Abstract
Numerous specimens of Ancyrocephaloides triacanthi Yamaguti, 1938 and A. chauhani Bychowsky & Nagibina, 1975 were collected from two triacanthid fishes, Triacanthus biaculeatus and Tripodichthys blochii, off Peninsular Malaysia. The two monogenean species are redescribed and considered to be the only valid species of Ancyrocephaloides Yamaguti, 1938. Examinations of these worms revealed new features, e.g. the presence of exudates (both net-like and bundle-like) and superficial grooves in the anchors in both species, which necessitated re-descriptions of the two species and amendments to the generic diagnosis. Both species have relatively small anchors with two lateral superficial grooves along the shaft and point, peduncular glands and four large, pyriform secretory reservoirs in the peduncular-haptoral region, each with a single tubular extension to an associated anchor, and net-like structures (exudate) attached to the anchors. The net-like structures are one of the external manifestations of the secretion produced in the peduncular glands and stored in the pyriform secretory reservoirs. When released within the gill-tissue of the host, the exudate is in the form of bundles which extend within the gill-filament. The small anchors convey secretions from the secretory reservoirs via lateral superficial grooves into the gills as the anchors pierce the host tissue for attachment. The secretion coagulates as left and right thread-like bundles of exudate within the gill tissues and is only apparent as nets when it is released into the surrounding water. The recurved point of the anchor and position of the point of exudation allow the nets to remain attached to the anchor point, even after the detachment of the anchors from the gill tissue. This exudate possibly acts somewhat like a ‘belay device’ or ‘safety belt’, preventing the parasite from being washed away by the respiratory current during the onset of its leech-like locomotion, as well as assist the relatively small anchors in attachment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ancyrocephaloides Yamaguti, 1938 was proposed by Yamaguti (1938) with A. triacanthi Yamaguti, 1938 from Triacanthus biaculeatus (Bloch) off Japan as the type- and only species. Only six nominal species of Ancyrocephaloides have been described to date, four species from T. biaculeatus (= T. brevirostris Temminck & Schlegel) and one each from two non-triacanthid species (Table 1). The generic diagnosis was amended by Bychowsky & Nagibina (1975) when they added A. chauhani Bychowsky & Nagibina, 1975 from T. biaculeatus collected in the Yellow and South China Seas and re-described A. triacanthi. Of the six reported species of Ancyrocephaloides, only A. triacanthi and A. chauhani are considered valid in this study (see below).
In the present investigation, two species of Ancyrocephaloides were collected from Triacanthus biaculeatus and another triacanthid, Tripodichthys blochii (Bleeker), from the waters off the coast of Peninsular Malaysia. The examination of this material revealed features, especially relating to unusual attachment mechanisms, not reported in previous studies, which indicated the need for re-descriptions and an amended generic diagnosis.
Materials and methods
Monogeneans from the gills and gill filaments with attached worms were collected from freshly killed and frozen specimens of Triacanthus biaculeatus and Tripodichthys blochii. Individual detached monogeneans were then cleaned by removing any attached tissue or debri with fine needles and placed on clean slides with a drop of water under a coverslip. Excess water was removed using blotting paper and the four corners of the coverslip were fixed using nail varnish to prevent it from moving. Ammonium picrate glycerine was added beneath the coverslip to fix and clear the specimens. The same process was also used for monogeneans still attached to gill filaments. For some specimens, formalin (10%) was introduced beneath the coverslip, and later ammonium picrate glycerine was added to clear the specimens and prevent them from drying. To prepare permanent, stained and unstained specimens, coverslips were removed from the above preparations and the parasites on the slides were washed several times in distilled water; some were then stained with Gomori’s trichrome to reveal the reproductive system. The stained and unstained specimens were dehydrated in a series of alcohols, cleared in xylene and mounted in Canada balsam. Gills with attached worms were also collected and preserved in neutralised formalin; they were then dehydrated, embedded in wax and sectioned at 5–6 μm. The sections were stained in Harris’ haematoxylin and eosin (H & E), dehydrated in an alcohol series, cleared in xylene and mounted in Canada balsam.
