Abstract
Heterocotyle sulamericana n. sp. is described from the gills of Dasyatis guttata (Bloch & Schneider) caught off the coast of Brazil near Rio de Janeiro. This species can be distinguished from all other members of Heterocotyle Scott, 1904 by a combination of the morphology of the male copulatory organ, which is a short, slightly curved, sclerotised tube with no accessory piece, and the haptor, which has a single ridge surmounting all septa. This is the first Heterocotyle species to be described from the southwestern Atlantic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Species of Heterocotyle Scott, 1904 are parasitic on the gills of their stingray hosts and are known to parasitise mainly members of the Dasyatidae Jordan. They have one central and eight peripheral haptoral loculi and are distinguished from other members of the Monocotylidae Taschenberg, 1879 by the presence of four accessory structures on the dorsal surface of the haptor and by a single or multiple ridges on the ventral surface of the haptoral septa (Chisholm et al., 1995; Chisholm & Whittington, 1996).
During a survey of Dasyatis guttata (Bloch & Schneider) in southwestern Atlantic waters off Rio de Janeiro, specimens of Heterocotyle were discovered. We determined these to be a new species which is described herein.
Materials and methods
Five specimens of the longnose stingray Dasyatis guttata were caught in fishing nets off Angra dos Reis on the Brazilian coast near Rio de Janeiro in October, 2003. The gills were immediately removed and examined in a Petri dish with filtered seawater under a stereomicroscope. Live monocotylids were removed from the gill filaments using fine needles, fixed slightly flattened under a coverslip and preserved in 70% ethanol. They were then stained in Mayer’s paracarmine, or Gomori’s trichrome, dehydrated in an ethanol series, cleared in beechwood creosote and mounted in Canada balsam. Some specimens were mounted in Hoyer’s medium in order that the sclerotised structures of the haptor and the male copulatory organ could be seen more readily. Specimens were examined using a Leica DM LS2 phase contrast microscope and illustrations were made using a drawing tube. Measurements were made with the aid of an ocular micrometer and are given in micrometres as the mean followed, in parentheses, by the range and the number of structures measured. Worm length is given as the body length including the haptor and the male copulatory organ length is the total straight line length.
For closer inspection of the ventral surface of the haptor, other specimens were stained with alcoholic chloridic carmine and examined using confocal laser scanning electron microscopy (CLSM) under a ZEISS LSM510 Meta with FluoView Version 3.2. The ventral surface of the haptor of the type-material (accession details below) of Heterocotyle americana Hargis, 1955, H. granulatae Young, 1967 and H. pseudominima Hargis, 1955 were also examined using CLSM.
The terminology used follows that of Chisholm & Whittington (1996). Type-material is deposited in the Helminthological Collection of Oswaldo Cruz Institute (CHIOC) at the Fundação Oswaldo Cruz (FIOCRUZ), Av. Brasil, 4365, Rio de Janeiro, Brazil and in the Australian Helminthological Collection (AHC) at the South Australian Museum (SAMA), North Terrace, Adelaide, South Australia 5000, Australia. For comparative purposes the holotype of H. americana (USNPC 49351), a paratype of H. granulatae (USNPC 61749) and the holotype of H. pseudominima (USNPC 49352) were examined.
-
Monocotylidae Taschenberg, 1879
-
Heterocotylinae Chisholm, Wheeler & Beverley-Burton, 1995
-
Heterocotyle Scott, 1904
Heterocotyle sulamericana n. sp.
Type-host: Dasyatis guttata (Bloch & Schneider) (Dasyatidae).
Type-locality: Off Angra dos Reis, Rio de Janeiro, Brazil (23°00′24″S, 44°19′05″W).
Site: Gills, between secondary lamellae.
Infection details: Three of five rays infected with 3, 10 and 26 monocotylids, respectively.
Type-material: Holotype CHIOC 3751a; 3 paratypes CHIOC 3751b-d; 4 vouchers CHIOC 3752a-c; 7 paratypes SAMA AHC 35233–35239.
Etymology: This species name reflects the geographical position of the type-locality.
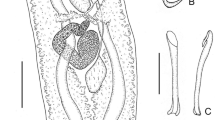
Description (Figs. 1, 2a–c, 3a)
[Based on 11 whole-mounts.] Body, including haptor, 513 (414–598, n = 11) long, 155 (114–209, n = 11) wide at level of ovary. Haptor 170 (150–192, n = 11) long, 182 (141–210, n = 11) wide, consisting of single central and 8 peripheral loculi (Figs. 1, 3a). Single sinuous ridge present around all septa (Figs. 1, 3a). Hamulus (Figs. 1, 2a) 39 (34–43, n = 11) long; handle thin, 18 (13–23, n = 11) long, embedded in tissue of septum between posterior and posterolateral loculi (Fig. 1); shaft short; point curved (Fig. 2a). Fourteen hooklets, 10 (9–11, n = 8) long, distributed in marginal membrane as illustrated (Fig. 1). Dorsal surface of haptor with 4 accessory structures, bearing sclerotised, striated anterior edge, situated dorsal to 4 posteriormost loculi (Fig. 1).
