Abstract
A new genus and species of tetraphyllidean cestode, Caulopatera pagei n. g., n. sp., is described from the grey carpetshark Chiloscyllium punctatum Müller & Henle in Moreton Bay, Australia. The new genus is placed in the Phyllobothriidae, subfamily Phyllobothriinae. Caulopatera n. g. is distinct from all other phyllobothriine genera in having stalked, circular, non-loculate bothridia that lack an apical sucker, testes that are restricted to the region anterior to the cirrus-sac and circum-medullary vitelline follicles. The new genus most closely resembles Carpobothrium Shipley & Hornell, 1906, with which it shares non-loculate, stalked, unhooked bothridia without an accessory sucker and testes that are entirely anterior to the cirrus-sac, but differs from it in that it lacks a slit-like opening in each bothridium and flaps surrounding the opening. The possession of bothridial stalks is consistent with two cestode orders, the Tetraphyllidea and the Rhinebothriidea. The morphology of the bothridial stalks is consistent with other tetraphyllidean genera, in that Caulopatera possesses triangular bothridial stalks surrounding the back of the bothridia, indicating that it belongs in the Tetraphyllidea senso stricto, rather than in the recently recognised Rhinebothriidea.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During helminthological surveys of carcharhiniform and orectolobiform sharks of Moreton Bay, off the coast of Queensland, Australia, several specimens of the grey carpetshark Chiloscyllium punctatum Müller & Henle were collected and examined for tetraphyllidean cestodes. To date, 10 tetraphyllidean species from three genera have been described or recorded from C. punctatum or C. cf. punctatum; the phyllobothriids Orectolobicestus lorettae Ruhnke, Caira & Carpenter, 2006 and O. tyleri Ruhnke, Caira & Carpenter, 2006, and the onchobothriids Spiniloculus mavensis Southwell, 1925, Yorkeria hilli Caira & Tracy, 2002, Y. izardi Caira, Jensen & Rajan, 2007, Y. kelleyae Caira & Tracy, 2002, Y. longstaffae Caira, Jensen & Rajan, 2007, Y. pusillulus Caira, Jensen & Rajan, 2007, Y. saliputium Caira, Jensen & Rajan, 2007 and Y. yubodohensis Caira, Jensen & Rajan, 2007. Of these 10 species, four have been recorded from Australia, all from C. cf. punctatum in Queensland waters, i.e. O. lorettae, Y. izardi and Y. longstaffae described from off Cairns (Ruhnke et al., 2006a; Caira et al., 2007) and S. mavensis described from Moreton Bay (Southwell, 1925) and later recorded from two other locations, off Heron Island (Williams, 1964) on the southern end of the Great Barrier Reef and from the waters off Mackay and Balgal (Caira, 1990). In addition to species of Orectolobicestus Ruhnke, Caira & Carpenter, 2006, Spiniloculus Southwell, 1925 and Yorkeria Southwell, 1927, species of Carpobothrium Shipley & Hornell, 1906, Pithophorus Southwell, 1925 and Acanthobothrium van Beneden, 1849 have been described from other species of Chiloscyllium Müller & Henle in the Indo-west Pacific (e.g. Shipley & Hornell, 1906; Subhapradha, 1955; Zaidi & Khan, 1976; Jensen & Caira, 2008). In the present study, tetraphyllideans from C. punctatum included a genus and species new to science. We describe and discuss the new genus in relation to other genera of its allocated family, the Phyllobothriidae Braun, 1900, and subfamily, the Phyllobothriinae Braun, 1900.
Materials and methods
Between April, 2006 and December, 2008, seven specimens of Chiloscyllium punctatum were collected from Moreton Bay, Queensland. Host molecular voucher specimens (tissue samples) from three of the C. punctatum were fixed in 100% ethanol and lodged in the Queensland Museum’s frozen tissue collection, Brisbane, Australia (Registration Numbers A006488–90). Spiral intestines were removed and kept on ice (<4 hours) for transport prior to examination. Each spiral intestine was opened longitudinally and examined under a dissecting microscope. Cestodes were removed, washed and subsequently killed in near-boiling vertebrate saline (0.85% NaCl solution). Worms were fixed in 10% formalin for morphological examination and scanning electron microscopy (SEM).