These temporary and permanent preparations were studied under bright-field and phase contrast microscopy. Images of the hard and soft parts were first captured using a Leica digital camera and measured using Leica QWin Plus software. The captured images were exported and drawn on a digitising tablet (WACOM) using Adobe Illustrator software. Measurements of the sclerotised parts (haptoral and reproductive hard-parts) were made on specimens flattened and fixed in ammonium picrate glycerine or permanently mounted, stained or unstained, in Canada balsam. The basic measurements taken are as indicated in Fig. 2 and are given as the mean and range (in parentheses) in micrometres; in the case of two-dimensional measurements, the length is given before the width. All dimensions were recorded for each specimens (numbers given in the text), except in the case of marginal hooks, where as many as possible were measured. Composite illustrations of the two Ancyrocephaloides species are also given.
In this paper the term secretory reservoirs refers to the pyriform structures with a long, thin extension or duct found in the peduncular-haptoral region, which store secretions from the peduncular glands. The term superficial groove refers to the superficial depressions found on the left and right sides of the shaft and point of the anchors. The convergence point is where the two superficial lateral grooves appear to meet on the upper surface of the recurved point, a short distance from the tip. The secretions are produced in the peduncle but secreted via the anchors, and they are herein known as haptoral secretions. Once released and coagulated, they are referred to as exudates, which are manifest as net-like structures in seawater or thread-like bundles in the gill-tissues.
Ancyrocephaloides triacanthi Yamaguti, 1938
Type-host: Triacanthus brevirostris Temminck & Schlegel (now T. biaculeatus (Bloch)).
Type-locality: Off Hamana-ko, Japan.
Other records: Yellow Sea, off China (Bychowsly & Nagibina, 1975); South China Sea, off Hong Kong (Bychowsky & Nagibina, 1975); Indian Ocean, off Colombo, Sri Lanka (Bychowsky & Nagibina, 1975).
Present material: Ex Triacanthus biaculeatus from off Pulau Langkawi, off Lumut, Perak and off Kuantan, Pahang; ex Tripodichthys blochii (new host record) from off Lumut, Perak.
Site: Gills.
Studied material: 50 specimens; 10 specimens measured.
Deposited material: 5 voucher specimens BM(NH) 2007.4.12.1 and 2007.4.12.4 in the Natural History Museum, London; 6 voucher specimens in the University of Malaya collection MZUM(P) 581–586(v).
Redescription (Figs. 1–4)
Body elongate, 1,043 (733–1,167) × 157 (139–172); anterior region with 3 pairs of head-organs of dactylogyrid type and 2 pairs of pigmented eye-spots; peduncle long, 134 (85–192) × 139 (125–147), tapers to 140 (114–163), with bi-lobed haptor. Mouth subterminal; pharynx 56 (35–63) × 51 (37–59), ovate; oesophagus short; intestinal bifurcation just anterior to male genital pore; caeca pass back laterally to reproductive organs and unite to form cyclocoel just posterior to testis. Numerous glands present in anterior of peduncle just posterior to caecal confluence; 4 large, prominent, pyriform secretory reservoirs at junction between peduncle and haptor, each extended into long tube which leads to an anchor.