Heterocotyle sulamericana n. sp. Whole-mounted specimen, ventral view, composite drawing. Abbreviations: alg, anterolateral gland-duct opening; amg, anteromedian gland-duct opening; cgp, common genital pore; e, eyespots as dispersed pigment granules; eb, ejaculatory bulb; h, marginal hooklet; ha, hamulus; i, intestinal caecum; mco, male copulatory organ; mm, marginal membrane; or, outer ring septum; ov, ovary; p, pharynx; r, sinuous ridge; sr, seminal receptacle; sv, seminal vesicle; t, testis; tvd, transverse vitelline duct; vd, vas deferens; vg, vagina; vi, vitellarium. Scale-bar: 100 μm
Confocal scanning laser electron microscopy of the ventral surface of the haptor of Heterocotyle spp. a. Close-up of the single sinuous ridge surmounting septa in Heterocotyle sulamericana n. sp.; b. Close-up of H. americana (holotype USNPC 49351) showing the 1/2/3 arrangement of sinuous septal ridges (arrow) associated with anteriormost loculi; c. Whole haptor of H. granulatae (paratype USNPC 61749) showing the single sinuous ridge surmounting all septa; d. Whole haptor of H. pseudominima (holotype USNPC 49352) showing the 1/2/3 arrangement of sinuous septal ridges (arrow). Scale-bars: a–b, 20 μm; c–d, 50 μm
Mouth ventral, subterminal. Anterior region with 2 pairs of anteromedian and 3 pairs of anterolateral gland-duct openings, but glands supplying and ducts leading to these openings not seen. Eyespots in form of dispersed pigment granules dorsolateral and anterodorsal to pharynx (Fig. 1). Pharynx muscular, 55 (45–66, n = 9) long, 39 (34–46, n = 9) wide (Fig. 1). Caeca simple, not confluent posteriorly.
Testis long, tubular, forming complete loop (Fig. 1). Vas deferens arises from left anterior margin of testis, curves to right, then to left and widens to form large seminal vesicle (Fig. 1). Duct from seminal vesicle narrows and opens into base of ejaculatory bulb (Fig. 1). Muscular ejaculatory bulb oval, 51 (46–55, n = 11) long, 30 (23–34, n = 11) wide. Sclerotised male copulatory organ short, curved 26 (23–27, n = 11) long, (Figs. 1, 2b); accessory piece absent.
Ovary median, encircles right intestinal caecum dorsoventrally and narrows to form oviduct (Fig. 1). Vitelline follicles extend in lateral fields from level of mid-region of pharynx to anterior margin of haptor; transverse vitelline ducts join to form common vitelline duct which enters oviduct at level of anteriormost region of ovary (Fig. 1). Vaginal pore unarmed, opens on left side of body at level of common genital pore (Fig. 1). Vaginal duct narrow; vaginal walls not sclerotised; vaginal sclerites absent. Seminal receptacle small. Mehlis’ gland not observed. Oötype passes anteriorly to open via common genital pore. Egg tetrahedral, 91 (74–106, n = 3) in side length (measured within oötype); short filament present at single pole (Fig. 2c).
Remarks
Heterocotyle sulamericana n. sp. can be distinguished from the other species in the genus by the morphology of the male copulatory organ, which is short and lacks an accessory piece associated with it. The only other species with a similar male copulatory organ is H. pseudominima Hargis, 1955. In addition, the testis in both of these species is tubular and forms a loop. However, our new species is easily differentiated from H. pseudominima by the arrangement of the sinuous ridge(s) on the haptor, which is single on all septa of H. sulamericana (Figs. 1, 3a) but has a 1/2/3 arrangement in H. pseudominima (Fig. 3d) (see Chisholm & Whittington, 1996, and ‘Discussion’ below).
The sinuous ridge(s) surmounting the septa of the haptor can be difficult to see under the light microscope. Examination of the haptors of the types of H. americana Hargis, 1955, H. granulatae Young, 1967 and H. pseudominima using CLSM confirmed the 1/2/3 septal ridge arrangement in H. americana (Fig. 3b) and H. pseudominima (Fig. 3d) and the highly sinuous single ridge arrangement in H. granulatae (Fig. 3c). Excellent results were obtained using CLSM, despite the fact this type-material had been mounted for about 50 years and was not stained with alcoholic chloridic carmine.