Specimens for morphological analysis were washed in tap-water, stained in Mayer’s haematoxylin, destained in a solution of 1.0% HCl and neutralised in 0.5% ammonium hydroxide solution. Specimens were then dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted in Canada balsam. Measurements were made using a SPOT Insight™ digital camera (Diagnostic Instruments, Inc.) mounted on an Olympus BH-2 compound microscope using SPOT™ imaging software. Measurements are in micrometres unless otherwise stated and are given as the range followed in parentheses by the mean, standard deviation, number of worms measured (n) and the total number of measurements taken (n) if multiple measurements were taken per worm. For two-dimensional measurements, length is given before width. Drawings were made with the aid of a drawing tube. Microthrix terminology follows Chervy (2009).
Specimens for SEM were dehydrated in a graded ethanol series and then dried in a critical point dryer (Balzers 11 120 A Critical Point Drier, Balzers Union Ltd, Balzers, Principality of Liechtenstein). Specimens were mounted on carbon tab pin stubs, sputter-coated with 20–30 nm of platinum (EIKO IB-5 Ion Coater, EIKO Engineering Company, Ibaraki, Japan) and stored in airtight containers with silica gel to absorb any residual moisture. Specimens were examined using JEOL JSM 6300F and JEOL JSM-6400F scanning electron microscopes (JEOL Ltd, Tokyo, Japan).
Caulopatera n. g.
Diagnosis
Tetraphyllidea, Phyllobothriidae, Phyllobothriinae. Worms euapolytic. Scolex with four circular, stalked bothridia; each bothridium with distinct rim, lacking apical sucker. Proximal bothridial surfaces covered with gladiate spinitriches; margin of distal bothridial surface (internal surface of rim) covered with lingulate spinitriches. Presence of cephalic peduncle unclear. Surface of proglottids covered with capilliform filitriches arranged into scutes. Proglottids acraspedote, only terminal proglottis mature. Testes numerous, restricted to region anterior to cirrus-sac, single layer deep. Cirrus-sac piriform to reniform, contains coiled and armed cirrus. Genital pores lateral, alternate irregularly. Genital atrium shallow. Vagina opens into genital atrium anterior to cirrus-sac. Ovary near posterior end of proglottis, H-shaped in dorsoventral view. Uterus saccate, ventral to vagina, extends anteriorly from between anterior lobes of ovary to ventrally overlap posterior portion of cirrus-sac. Uterine duct present, enters anterior third of uterus. Vitellarium follicular, circum-medullary, extends entire length of mature proglottis, absent dorsal to vas deferens, ventral to uterus, both dorsal and ventral to ovary and partly absent both dorsal and ventral to cirrus-sac. Excretory ducts lateral. Parasites of Chiloscyllium. Type- and only known species: C. pagei n. sp.
Etymology: The generic name is from the Latin caulis, stalk, and patera, saucer, referring to the appearance of the bothridia. Its gender is feminine.
Caulopatera pagei n. sp.
Type-host: Chiloscyllium punctatum Müller & Henle (Orectolobiformes: Hemiscylliidae), grey carpetshark.
Type-locality: Off St. Helena Island (27°23′S, 153°14′E), Moreton Bay, Queensland, Australia.
Site: Spiral intestine.
Prevalence: In 2 of 7 hosts.
Type-material: Holotype (QM G 231742) and 14 paratypes (QM G 231743–56) deposited in the Queensland Museum, Brisbane, Australia.
Etymology: This species is named for John Page, whose friendship and continual assistance with the collection of elasmobranchs has been greatly appreciated.