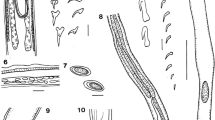
Ancyrocephaloides triacanthi Yamaguti, 1938. 1. Composite illustration (dorsal view). 2. Sclerotised hard-parts: a. ventral anchor with groove (gr); b. ventral bar; c. dorsal anchor with groove (gr); d. dorsal bar; e1, e2. marginal hooks; f1. copulatory organ; f2. distal part of copulatory tube. Abbreviations indicate the parameters measured (ir, inner root; il, inner length; or, outer root; ol, outer length; pt, point; ie, inner end; oe, outer end)
Light microscope image of the haptor of Ancyrocephaloides triacanthi Yamaguti, 1938 showing the secretory reservoirs (SR) and lower left dorsal anchor with an attached net-like structure (N). Scale-bar: 20 μm
Light microscope image of the net-like structures (N) of Ancyrocephaloides triacanthi Yamaguti, 1938 attached close to the tip of the anchor points of the dorsal (DA) and ventral (VA) anchors; also showing the marginal hooks (M) on one of the lateral lobes of the haptor and a lateral groove (G) in an anchor point. Scale-bar: 10 μm
Haptor bi-lobed, 101 (71–144) × 206 (184–232), well set off from body, armed with 14 larval-type marginal hooks and 2 pairs of anchors. Marginal hooks 12 (10–13) in length. Anchors of similar shape and size, set apart on haptoral lobes, with cap-like pieces on inner and outer roots, with lateral superficial grooves running along both sides of the shaft and recurved point and converging close to tip of point; filament loop present; dorsal anchor, with inner root 6 (4–8), inner length 17 (16–18), outer length 14 (13–15), smaller outer root 3 (2–4), recurved point 13 (10–14); ventral anchor with inner root of 5 (4–6), inner length 16 (15–16), outer length 15 (14–17), smaller outer root 3 (2–4), recurved point 13 (11–14). Four connecting bars, 2 dorsal and 2 ventral; dorsal bar 17 (15–18), outer end round, 3 (2–4) wide, inner end 2 (2–3) wide; ventral bar 14 (13–16), outer end 3 (2–3) wide, inner end 2 (1–3) wide, with protuberance close to one end and furrow along mid-section. Exudates (see below) are normally attached to anchor point (Figs. 3, 4) (may become detached if not carefully collected).
Testis ovoid, postovarian. Vas deferens leaves antero-medial region of testis, ascends intercaecally before looping sinistral caecum to ventral side, then distends to form seminal vesicle; as latter ascends, it narrows, passes forward to level of intestinal bifurcation, reflexes, descends and opens into copulatory tube. Copulatory organ 251 (238–279) long, lacks accessory piece, consists of 3 distinct parts: funnel-like proximal part 9 (6–12) long, 11 (9–12) wide; uncoiled proximal tube 28 (25–31) long, narrows from 9 (7–11) to 4 (3–5); followed by filiform, coiled (4 times, clockwise in ventral view) distal tube 213 (197–240) long, tapers from 3 (2–4) to 2 (1–2). In un-flattened worms, coils of distal copulatory tube arranged compactly.
Ovary in mid-body; oviduct leaves anterior extremity of ovary, receives vaginal duct and duct from vitelline reservoir, ascends forming oötype which is surrounded by Mehlis’ gland, continues anteriorly as uterus to uterine pore near copulatory organ. Vaginal pore dextrally sub-marginal at same level as oviduct; seminal receptacle not observed. Left and right vitelline ducts open into muscular vitelline reservoir located antero-ventral to ovary.
Comments
Bychowsky & Nagibina (1975) redescribed A. triacanthi, noting the presence of four connecting bars, which were not observed in the original description given by Yamaguti (1938), and stating that lateral furrows on the anchor (seen in A. chauhani) were absent. Lateral grooves were, however, observed on the anchors of the present specimens of A. triacanthi. In addition, net-like structures were observed in the new material closely associated with the four anchors (see Fig. 3 and ‘Discussion’). Additional newly reported features include the presence of a vitelline reservoir.
Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975
Type-host: Triacanthus brevirostris Temminck & Schlegel (now T. biaculeatus (Bloch)).
Type-locality: Yellow Sea, off China.
Other locality: South China Sea, off Hong Kong (Bychowsky & Nagibina, 1975).
Present hosts: Triacanthus biaculeatus (Bloch) off Langkawi, Kedah.
Site: Gills.
Present material: 17 specimens studied; 17 specimens measured.
Deposited material: 3 voucher specimens BM(NH) 2007.4.12.2 and 2007.4.12.5–6 in the Natural History Museum, London; 6 voucher specimens in the University of Malaya collection MZUM(P) 587–592(v).
Redescription (Figs. 5–12)
Body elongate, 1,740 (1,572–2,015) × 236 (176–356); anterior region with 3 pairs of head-organs of dactylogyrid type and 2 pairs of pigmented eye-spots. Mouth subterminal; pharynx ovate; oesophagus short; intestine forms 2 caeca; caeca pass back laterally to reproductive organs and unite to form cyclocoel just posterior to testis. Peduncle long, indistinctly delineated but clearly delimited by contents, 447 (409–517) in length, narrowing from 284 (202–517) to 173 (159–181) at junction with haptor, contains large peduncular glands surrounding 4 large, prominent, pyriform secretory reservoirs, each of which narrows posteriorly to form tube that leads to an anchor.
Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975. 5. Composite illustration (ventral view). 6. Sclerotised hard-parts: a. ventral anchor with groove (gr); b. ventral bar; c. dorsal anchor with groove (gr); d. dorsal bar; e1, e2. marginal hooks; f1–f2. different views of copulatory organ. Abbreviations: ie, inner end; oe, outer end
Light microscope image of the haptor of Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975, showing the dorsal and ventral anchors (A) on the haptoral lobes (L), with net-like structures (N) and thread-like exudates in bundles (B) attached to the anchors on the left. The gill-tissue is removed. Scale-bar: 40 μm
Light microscope image of the haptor of Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975, indicating the possible junction (J) of a tubular extension (T) of the secretory reservoir and an anchor (A). Scale-bar: 10 μm
Histological section of Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975 attached to the gill of Triacanthus biaculeatus, showing the penetration of an anchor (A), secretion (exudate) (S) in the gill tissue (GT) and secretion (ST) in a tubular extension of a secretory reservoir. Scale-bar: 20 μm
Light microscope image of the haptor of Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975, showing net-like structures (N) attached to the dorsal (DA) and ventral anchors (VA) near their tip and lateral grooves (GR) on the shaft and point. Scale-bar: 10 μm
Light microscope image of the haptor of Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975, showing net-like structures (N) attached near the tips of the dorsal (DA) and ventral anchors (VA) and to the thread-like bundles (B) from the gill tissue (the actual gill-tissue is removed). Scale-bar: 20 μm
Light microscope image of the haptor of Ancyrocephaloides chauhani Bychowsky & Nagibina, 1975, showing the left and right bundles of coalesced thread-like exudates (B) (with gill tissues removed), anchors attached to net-like exudates (N) and the secretory reservoirs (SR) with long tubular extensions (T) leading to the anchors. Scale-bar: 40 μm
Bi-lobed haptor set off from body, 106 (84–121) × 250 (191–293), with 14 larval-type marginal hooks of 14(13–15) in length and 2 pairs of small, stout anchors of similar shape and size; anchors set apart, with cap-like pieces on inner and outer roots, shaft and recurved point with 2 superficial lateral grooves which converge near tip of point; filament loop present; dorsal anchors with inner root 8 (6–9), inner length 23 (22–24), outer root 3 (2–4), outer length 17 (15–18), point 20 (16–21); ventral anchors with inner root 6 (4–8), inner length 21 (19–22), outer root 4 (3–5), outer length 19 (17–22), point 20 (15–25). Exudates (see below) are normally attached to anchor points (Figs. 7–9, 11, 12) (may be detached if not carefully collected). Four connecting bars; 1 for each anchor; dorsal bars 15 (14–17), with rounded outer end 5 (4–6) wide, inner end 3 (2–3) wide; ventral bars 20 (19–22), with outer end 2 (2–3) wide, inner end 3 (2–3) wide; groove present in middle of ventral bars and protuberance close to one end.
Testis subovoid, postovarian. Vas deferens leaves antero-medial region of testis, ascends intercaecally, loops left intestinal caecum, then distends to form large seminal vesicle, which ascends and narrows, then reflexes and descends to open into copulatory tube. Copulatory organ 92 (86–98) long, lacks accessory piece; copulatory tube ham-shaped proximally, consists of funnel-shaped proximal part of 27 (22–29) in length, with 20 (18–23) wide opening narrowing to 15 (14–15); tube 32 (27–36), narrowing from 15 (14–15) in diameter to 5 (4–7), curved (90°) in subdistal expanded section, with short, tapered, scoop-like (but not filiform) extremity, 32 (27–41) in length.
Elongate ovary in mid-body; oviduct arises anteriorly, receives vaginal duct and duct from medial vitelline reservoir, ascends forming oötype which is surrounded by Mehlis’ gland, continues anteriorly as uterus; uterine pore immediately posterior to intestinal bifurcation. Vaginal pore dextrally submarginal at same level as oviduct; vaginal duct descends to open into oviduct; no seminal receptacle observed. Left and right main vitelline ducts lead into muscular vitelline reservoir located antero-ventrally to ovary.
