in an ethanol series, cleared in methyl salicylate and mounted on slides in Canada balsam. Palm’s (2004) monograph and two original descriptions (Beveridge & Justine, 2007; Schaeffner & Beveridge, 2012a) were used for identification; classification follows Palm (2004). Measurements were taken using an ocular micrometer and are in micrometres unless otherwise stated. Measurements are presented in the text as the range followed by the mean and standard error in parentheses. The number of cestodes examined (N) and the total number of measurements (n) in cases when more than one measurement was taken per worm, are also provided. Drawings were made with a drawing tube attached to an hp NP-21 microscope. Vitelline follicles are shown on the lateral margins of the proglottids only.
Voucher specimens have been deposited in the Natural History Museum of Iran, Tehran, Iran (MMTT) as follows: Pterobothrium lesteri Campbell & Beveridge, 1996 (MMTT 4150); Otobothrium carcharidis (Shipley & Hornell, 1906) (MMTT 4153-56); Eutetrarhynchus platycephali Palm, 2004 (MMTT 4157); Parachristianella indonesiensis Palm, 2004 (MMTT 4158-62); Pa. monomegacantha Kruse, 1959 (MMTT 4160-61); Prochristianella mooreae Beveridge, 1990 (MMTT 4162-63). Type-specimens of Parachristianella garshaspi n. sp. have been deposited in the Natural History Museum of Iran and Museum d’Histoire Naturelle, Geneva, Switzerland (MHNG).
Results
Table 1 presents the prevalence and intensity of the trypanorhynch cestodes recovered from the elasmobranch hosts in the Gulf of Oman. A total of seven species of the families Pterobothriidae Pintner, 1931, Otobothriidae Dollfus, 1942 and Eutetrarhynchidae Guiart, 1927 was identified, all of which represent new locality records. Furthermore, five new host records were established (Table 1). The maximum intensity (29) was recorded for Pa. indonesiensis in Ps. sephen. A single otobothriid species, Otobothrium carcharidis (Shipley & Hornell, 1906), was found in two species of carcharhinid sharks. This is the second otobothriid species reported from the Gulf of Oman, the first being O. penetrans Linton, 1907 from Tylosurus crocodilus crocodilus (Peron & LeSueur) (Palm, 2004). The family Eutetrarhynchidae represents the most diverse group in the present collection with five out of the seven species found (E. platycephali, Pa. indonesiensis, Pa. monomegacantha, P. mooreae and P. garshaspi n. sp.). Parachristianella indonesiensis showed the widest host range (Table 1). Prochristianella garshaspi n. sp. represents the 20nd species of Prochristianella and is described below.
Prochristianella garshaspi n. sp.
Type-host: Pastinachus sephen (Forsskål) (Myliobatiformes: Dasyatidae).
Other host: Rhinoptera sp. (Myliobatiformes: Myliobatidae).
Type-locality: The Gulf of Oman, Iran (25°12′N–25°17′N, 60°8′E–60°38′E).
Site in host: Spiral intestine.
Type-material: Holotype in MMTT (No. 4172; 1 slide); paratypes in MMTT (Nos 4164–71; 10 slides with 8 specimens) and MHNG (No. PLAT-82735; 3 specimens).
Etymology: The specific name garshaspi is derived from Garshasp, a Persian mythical hero, whose spiked club was similar to the tentacles of these worms.