Description (Figs. 1–10)
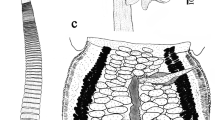
[Based on 15 whole-mounts of mature specimens and 10 mature specimens prepared for SEM.] Worms euapolytic, 4.43–7.06 (6.05 ± 0.84; n = 10) mm long; maximum width 566–879 (710 ± 88; n = 13) at scolex. Scolex tetrabothridiate (Figs. 3, 4, 8), 324–526 (421 ± 83; n = 9) long. Bothridia stalked, circular, non-loculate, lacking marginal loculi, 304–506 (388 ± 57; n = 11; n = 22) in diameter, with distinct rim, lacking apical sucker. Bothridial stalk muscles attached to bothridium in ring at its base (Figs. 9, 10). Proximal bothridial surfaces covered with gladiate spinitriches (Fig. 5); filitriches on proximal bothridial surface not observed. Margin of distal bothridial surface (internal surface of rim) covered with small lingulate spinitriches (Fig. 6); filitriches on distal bothridial surface not observed. Microtriches on centre of distal bothridial surface not observed. Distal portion of bothridial stalks covered with gladiate spinitriches; microtriches on proximal portion of bothridial stalks not observed (Fig. 7). Presence of cephalic peduncle unclear. Surface of strobila covered with capilliform filitriches arranged into scutes.
Scanning electron micrographs and photomicrographs of Caulopatera pagei n. sp. 4. Scolex. 5. Proximal bothridial surface. 6. Margin of distal bothridial surface (internal surface of rim). 7. Distal portion of bothridial stalks; white arrow indicates proximal extent of gladiate spinitriches. 8. Scolex of paratype (QM G 231743). 9. Bothridia of holotype (QM G 231742). 10. Bothridia of paratype (QM G 231746); black arrows in 9–10 indicate attachment positions of caudal stalk. Scale-bars: 4, 100 μm; 5, 6, 1 μm; 7, 10 μm; 8–10, 100 μm
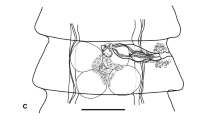
Proglottids acraspedote, 8–11 (10 ± 1; n = 10) in number. Immature proglottids 7–10 (9 ± 1; n = 10) in number. Terminal proglottis mature, much longer than wide (Fig. 2), 1,896–2,819 (2,289 ± 281; n = 15) × 344–468 (406 ± 32; n = 15); length to width ratio 4.79–7.12:1 (5.65 ± 0.69; n = 15). Testes oval in dorsoventral view, 54–88 (76 ± 8; n = 14) in number in terminal proglottis, single layer deep, restricted to region anterior to cirrus-sac, not extending posterior to genital pore, 29–51 (40 ± 4; n = 15; n = 135) × 41–75 (60 ± 6; n = 15; n = 135); length to width ratio 0.46–0.89:1 (0.67 ± 0.1; n = 15; n = 135). Cirrus-sac pyriform to reniform, curves posteriorly, contains coiled cirrus, 238–364 (314 ± 39; n = 15) × 163–242 (191 ± 24; n = 15); length to width ratio 1.29–2.17:1 (1.66 ± 0.22; n = 15). Cirrus armed with small microtriches. Vas deferens medial, overlaps cirrus-sac dorsally, extends in tight coils posteriorly along mid-line of proglottis for distance approximately equal to length of cirrus-sac from posterior margin of cirrus-sac, dorsal to vagina. Vagina medial, extends from genital atrium along anterior cirrus-sac margin, overlaps part of cirrus-sac ventrally, then passes posteriorly along mid-line of proglottis to ovary. Genital atrium shallow. Genital pores lateral, alternate irregularly, 60–69% (66 ± 2; n = 15) of proglottis length from posterior end of proglottis. Ovary near posterior end of proglottis, lobulate, H-shaped in dorsoventral view, 160–310 (227 ± 41; n = 15) × 166–262 (207 ± 24; n = 15). Ovarian bridge asymmetrically frontal. Ovicapt at posterior margin of ovarian bridge, 42–64 (54 ± 7; n = 15) in diameter. Mehlis’ gland posterior to ovary, sometimes bordering posterior margins of ovarian lobes, 80–117 (95 ± 12; n = 10) × 83–119 (97 ± 11; n = 10). Vitellarium follicular, circum-medullary, extends entire length of mature proglottis, absent dorsal to vas deferens, ventral to uterus, both dorsal and ventral to ovary and partly absent both dorsal and ventral to cirrus-sac, sometimes medially absent dorsal to testes; follicles 19–46 (34 ± 5; n = 15; n = 72) × 45–95 (62 ± 10; n = 15; n = 72). Uterus thick-walled, saccate, ventral to vagina, median, extends anteriorly from between anterior lobes of ovary to ventrally overlapping posterior portion of cirrus-sac in mature proglottids. Uterine duct dorsal to uterus, median, extends anteriorly, enters anterior third of uterus just posterior to posterior margin of cirrus-sac. Eggs not seen. Excretory ducts lateral, 2 dorsal and 2 ventral.