Comments
This species resembles A. triacanthi in possessing similar small (relative to the body size), robust anchors, with cap-like pieces on the roots, superficial grooves along the shaft and anchor point, and four connecting bars. In both species, net-like structures were observed attached close to the tips of the anchor point. The pyriform secretory reservoirs are the ‘large oval elongated glands’ of Bychowsky & Nagibina (1975). However, A. chauhani differs from A. triacanthi in the morphology of the copulatory tube (cf. Figs. 2d and 5d), in being 1.5–2 times larger and by having larger anchors and secretory reservoirs.
According to Bychowsky & Nagibina (1975), there is a bubble-like seminal receptacle; but in the present specimens no such structure was observed. However, we were able to discern a medial vitelline reservoir located antero-ventrally to the ovary. Furthermore, the vas deferens was observed to ascend intercaecally past the level of the ovary, coil around the left intestinal caecum to the ventral side and then distend to form the seminal vesicle, whereas Bychowsky & Nagibina (1975) described the vas deferens as not looping the left caecum. Pyriform secretory reservoirs have been observed by all the previous authors. However, none of them reported the net-like or other structures attached to the anchor points.
Haptoral exudates (net-like structures and thread-like bundles) (Figs. 7–12)
In the two species of Ancyrocephaloides examined in this study, the haptor is distinctly bi-lobed and the anchor pairs are widely separated in the haptoral lobes (Figs. 1, 3, 7). Each of the secretory reservoirs has a tubular extension (duct) which leads to the anchor at a point near the outer root (Fig. 8), although the exact point of connection could not be determined. We were not able to locate any pore or aperture in the anchors or see the reservoir duct extending directly to the superficial lateral grooves on the anchors, but, as it seems clear that the secretions are introduced into the gill tissues by the anchors (Fig. 9), the secretion may pass through an internal bifurcate canal to the lateral grooves on the anchor (a single medial duct has been seen in TEM sections of the proximal shaft of a species of Bravohollisia Bychowsky & Nagibina, 1970 by Wong et al., in press). After being directed into left and right superficial grooves, the secretions appear to discharge together a short distance from the tip of the anchor point, as indicated by the attachment of the resultant exudate (Figs. 3, 4, 7). Careful examination of the anchors indicates that superficial grooves do occur on both sides of the anchor (Figs. 2a-b, 4a-b, 10) and that these two grooves appear to meet and open together (point of convergence) close to the tip of the anchor point, as suggested in Fig. 10. The presence of two superficial lateral grooves is also indicated by the facts that grooves are observed no matter how the anchor is orientated (Fig. 10) and that similar structures have been observed on the anchors of species of the related Bravohollisia and Caballeria Bychowsky & Nagibina, 1970, which also use haptoral exudates for attachment (Lim, 1995). The possibility that there is only a single central canal rather than two superficial grooves has also been considered for members of these two genera; however, in Bravohollisia deep lateral indentations are clearly visible in TEM cross-sections (Wong et al., in press), indicating that two lateral superficial grooves are present.
When the secretions from the secretory reservoirs are introduced into the gill tissues by the insertion of the anchors (Fig. 9), those from the anchors (dorsal and ventral) on the same lobe of the haptor coalesce to form a single thread-like bundle which spreads for some distance within the gill-filament (Figs. 7, 8, 11), resulting in two long bundles (left and right) of exudate per worm. When these same secretions are released into seawater (Fig. 10), they coagulate and the resulting exudate forms a net-like structure.
Histological sections show the presence of red-stained (H & E) secretions surrounding the tip of the anchors inside the gill tissue, which appear identical to the product in the secretory reservoirs (Fig. 9). These secretions in the gill tissues (S in Fig. 9) are in the same position as seen in LM observations and TEM sections of Bravohollisia rosetta Lim, 1995 and B. gussevi Lim, 1995 (Wong et al., in press). This further supports the notion that the secretions from the reservoirs are introduced into the gill tissue by the anchors.