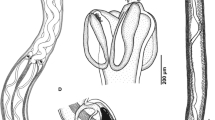
Description (Figs. 1, 2)
[Based on 9 mature specimens.] Slender cestodes, 2.5–8.0 (4.8 ± 0.1, N = 7) mm long, with 8–11 proglottids. Scolex 810–1,224 (1,003 ± 42, N = 7) long, acraspedote (Fig. 1A), lacking microscopically visible microtriches; scolex width at pars bothrialis 89–259 (158 ± 19, N = 8), scolex width at pars vaginalis 113–203 (154 ± 9, N = 9), scolex width at pars bulbosa 146–227 (173 ± 8, N = 9). Bothria 2 in number, oval, 139–405 (233 ± 30, N = 9, n = 11) long, 164–220 (185 ± 13, N = 4) wide, with free margins and posterior notch; bothrial pits absent. Pars bothrialis 139–208 (177 ± 7, N = 9) long; pars vaginalis 376–753 (609 ± 40, N = 9) long; tentacle sheaths sinuous. Pars bulbosa 327–421 (382 ± 9, N = 9) long; prebulbar organs present; bulbs elongate, 341–406 (372 ± 6, N = 9, n = 12) long, 44–69 (61 ± 2, N = 9, n = 13) wide; bulb length/width ratio 1 : 5–9 (6, N = 9, n = 11). Scolex ratio (pars bothrialis : pars vaginalis : pars bulbosa) 1 : 2.0–4.5 (3.5, N = 9) : 1.2–1.8 (1.6, N = 9); retractor muscles originate at posterior extremity of bulbs; gland-cells attached to retractor muscle within bulb at posterior part; pars post-bulbosa absent.
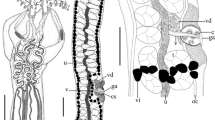
Prochristianella garshaspi n. sp. A, scolex; B, mature proglottid; C, terminal genitalia. Abbreviations: c, cirrus; cs, cirrus sac; esv, external seminal vesicle; ga, genital atrium; mg, Mehlis’ gland; o, ovary; oc, osmoregulatory canal; t, testis; u, uterus; v, vagina; vd, vas deferens; vi, vitelline follicle; vs, vaginal sphincter. Scale-bars: A, B, 100 μm; C, 10 μm
Tentacles long, with characteristic basal armature; tentacle width at metabasal region 14–18 (15, N = 9); basal swelling present (Figs. 1A, 2A–C), with maximum width 22–34 (26 ± 1, N = 9). Hook rows 13 (Fig. 2A–C); first row with enlarged uncinate hooks, 4–8 (6, N = 9, n = 12) long, base 4–7 (5, N = 9, n = 12) long on antibothrial surface, 6–9 (7, N = 9, n = 12) long, base 5–8 (6, N = 9, n = 15) long on bothrial surface; second row of smaller uncinate hooks, 3–6 (5, N = 8, n = 15) long, base 2–5 (4, N = 8, n = 17) long; rows 3–6 of hooks spiniform, originating on antibothrial surface and terminating on bothrial surface, 5–7 (6, N = 9, n = 15) long, base 2–4 (3, N = 9, n = 15) long, terminal hooks with broad base on bothrial surface in row 6; rows 7–8 of hooks spiniform on antibothrial surface with slightly recurved tip, 4–6 (5, N = 9) long, base 1–2 (2, N = 9) long, hooks become larger along row from antibothrial to bothrial surface, terminating in robust and large billhooks on bothrial surface, 4–8 (6, N = 8, n = 14) long; next row consists of smaller billhooks on antibothrial surface, 1–4 (3, N = 5, n = 6) long, base 2–4 (3, N = 6) long, hooks become larger towards bothrial surface, 4–6 (5, N = 6) long, base 5–8 (6, N = 7, n = 9) long; rows 10–13 consist of small billhooks on antibothrial surface, 2–4 (3, N = 6) long, base 3–4 (3, N = 6) long; rows terminate in spiniform hooks with narrow bases on bothrial surface, 2–6 (4, N = 7, n = 10) long, base 2–6 (4, N = 6, n = 7) long.