Discussion
Caulopatera n. g. is placed in the Phyllobothriidae Braun, 1900 and is consistent with the familial diagnosis given by Euzet (1994) and amended by McKenzie & Caira (1998). Caulopatera lacks the scolex with a single, undivided, globular apical organ of the Disculicipitidae Joyeux & Baer, 1936 and the four cup-shaped, glandular organs of the Prosobothriidae Baer & Euzet, 1955, possessing instead a scolex consisting of four muscular bothridia. The bothridia of Caulopatera are not subdivided by septa, like those of the Serendipidae Brooks & Barriga, 1995, and lack the bothridial hooks of the Onchobothriidae Braun, 1900. The proglottids of Caulopatera are hermaphroditic, unlike the dioecious proglottids of the Dioecotaeniidae Schmidt, 1969, and the vitellarium extends the entire length of the mature proglottis, rather than being confined to the posterior part of the proglottis, as in the Chimaerocestidae Williams & Bray, 1984.
Following the recent elevation of the phyllobothriid subfamily Rhinebothriinae Euzet, 1956 to ordinal status by Healy et al. (2009), there are four subfamilies within the Phyllobothriidae. We propose the placement of Caulopatera in the Phyllobothriinae Braun, 1900 [contrary to much recent literature, we have recorded the authority here as Braun, 1900 in accordance with Article 36.1 (Statement of the Principle of Coordination applied to family-group names) of the International Code of Zoological Nomenclature (ICZN, 1999)]. Caulopatera lacks the apical glandulomuscular myzorhynchus possessed by the Echeneibothriinae de Beauchamp, 1905, lacks the characteristic metascolex of the Thysanocephalinae Euzet, 1953, and lacks the bothridial loculi of the Triloculariinae Yamaguti, 1959. Caulopatera is completely consistent with the diagnosis of the Phyllobothriinae given by Euzet (1994) and as amended by McKenzie & Caira (1998).
Caulopatera can be differentiated from the other genera of the Phyllobothriinae as follows (five other phyllobothriid genera, Anindobothrium Marques, Brooks & Lasso, 2001, Caulobothrium Baer, 1948, Nandocestus Reyda, 2008, Orectolobicestus Ruhnke, Caira & Carpenter, 2006 and Pararhinebothroides Zamparo, Brooks & Barriga, 1999, not currently assigned to a subfamily are also considered here). The new genus differs from Anindobothrium, Bibursibothrium McKenzie & Caira, 1998, Calyptrobothrium Monticelli, 1893, Cardiobothrium McKenzie & Caira, 1998, Ceratobothrium Monticelli, 1892, Crossobothrium Linton, 1889, Dinobothrium van Beneden, 1889, Flexibothrium McKenzie & Caira, 1998, Gastrolecithus Yamaguti, 1952, Guidus Ivanov, 2006, Marsupiobothrium Yamaguti, 1952, Monorygma Diesing, 1863, Nandocestus, Orectolobicestus, Orygmatobothrium Diesing, 1863, Paraorygmatobothrium Ruhnke, 1994 and Phyllobothrium van Beneden, 1850 in the absence of an apical sucker on each bothridium. Caulopatera possesses stalked, circular bothridia with a distinct rim, which differentiates it from Pithophorus, which possesses sessile, tubular bothridia, and from Scyphophyllidium Woodland, 1927, which possesses globular, pouch-shaped bothridia. The bothridia of Caulopatera lack a central sucker and are not separated by a cruciform apex, differentiating it from Clistobothrium Dailey & Vogelbein, 1990, and do not possess marginal or medial loculi, differentiating it from Caulobothrium, Pararhinebothroides and Ruhnkecestus Caira & Durkin, 2006. Caulopatera differs from Anthobothrium van Beneden, 1850 in having a circum-medullary vitellarium, testes restricted to the region anterior of the cirrus-sac and proglottids that are acraspedote.