Discussion
The haptoral secretions/exudates
The presence of left and right bundles of thread-like exudate in the gill-tissue (Fig. 12) provides a possible explanation for the role or roles of the copious haptoral secretions in the attachment process of the worm. The images (Figs. 7, 11, 12) of the worm hanging on to the gill-tissues by the net-like structures and bundles of exudate suggest that together these two forms of the exudate function like a kind of ‘belay-device’ or ‘safety belt’ (see also Wong et al., in press) to help prevent the worm from being washed away by the strong respiratory current of the host, especially during the initial stage of the worm’s locomotion when the anchors disengage and become detached from the gill-tissues. It is also possible that the presence of the flexible nets will reduce stress upon, and thus result in less damage to, the gill-tissue of the host than would be caused by the more rigid anchors pulling against these tissues. The coagulated secretory product may also be somewhat elastic; if so, this would enable the worm to extend its body further anteriorly for the temporary engagement of their head organs (Wong et al., 2006) during locomotion. Once the head organs are firmly attached, the anchors can be withdrawn into the haptor, thus completely cutting off the flow of secretion and disconnecting the worm from the exudate. The function of the exudates as accessory attachment mechanisms would explain how the rather small anchors of these worms are able to secure this relatively large worm in position.
The species of Ancyrocephaloides
Four of the six nominal species of Ancyrocephaloides are not considered valid in this work (Table 1). Gupta & Sharma (1979) described A. yamagutii Gupta & Sharma, 1979 and A. mizellei Gupta & Sharma, 1979 from non-triacanthid hosts, Dermogenys (= Hemiramphus) brachynopterus (Bleeker) (Hemirhamphidae) and Eupleurogrammus (= Trichiurus) muticus (Gray) (Trichiuridae), respectively, caught off Pamban, Tamilnadu, India. In view of their hosts, the limited description and resemblance to A. chauhani and A. triacanthi, respectively, they are treated here as species inquirendae, pending further research. The illustrations of A. indicus Karyakarte & Das, 1979 from T. biaculeatus off Ratnagiri, Maharashtra, India (see Karyakarte & Das, 1979) indicate that this species is a synonym of A. chauhani. Similarly, we believe that the illustrations of A. chilkai Agrawal & Yadav, 1996 from T. biaculeatus in Balugan, Chilka Lake, Orissa, India (see Agrawal & Yadav, 1996) strongly suggest that this species is a synonym of A. triacanthi. Hence, only A. triacanthi and A. chauhani are considered here to be good species.
Amendments to generic diagnosis of Ancyrocephaloides
There are several, and some unusual, aspects of the morphology of these worms which require comment. The coiled, distal, fliliform end of the copulatory organ of A. triacanthi could be considered a basic modification (elongation) of the distal, tapering, scoop-like part of the copulatory organ of A. chauhani (cf. Figs. 2d and 5d). A medial muscular vitelline reservoir situated antero-ventral to the ovary is observed in both species. Furthermore, they possess a long peduncle with numerous glands and a bi-lobed haptor with small anchors. The haptor has four bars and four anchors with a sclerotised cap on each root and superficial grooves on both sides of the shaft and recurved point which converge a short distance from the tip of the anchor point. Large pyriform secretory reservoirs release secretions which coagulate to form net-like structures externally or thread-like bundles within the host tissues. The net-like structures, which appear at the point of convergence of the grooves on the anchors, were not reported by previous authors (Yamaguti, 1938; Bychowsky & Nagibina, 1975). Bychowsky & Nagibina (1975) noted, in their amendment to the generic diagnosis, that the vagina is sinistral, although they indicated the vaginal position as dextral in their description. In view of these comments, the generic diagnosis is amended below to include these new data.