Metabasal armature heteroacanthous typical, heteromorphous; hook rows in ascending half spirals, with 7–9 falcate, hollow hooks; rows commence on antibothrial surface and terminate on bothrial surface; hooks 1(1′) 3–6 (4, N = 9, n = 13) long, base 1–4 (3, N = 9, n = 11) long; hooks 2(2′) 6–10 (8, N = 9, n = 12) long, base 2–5 (3, N = 9) long; hooks 3(3′) 7–10 (9, N = 9) long, base 2–5 (3, N = 9) long; hooks 4(4′) 7–10 (8, N = 9) long, base 2–4 (3, N = 9) long; hooks 5(5′) 6–10 (7, N = 9) long, base 2–3 (2, N = 9) long; hooks 6(6′) 5–8 (6, N = 9) long, base 1–2 (2, N = 9) long; hooks 7(7′) 5–8 (6, N = 9) long, base 1–2 (2, N = 9) long; hooks 8(8′) 4–6 (5, N = 9) long, base 1–2 (1, N = 9) long; hooks 9(9′) 4–5 (4, N = 3) long, base 1–2 (1, N = 3) long. Principle hooks increase in size from antibothrial surface towards internal and external surfaces, then diminish in size towards bothrial surface.
Segments acraspedote; mature proglottids 284–1,944 (819 ± 125, N = 9, n = 18) long, with maximum width 162–373 (221 ± 23, N = 8, n = 9). Genital pores postequatorial, 284–891 (607 ± 64, N = 9, n = 11) from posterior margin of proglottid. Testes inter-vascular, 44–99 (71 ± 4, N = 9, n = 19) long, 38–56 (48 ± 3, N = 9, n = 10) wide, arranged in two columns, 42–49 (44 ± 1, N = 8) in number, 21–25 (22 ± 1, N = 7) antiporal, 13–16 (15 ± 3, N = 8) pre-vaginal, 5–8 (6, N = 9) post-vaginal; vas deferens coils posteriorly; external seminal vesicle 56–79 (69 ± 5, N = 4) long, 20–44 (36 ± 4, N = 6) wide; cirrus-sac ovoid, 64–74 (N = 2) long, 20–49 (33 ± 3, N = 8, n = 10) wide; cirrus unarmed. Ovary H-shaped in dorso-ventral view, ovarian lobes 267–405 (338 ± 21, N = 7) long, 20–39 (30 ± 3, N = 6) wide; Mehlis’ gland posterior to ovarian isthmus, 42–56 (49 ± 3, N = 4) in diameter; vagina sinuous, relatively uniform in width, enters genital atrium at posterior level of cirrus-sac, surrounded by sphincter in distal part; uterus median, extends to anterior part of proglottid; vitelline follicles circumcortical, 20–34 (25 ± 1, N = 9, n = 17) in diameter. Gravid proglottids observed only in one worm, 2,040–2,245 (n = 2) long, 347–653 (n = 2) wide.
Remarks
Prochristianella garshaspi is allocated to the genus Prochristianella on the basis of possessing two bothria, an heteroacanthous typical armature with relatively few hooks in each principal row, the hollow hooks increasing in size from antibothrial and then decreasing toward bothrial surface of the tentacle, the divergent hook files 1(1′), a distinct space between the rows of principal hooks, a basal swelling, a characteristic basal armature with characteristically shaped bill hooks increasing in size toward the bothrial surface and a prebulbar organ.
The lack of microscopically visible microtriches on the scolex in P. garshaspi distinguishes it from P. hispida (Linton, 1890) Campbell & Carvajal, 1975, P. clarkeae Beveridge, 1990, P. thalassia (Kovaks & Schmidt, 1980) Beveridge, 1990, P. multidum Friggens & Duszynski, 2005 and P. cairae Schaeffner & Beveridge, 2012. The new species can be easily distinguished from P. heteracantha Dailey & Carvajal, 1976 by the number of testes (42–49 vs 28–35) and from P. odonoghuei Beveridge, 1990 in possessing an external seminal vesicle. Prochristianella garshaspi differs from P. fragilis Heinz & Dailey, 1974 in the number of testes (42–49 vs 50–60), in the length of pars vaginalis (113–203 vs 540–770), and in the number of rows on the basal swelling (13 vs 5). The new species differs from P. jensenae Schaeffner & Beveridge, 2012 in possessing gland-cells within the tentacular bulbs and from P. kostadinovae Schaeffner & Beveridge, 2012 in the number of hooks per half spiral row (7–9 vs 10). The species described here differs from P. scholzi Schaeffner & Beveridge, 2012 in that hooks 5(5′) are smaller than hooks 4(4′) and larger than hooks 6(6′), whereas P. scholzi possesses much smaller hooks 4(4′) than the neighbouring hooks 3(3′) and 5(5′). Prochristianella garshaspi can be distinguished from P. aciculata Beveridge & Justine, 2010 by the shape of the first hook of each principal row on the antibothrial surface (falcate vs small linear with tiny, hooked extremity).