The new genus most closely resembles Carpobothrium, which also parasitises Chiloscyllium species, including C. punctatum. Both Caulopatera and Carpobothrium have non-loculate, stalked, unhooked bothridia without an accessory sucker and testes that are entirely anterior to the cirrus-sac (Shipley & Hornell, 1906; Subhapradha, 1955). Caulopatera differs from Carpobothrium, however, in that it lacks the slit-like opening in each bothridium and the one to two flaps surrounding the opening (Shipley & Hornell, 1906; Euzet, 1994). Caulopatera also differs from Carpobothrium in that it lacks a pair of bothridial muscular pads, which in Carpobothrium surround the slit-like opening.
Caulopatera possesses bothridial stalks, a feature which is consistent with two orders, the Tetraphyllidea and the Rhinebothriidea. In the recent elevation of the Rhinebothriinae to ordinal status, Healy et al. (2009) used the possession of bothridial stalks as a morphological feature to differentiate Rhinebothriidea from other orders. These authors noted that, although species of the Tetraphyllidea and Rhinebothriidea both possess bothridial stalks, the morphology of the stalks differed between the two orders. It was proposed that the stalks of the phyllobothriine genera Marsupiobothrium and Clistobothrium differed from those of the new order in that they were triangular and surrounded the entire back of the bothridia, whereas the stalks of rhinebothriideans are almost rectangular and attached to the centre of the back of the bothridia (Healy et al., 2009). Caulopatera possesses triangular bothridial stalks surrounding the back of the bothridia, and is thus consistent with the Tetraphyllidea sensu stricto (Figs. 9, 10).
The circum-medullary vitellarium and absence of testes posterior to the genital pore in Caulopatera pagei n. sp. are interesting, as these are features rarely found in phyllobothriine species. Circum-medullary vitelline follicles are more common in the Disculicipitidae, Prosobothriidae, Serendipidae and the onchobothriid genus Platybothrium Linton, 1890 (see Euzet, 1994; Brooks & Barriga, 1995; Caira et al., 2001; Healy, 2003). Within the Phyllobothriinae, circum-medullary vitelline follicles have been recorded only from Monorygma, with all other phyllobothriines possessing lateral vitelline follicles, with the exception of Gastrolecithus, which has a ventral vitellarium (Euzet, 1994; Caira et al., 2001). Circum-medullary vitelline follicles have also been recorded from Nandocestus, which is currently not allocated to a subfamily (Reyda, 2008). Although some species possess quite extensive lateral bands of vitelline follicles (e.g. Clistobothrium montaukense Ruhnke, 1993 (emend.), Paraorygmatobothrium janineae Ruhnke, Healy & Shapero, 2006 and P. kirstenae Ruhnke, Healy & Shapero, 2006), they are still separated medially (Ruhnke, 1993; Ruhnke et al., 2006b). The absence of testes posterior to the cirrus-sac is also a distinctive feature of this new genus. This condition is not common in the Phyllobothriinae and has been reported from a few species of Calyptrobothrium, Carpobothrium, Pithophorus and Gastrolecithus (Subhapradha, 1955; Zaidi & Khan, 1976; Caira et al., 2001; Tazerouti et al., 2007). Elsewhere within the Phyllobothriidae this condition has been reported commonly from the subfamilies Echeneibothriinae and Triloculariinae (Euzet, 1994; Caira et al., 2001).
There is some uncertainty regarding the identity of the host of Caulopatera pagei. A definitive identification will require combined morphological and molecular investigation of the potential species complex that is Chiloscyllium punctatum. The range of C. punctatum is unusually extensive for a demersal elasmobranch, being found throughout the Indo-west Pacific, from eastern India to Japan and northern Australia (Last & Stevens, 2009). Recent collection and examination of this species from Malaysian Borneo uncovered morphological inconsistencies relative to specimens from Australia (Caira et al., 2007). Caira et al. (2007) noted that putative specimens of this species from Malaysian Borneo have some differences in markings from those from Australia and, on the basis of this and discussions with elasmobranch taxonomists, recommended that Australian specimens should be referred to as C. cf. punctatum. As the taxonomic status of this species complex is yet to be resolved, we follow Last & Stevens (2009), and the recommendations of P. Last (pers. com), and record C. punctatum as the type- and only host of Caulopatera pagei, but remain open to the possibility of the evolution of the concept of this species. Molecular specimens of C. punctatum from Moreton Bay have been lodged in the Queensland Museum to aid in a more definitive host identification once the potential Chiloscyllium punctatum species complex is resolved.