Ancyrocephaloides Yamaguti, 1938
Generic diagnosis. Monogeneans (Dactylogyridea: Ancyrocephalidae (sensu Gussev, 1976)) with 3 pairs of head-organs, 2 pairs of eye-spots and bi-lobed haptor separated from body proper by indistinctly delineated peduncle. Mouth subterminal; pharynx oval; oesophagus short; intestinal bifurcation just anterior to copulatory organ; caeca unite between testis and peduncle to form cyclocoel. Haptor with 4 relatively small recurved anchors, 4 small but distinctive connecting bars and 14 marginal hooks; anchors with superficial grooves on either side of recurved point. Peduncle with large secretory glands. Posterior to peduncular glands and extending into haptor are 4 large pear-shape or pyriform secretory reservoirs, each with single posterior extension (duct) leading to each of 4 anchors; this secretion passes along 2 lateral grooves in anchor shaft and point to be released short distance from tip of point; secretion forms net-like structure if released in seawater or thread-like bundles when released into host gill-tissue. Large testis postovarian; vas deferens arises from anterior region of testis to loop left intestinal caecum, then distends to form large seminal vesicle which tapers anteriorly to open into simple copulatory organ without accessory piece. Ovary smaller than testis; oviduct arises from anterior ovary, receives duct from vagina and vitelline ducts from vitelline reservoir, forms oötype which is surrounded by Mehlis’ gland; uterus continues to uterine pore located near male pore. Vaginal pore dextrally submarginal. Parasitic on members of Triacanthidae. Type-species: A. triacanthi Yamaguti, 1938. Other valid species, A. chauhani Bychowsky & Nagibina, 1975.
References
Agrawal, N., & Yadav, V. S. (1996). Studies on three monogeneans on Triacanthus brevirostris (Temm. & Schleg) from Chilka Lake, Orissa. Indian Journal of Helminthology, 13, 56–63 (NS).
Bychowsky, B. E., & Nagibina, L. F. (1975). New data about genus Ancyrocephaloides Yamaguti, 1938 (Dactylogyridae, Ancyrocephalinae). In K. K. Tiwari, C. B. L. Srivastava, & R. B. S. Chauhan (Eds.), Commemorative volume (pp. 68–73). Orissa: Zoological Society of India.
Gupta, S. P., & Sharma, R. K. (1979). Two new monogenetic Trematoda of the genus Ancyrocephaloides Yamaguti, 1938 from marine fishes of Pamban, Tamilnadu. Indian Journal of Helminthology, 31, 36–44.
Karyakarte, P. P., & Das, S. R. (1979). A new species of monogenetic trematode Ancyrocephaloides (Monopisthocotylea, Dactylogyridae) from the fish Triacanthus brevirostris in India. Rivista di Parassitologia, 40, 9–12.
Lim, L. H. S. (1995). Bravohollisia Bychowsky & Nagibina, 1970 and Caballeria Bychowsky & Nagibina, 1970 (Monogenea: Ancyrocephalidae) from Pomadasys hasta (Bloch) (Pomadasyidae), with the description of a new attachment mechanism. Systematic Parasitology, 32, 211–224.
Wong, W. L., Brennan, G. P., Halton, D. W., & Lim, L. H. S. (2006). Fine structure of the head organ (anterior adhesive apparatus) of Bravohollisia gussevi Lim, 1995 (Monogenea: Ancyrocephalidae). Parasitology, 132, 427–438.
Wong, W. L., Brennan, G. P., Halton, D. W., Maule, A. G., & Lim, L.H.S. (in press). Secretory products of the haptoral reservoirs and peduncular glands of Bravohollisia rosetta Lim, 1995 and B. gussevi Lim, 1995 (Monogenea: Ancyrocephalidae). Invertebrate Biology.
Yamaguti, S. (1938). Studies on the helminth fauna of Japan. Part 24. Trematodes of fishes. Japanese Journal of Zoology, 8, 15–74.
Acknowledgements
We would like to thank Mr Liew K.S. for preparing the specimens and for helping with the fieldwork and Mr Wong W.L. for assistance with the histology. We are also grateful to two reviewers whose comments greatly improved the manuscript. This work is made possible by an R&D grant from the Malaysian Government to the senior author.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, L.H.S., Gibson, D.I. Redescriptions of species of Ancyrocephaloides Yamaguti, 1938 (Monogenea: Ancyrocephalidae) from triacanthid fishes caught off Peninsular Malaysia and a report of their haptoral secretions. Syst Parasitol 69, 59–73 (2008). https://doi.org/10.1007/s11230-007-9112-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-007-9112-8