The number of hooks per half spiral row in the metabasal region in P. garshaspi is smaller than in P. omunae Beveridge & Justine, 2010 (7–9 vs 13–15). Prochristianella garshaspi can be distinguished from P. mooreae by possessing an external seminal vesicle and 7–9 hooks rather than 10–12 hooks per half spiral row in the metabasal armature; from P. butlerae Beveridge, 1990 in possessing the hollow hooks and an external seminal vesicle; from P. minima Heinz & Dailey, 1974 in possessing a shorter basal swelling (13 vs 18–20 hook rows) and in the number of testes (42–49 vs 18–25); and from P. glaber (Dollfus, 1969) Palm, 2004 in the length of the scolex (810–1,224 vs 4,000) and in the number of hooks per half spiral row (7–9 vs 10). The presence of hollow hooks and the number of testes (42–49 vs 63, respectively) differentiate P. garshaspi from P. tumidula (Linton, 1890) Campbell & Carvajal, 1975. Finally, Prochristianella garshaspi differs from P. papillifer (Poyarkoff, 1909) Dollfus, 1957 in having an external seminal vesicle.
Discussion
The connection of the Persian Gulf to the Indian Ocean through the Gulf of Oman supports the expectation of similarity between the trypanorhynch faunas of these two water bodies. To date, nine of the 13 species found in the Gulf of Oman have also been reported from the Persian Gulf (see El Naffar et al., 1992; Palm, 2004; Haseli et al., 2010, 2011). One of the reasons for this similarity is that host specificity of the members of the order, and especially of the larval stages, is relatively low (Palm & Caira, 2008). The family Eutetrarhynchidae showed the greatest diversity in both regions. Certainly, life-cycles of the members of this family and their low host specificity play role in this case. The life-cycles of eutetrarhynchids include three hosts, with copepods and benthic or coastal invertebrates acting as first and second intermediate hosts, respectively, and elasmobranch fishes acting as final hosts (Palm, 2004). Further studies on the invertebrate fauna from the two water bodies off southern Iran are needed to explain why the Eutetrarhynchidae represents the most diverse trypanorhynch group within both regions.
Of the new locality records, P. mooreae is not only a new record from the Gulf of Oman but also a new record for the Indian Ocean. This species was described by Beveridge (1990) from Dasyatis brevicaudata (Hutton) from Spencer Gulf (Australia), D. fluviorum Ogilby from Moreton Bay (Australia), and Parascyllium ferrugineum McCulloch from Holdfast Bay (South Africa). It is worth mentioning that P. mooreae has previously only been reported from temperate regions.