Considering the relatively limited helminthological examination C. punctatum has received through most of its Australian range, and the rich fauna of other orectolobiform sharks that can be found off the Australian coast, Caulopatera may be a more widely distributed and speciose genus than currently understood. In addition to C. punctatum, a further 22 species of orectolobiform sharks can be found in Australian waters, including many similarly benthic species from the families Brachaeluridae, Hemiscylliidae, Parascylliidae and Orectolobidae (see Last & Stevens, 2009). Although the tetraphyllidean fauna of some of these orectolobiform species has been the focus of several studies (e.g. Butler, 1987; Campbell & Beveridge, 2002; Caira et al., 2004; Ruhnke et al., 2006a; Caira et al., 2007), more comprehensive sampling of these often endemic Australian sharks, as well as more extensive sampling of C. punctatum from regions along Australia’s northern coastline, may lead to the discovery of more species of Caulopatera and expand our understanding of the new genus.
References
Brooks, D. R., & Barriga, R. (1995). Serendip deborahae n. gen., n. sp. (Eucestoda: Tetraphyllidea: Serendipidae n. fam.) in Rhinoptera steindachneri Evermann, Jenkins, 1891. (Chondrichthyes: Myliobatiformes: Myliobatidae) from southeastern Ecuador. Journal of Parasitology, 81, 80–84.
Butler, S. A. (1987). Taxonomy of some tetraphyllidean cestodes from elasmobranch fishes. Australian Journal of Zoology, 35, 343–371.
Caira, J. N. (1990). The tapeworm Spiniloculus mavensis (Tetraphyllidea: Onchobothriidae) from the brownbanded bambooshark in Australia. Australian Journal of Zoology, 37, 705–710.
Caira, J. N., Jensen, K., & Healy, C. J. (2001). Interrelationships among tetraphyllidean and lecanicephalidean cestodes. In: Littlewood, D. T. J., & Bray, R. A. (Eds) Interrelationships of the Platyhelminthes. London: Taylor & Francis, pp. 135–158.
Caira, J. N., Jensen, K., & Rajan, C. (2007). Seven new Yorkeria species (Cestoda: Tetraphyllidea) from Borneo and Australia and their implications for identification of Chiloscyllium (Elasmobranchii: Orectolobiformes) species. Journal of Parasitology, 93, 357–376.
Caira, J. N., Tracy, R., & Euzet, L. (2004). Five new species of Pedibothrium (Tetraphyllidea: Onchobothriidae) from the tawny nurse shark, Nebrius ferrugineus, in the Pacific Ocean. Journal of Parasitology, 90, 286–300.
Campbell, R. A., & Beveridge, I. (2002). The genus Acanthobothrium (Cestoda: Tetraphyllidea: Onchobothriidae) parasitic in Australian elasmobranch fishes. Invertebrate Systematics, 16, 237–344.
Chervy, L. (2009). Unified terminology for cestode microtriches: a proposal from the International Workshops on Cestode Systematics in 2002–2008. Folia Parasitologica, 56, 199–230.
Euzet, L. (1994). Order Tetraphyllidea Carus, 1863. In: Khalil, L. F., Jones, A., & Bray, R. A. (Eds) Keys to the cestode parasites of vertebrates. Wallingford: CAB International, pp. 149–194.
Healy, C. J. (2003). A revision of Platybothrium Linton, 1890 (Tetraphyllidea: Onchobothriidae), with a phylogenetic analysis and comments on host-parasite associations. Systematic Parasitology, 56, 85–139.