Discussion
This is the first record of a Heterocotyle species from the southwestern Atlantic. With the description of H. sulamericana n. sp. there are now 19 described species of Heterocotyle. The easiest way to identify Heterocotyle species is by examining the morphology of the sclerotised male copulatory organ. A combination of its shape and length, the presence or absence of spines and the presence/absence and morphology of an accessory piece are all useful characters for identification. As demonstrated above, the morphology of the sinuous ridge(s) on the ventral surface of the haptoral septa can also aid identification. Six species, including H. armata Timofeeva, 1983, H. capricornensis Chisholm & Whitttington, 1996, H. confusa Timofeeva, 1983, H. forcifera Neifar, Euzet & Ben Hassine, 1999, H. granulatae and H. sulamericana have been described with only a single sinuous ridge surmounting the septa. The remaining 13 species have the 1/2/3 sinuous ridge arrangement, which consists of: a single sinuous ridge on the inner and outer ring and three posterior septa; a double sinuous ridge on the two lateral septa; and three sinuous ridges surmounting the three anterior septa (Chisholm & Whittington, 1996). The species with this arrangement include: H. americana, H. capapei Neifar, Euzet & Ben Hassine, 2000, H. chinensis Timofeeva, 1983, H. dasyatis (Yamaguti, 1965) Chisholm, 1995, H. minima (MacCallum, 1916) Price, 1938, H. mokhtarae Neifar, Euzet & Ben Hassine, 1999, H. pastinacae Scott, 1904, H. pseudominima, H. scotti Neifar, Euzet & Ben Hassine, 1998, H. similis Neifar, Euzet & Ben Hassine, 1998, H. striata Neifar, Euzet & Ben Hassine, 1999, H. taeniuropi Cao, Ding, Zhang & Liu, 2010 and H. tokoloshei Vaughan & Chisholm, 2010 .
Vaughan & Chisholm (2010) used the proteolytic digestion technique described by Vaughan et al. (2008) to examine the haptor of H. tokoloshei. They noted that the degree of digestion of the sinuous ridge on the septa was equal to other non-sclerotised parts of the haptor and concluded that the sinuous ridge on the septa may in fact not be sclerotised. Given these results, we have not used the term ‘sclerotised’ when referring to the sinuous ridge(s) here, although recognising that the composition of the ridge(s) needs to be verified in other Heterocotyle species.
The anterior glands of Heterocotyle species are best viewed in live material using phase contrast optics. We could see two pairs of median and three pairs of anterolateral gland-duct openings, but could not find their associated glands or ducts in the fixed material we examined. The arrangement of glands and ducts probably follows the pattern outlined by Chisholm & Whittington (1996) for Heterocotyle species. Thus the two median pairs likely arise from the anteromedian and the anterolateral glands, and the anterolateral openings likely arise from the lateral glands, but this needs to be confirmed.
The type-host, Dasyatis guttata, is closely related to D. americana Hildebrand & Schroeder. The distributions of these two fish species overlap in the western Atlantic from the Gulf of Mexico to southern Brazil. Chisholm & Whittington (1996) demonstrated that species of Heterocotyle are not strictly host-specific and also that more than one species of Heterocotyle can infect a single host species. H. americana has been described from D. americana (see Hargis, 1955; Chisholm & Whittington, 1996), but it is possible that H. sulamericana may also infect this host. A survey of D. americana in this region is required to determine whether or not this is the case.
References
Chisholm, L. A., Wheeler, T. A., & Beverley-Burton, M. (1995). A phylogenetic analysis and revised classification of the Monocotylidae Taschenberg, 1879 (Monogenea). Systematic Parasitology, 32, 159–191.
Chisholm, L. A., & Whittington, I. D. (1996). A revision of Heterocotyle (Monogenea: Monocotylidae) with a description of Heterocotyle capricornensis n. sp. from Himantura fai (Dasyatididae) from Heron Island, Great Barrier Reef, Australia. International Journal for Parasitology, 26, 1169–1190.
Hargis, W. J., Jr. (1955). Monogenetic trematodes of Gulf of Mexico fishes. Part V. The superfamily Capsaloidea. Transactions of the American Microscopical Socety, 74, 203–225.
Vaughan, D. B., & Chisholm, L. A. (2010). Heterocotyle tokoloshei sp. nov. (Monogenea: Monocotylidae) from the gills of Dasyatis brevicaudata (Dasyatidae) kept in captivity at Two Oceans Aquarium, Cape Town, South Africa: description and notes on treatment. Acta Parasitologica, 55, 108–114.
Vaughan, D., Chisholm, L. A., & Christison, K. (2008). Overview of South African Dendromonocotyle (Monogenea: Monocotylidae), with descriptions of 2 new species from stingrays (Dasyatidae) kept in public aquaria. Zootaxa, 1826, 26–44.
Acknowledgements
We are grateful to Dr Pedro Figueiredo, Dr Carlos Elisyo Alhanat and Dr Aderval Vaz from ‘Eletronuclear’ for access to fishing facilities near the Angra dos Reis Nuclear Power Station. This study was financially supported by the Projects PROCAD NF/2009, PAPES V Fiocruz/CNPq, PROEP 2011 Fiocruz/CNPq and FAPERJ.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santos, C.P., Santos, A.L., Cunha, R. et al. A new species of Heterocotyle Scott, 1904 (Monogenea: Monocotylidae) from the gills of Dasyatis guttata (Dasyatidae) in southwestern Atlantic waters off Rio de Janeiro, Brazil. Syst Parasitol 81, 65–70 (2012). https://doi.org/10.1007/s11230-011-9328-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-011-9328-5