In addition to P. mooreae, P. garshaspi is the second species of the genus reported from the Gulf of Oman. The genus Prochristianella was first erected by Dollfus (1946) with two species, P. papillifer and P. tenuispinis (Linton, 1890) Dollfus, 1946, based on the presence of a basal swelling and principle hooks in the metabasal armature first increasing in size from the internal and then decreasing in size towards the external surface of the tentacle. Beveridge (1990) redefined the genus, described P. butlerae, P. clarkeae, P. mooreae and P. odonoghuei, and recognised other species of Prochristianella, including P. papillifer, P. minima, P. tenuispinis, P. tumidula, P. thalassia, P. fragilis and P. hispida. Prochristianella spinulifera was described by Beveridge & Jones (2000) but due to its similarity with Dollfusiella elongata Beveridge, Neifar & Euzet, 2004, Beveridge et al. (2004) transferred P. spinulifera to Dollfusiella Campbell & Beveridge, 1994 as D. spinulifera (Beveridge & Jones, 2000). These authors also re-examined the type specimens of P. tenuispinis and transferred the species to Dollfusiella as D. tenuispinis (Linton, 1890), concluded that in the species of the genus Prochristianella, principal hooks commence on the antibothrial surface and terminate on the bothrial surface, and revised the definitions of the related genera Dollfusiella and Prochristianella (see Beveridge et al., 2004). Palm (2004) described P. macracantha (syn. of P. butlerae; see Schaeffner & Beveridge, 2012b), transferred Eutetrarhynchus glaber Dollfus, 1969 to the genus Prochristianella and considered P. heteracantha as a valid species. Seven new species of Prochristianella have been described since 2004, namely P. multidum Friggens & Duszynski, 2005, P. aciculata Beveridge & Justine, 2010, P. omunae Beveridge & Justine, 2010, P. cairae Schaeffner & Beveridge, 2012, P. jensenae Schaeffner & Beveridge, 2012, P. kostadinovae Schaeffner & Beveridge, 2012, and P. scholzi Schaeffner & Beveridge, 2012. Historically, the state of separation of hook files 1 and 1′ along with the gradation of hook sizes along the principal rows have been used to distinguish Prochristianella from Dollfusiella (see Dollfus, 1946; Beveridge, 1990; Beveridge & Jones, 2000; Beveridge et al., 2004; Palm, 2004; Beveridge & Justine, 2010). Moreover, most of the valid species of the latter genus possess similarly-sized principal hooks. However, Dollfusiella spinulifera, D. elongata and D. spinifer (Dollfus, 1969) possess morphological features intermediate between the two genera, with a slight gradation in hook sizes as well as the hook files 1 and 1′ being almost abut. In the case of P. garshaspi there is a distinct space between hooks 1 and 1′, the gradation of hook sizes along the principal rows is significant and the hooks are not densely spaced. According to the recent phylogenetic studies of the order Trypanorhyncha, the monophyly of the two genera, Dollfusiella and Prochristianella is still ambiguous (see Palm et al., 2009; Olson et al., 2010). If further phylogenetic studies based on DNA sequence data can prove the monophyly of each genus, then it will be possible to see whether the state of separation of the hooks 1 and 1′ and the gradation of hook sizes along the principal rows of the metabasal armature can be considered as synapomorphic characters for the genus Prochristianella.
In this investigation, the dominant elasmobranch species from the Iranian side of the Gulf of Oman were examined and it should be kept in mind that this region has notable diversity of sharks and rays and that further studies, especially in the southern part are certainly needed to bring to light more species of this marvellous order.
References
Assadi, H., & Dehghani, R. (1997). Atlas of the Persian Gulf and the Sea of Oman fishes. Tehran: Iranian Fisheries Research and Training Organization, 226 pp.
Beveridge, I. (1990). Taxonomic revision of Australian Eutetrarhynchidae Guiart (Cestoda : Trypanorhyncha). Invertebrate Taxonomy, 4, 785–845.
Beveridge, I., & Jones, M. K. (2000). Prochristianella spinulifera n. sp. (Cestoda: Trypanorhyncha) from Australian dasyatid and rhinobatid rays. Systematic Parasitology, 47, 1–8.
Beveridge, I., & Justine, J.-L. (2007). Redescriptions of four species of Otobothrium Linton, 1890 (Cestoda: Trypanorhyncha), including new records from Australia, New Caledonia and Malaysia, with the description of O. parvum n. sp. Zootaxa, 1587, 1–25.
Beveridge, I., & Justine, J.-L. (2010). Two new species of Prochristianella Dollfus, 1946 (Platyhelminthes, Cestoda) from the blue-spotted stingray, Neotrygon kuhlii (Müller & Henle, 1841) off New Caledonia. Zoosystema, 32, 643–652.
Beveridge, I., Neifar, L., & Euzet, L. (2004). Eutetrarhynchid cestodes from Atlantic and Mediterranean elasmobranch fishes, with the description of two new species of Dollfusiella Campbell & Beveridge, 1994 and redescriptions of Prochristianella papillifer (Poyarkoff, 1909) Dollfus, 1957 and Parachristianella trygonis Dollfus, 1946. Systematic Parasitology, 59, 81–102.