Healy, C. J., Caira, J. N., Jensen, K., Webster, B. L., & Littlewood, D. T. J. (2009). Proposal for a new tapeworm order, Rhinebothriidea. International Journal for Parasitology, 39, 497–511.
ICZN (1999). International code of zoological nomenclature. Fourth edition. London: International Trust for Zoological Nomenclature, 306 pp.
Jensen, K., & Caira, J. N. (2008). A revision of Uncibilocularis Southwell, 1925 (Tetraphyllidea: Onchobothriidae) with the description of four new species. Comparative Parasitology, 75, 157–173.
Last, P. R., & Stevens, J. D. (2009). Sharks and rays of Australia. Collingwood: CSIRO Publishing, 644 pp.
McKenzie, V. J., & Caira, J. N. (1998). Three new genera and species of tapeworms from the longnose sawshark, Pristiophorus cirratus, with comments on their modes of attachment to the spiral intestine. Journal of Parasitology, 84, 409–421.
Reyda, F. B. (2008). Intestinal helminths of freshwater stingrays in southeastern Peru, and a new genus and two new species of cestode. Journal of Parasitology, 94, 684–699.
Ruhnke, T. R. (1993). A new species of Clistobothrium (Cestoda: Tetraphyllidea), with an evaluation of the systematic status of the genus. Journal of Parasitology, 79, 37–43.
Ruhnke, T. R., Caira, J. N., & Carpenter, S. D. (2006a). Orectolobicestus n. g. (Cestoda: Tetraphyllidea), with the description of five new species and the transfer of Phyllobothrium chiloscyllii to the new genus. Systematic Parasitology, 65, 215–233.
Ruhnke, T. R., Healy, C. J., & Shapero, S. (2006b). Two new species of Paraorygmatobothrium (Cestoda: Tetraphyllidea) from weasel sharks (Carcharhiniformes: Hemigaleidae) of Australia and Borneo. Journal of Parasitology, 92, 145–150.
Shipley, A. E., & Hornell, J. (1906). Cestode and nematode parasites from the marine fishes of Ceylon. Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar, Part 5, 43–96.
Southwell, T. (1925). A monograph on the Tetraphyllidea with notes on related cestodes. Memoirs of the Liverpool School of Tropical Medicine (New Series), No. 2, 1–368.
Subhapradha, C. K. (1955). Cestode parasites of fishes of Madras Coast. Indian Journal of Helminthology, 7, 41–132.
Tazerouti, F., Euzet, L., & Kechemir-Issad, N. (2007). Redescription de trois espèces de Calyptrobothrium Monticelli, 1893 (Tetraphyllidea: Phyllobothriidae) parasites de Torpedo marmorata et T. nobiliana (Elasmobranchii: Torpedinidae). Remarques sur leur spécificité parasitaire et sur la position taxonomique des espèces auparavant attribuées à C. riggii Monticelli, 1893. Systematic Parasitology, 67, 175–185.
Williams, H. H. (1964). Some new and little known cestodes from Australian elasmobranchs with a brief discussion on their possible use in problems of host taxonomy. Parasitology, 54, 737–748.
Zaidi, D. A., & Khan, D. (1976). Cestodes of fishes from Pakistan. Biologia, 22, 157–179.
Acknowledgements
We thank John Page, Joanna Stead, Dr Stephen Taylor and Jeremy F.P. Ullmann for their assistance in the collection of elasmobranch specimens, Dr Susan Theiss for assistance with SEM, and Dr Nicole Gunter and Dr Terrence Miller for providing helpful comments on the manuscript. We also thank the Tangalooma Wild Dolphin Resort for support of a broader project to MBB, of which this was a part. Sampling was conducted under DPI&F General Fisheries Permit PRM03951I. All procedures were approved by the UQ Animal Ethics Committee.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cutmore, S.C., Bennett, M.B. & Cribb, T.H. A new tetraphyllidean genus and species, Caulopatera pagei n. g., n. sp. (Tetraphyllidea: Phyllobothriidae), from the grey carpetshark Chiloscyllium punctatum Müller & Henle (Orectolobiformes: Hemiscylliidae). Syst Parasitol 77, 13–21 (2010). https://doi.org/10.1007/s11230-010-9252-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-010-9252-0