Campbell, R. A., & Beveridge, I. (1994). Order Trypanorhyncha Diesing, 1863. In: Khalil, L. F., Jones, A. & Bray, R. A. (Eds.) Keys to the cestode parasites of vertebrates. Wallingford: Commonwealth Agricultural Bureaux International, pp. 51–148.
Compagno, L. J. V. (1984). Sharks of the World. FAO Fisheries Synopsis No. 125. Rome: Food and Agriculture Organization of the United Nations, 655 pp.
Dollfus, R.-P. (1946). Notes diverses sur les Tétrarhynques. Mémoires du Muséum National d’Histoire Naturelle, Paris, Nouvelle Série, 22, 179–220.
El Naffar, M. K. I., Gobashy, A., El-Etreby, S. G., & Kardousha, M. M. (1992). General survey of helminth parasite genera of Arabian Gulf fishes (coast of United Arab Emirates). Arab Gulf Journal of Scientific Research, 10, 99–110.
Haseli, M., Malek, M., & Palm, H. W. (2010). Trypanorhynch cestodes of elasmobranchs from the Persian Gulf. Zootaxa, 2492, 28–48.
Haseli, M., Malek, M., Valinasab, T., & Palm, H. W. (2011). Trypanorhynch cestodes of teleost fish from the Persian Gulf, Iran. Journal of Helminthology, 85, 215–224.
Moore, A. B. M. (2011). Elasmobranchs of the Persian (Arabian) Gulf: ecology, human aspects and research priorities for their improved management. Reviews in Fish Biology and Fisheries, 22, 35–61.
Olson, P. D., Caira, J. N., Jensen, K., Overstreet, R. M., Palm, H. W., & Beveridge, I. (2010). Evolution of the trypanorhynch tapeworms: Parasite phylogeny supports independent lineages of sharks and rays. International Journal for Parasitology, 40, 223–242.
Palm, H. W. (2004). The Trypanorhyncha Diesing, 1863. Bogor: IPB-PKSPL Press, 710 pp.
Palm, H. W., & Caira, J. N. (2008). Host specificity of adult versus larval cestodes of the elasmobranch tapeworm order Trypanorhyncha. International Journal for Parasitology, 38, 381–388.
Palm, H. W., Waeschenbach, A., Olson, P. D., & Littlewood, D. T. J. (2009). Molecular phylogeny and evolution of the Trypanorhyncha Diesing, 1863 (Platyhelminthes: Cestoda). Molecular Phylogenetics and Evolution, 52, 351–367.
Randall, J. E. (1995). Coastal fishes of Oman. Honolulu: University of Hawaii Press, 439 pp.
Schaeffner, B. C., & Beveridge, I. (2012a). Cavearhynchus, a new genus of tapeworm (Cestoda: Trypanorhyncha: Pterobothriidae) from Himantura lobistoma Manjaji-Matsumoto & Last, 2006 (Rajiformes) off Borneo, including redescriptions and new records of species of Pterobothrium Diesing, 1850. Systematic Parasitology, 82, 147–165.
Schaeffner, B. C., & Beveridge, I. (2012b). Prochristianella Dollfus, 1946 (Trypanorhyncha: Eutetrarhynchidae) from elasmobranchs off Borneo and Australia, including new records and the description of four new species. Zootaxa, 3505, 1–25.
Acknowledgments
Financial support was provided through the Centre of Research Affairs of the University of Guilan. Thanks are due to Dr. Shabanipour and Dr. Norastehnia who provided laboratory facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haseli, M. Trypanorhynch cestodes from elasmobranchs from the Gulf of Oman, with the description of Prochristianella garshaspi n. sp. (Eutetrarhynchidae). Syst Parasitol 85, 271–279 (2013). https://doi.org/10.1007/s11230-013-9425-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-013-9425-8