Abstract
The onchobothriid tapeworm genus Megalonchos Baer & Euzet, 1962 is revised and the generic diagnosis amended based on the examination of some of Southwell’s material of M. mandleyi (Southwell, 1927) Baer & Euzet, 1962, the type-specimens of M. dubius Prudhoe, 1969 and M. musteli Prudhoe, 1969, and material of two new species, M. sumansinghai n. sp. and M. shawae n. sp., collected from the snaggletooth shark Hemipristis elongatus off northern Australia. Based on their possession of two pairs of uni-pronged hooks (rather than one pair of bi-pronged hooks) and possession of, rather than lack of, post-vaginal testes, M. dubius and M. musteli are transferred to Biloculuncus Nasin, Caira & Euzet, 1997 as B. dubius (Prudhoe, 1969) n. comb. and B. musteli (Prudhoe, 1969) n. comb. Both new species of Megalonchos differ from M. mandleyi in their possession of conspicuously smaller hooks and shorter cephalic peduncles. The new species are readily distinguished from one another in that, whereas the pores of the axial prongs of the medial and lateral hooks are located well anterior to the middle of the prong in M. sumansinghai n. sp., they are well posterior to the middle of the prongs in M. shawae n. sp. In addition, the base of the lateral hook is longer relative to that of the medial hook in the latter species than it is in the former species. The proglottid anatomy of valid species of Megalonchos is described for the first time, and the lack of post-vaginal testes is confirmed for the genus. In addition, members of this genus appear to be characterised by a sacciform uterus that extends only to the level of the cirrus-sac and an ovary that is H-shaped in frontal view and bilobed in cross-section. Species of Megalonchos have now been reported from two of the eight known species of hemigaleid sharks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1927, Southwell described the tetraphyllidean tapeworm Uncibilocularis mandleyi (Southwell, 1927) from the spiral intestine of a hemigaleid shark, Hemigaleus balfouri Day [=Chaenogaleus macrostoma (Bleeker)], off Sri Lanka. Because all of his specimens were immature, Southwell was unable to describe the proglottid anatomy of his new taxon. Southwell (1930) subsequently provided additional information on the scolex of this species, but once again from immature specimens. Based on their examination of some of Southwell’s material, Baer & Euzet (1962) noted that, although proglottid anatomy was difficult to discern in these specimens, it was possible to confirm that the species lacked post-vaginal testes. On the basis of this feature and the unusual form of the hooks, Baer & Euzet erected Megalonchos Baer & Euzet, 1962. To our knowledge, M. mandleyi (Southwell, 1927) Baer & Euzet, 1962 is known only from Southwell’s immature specimens and, as a consequence, its proglottid anatomy remains poorly known.
In 1969, Prudhoe described Megalonchos musteli Prudhoe, 1969 and M. dubius Prudhoe, 1969 from the gummy shark Mustelus antarcticus Günther collected from the Derwent Estuary at Hobart, Tasmania, Australia by the British, Australian and New Zealand Antarctic Research Expedition (B.A.N.Z.A.R.E.). Prudhoe (1969) also provided the first formal diagnosis for the genus based on morphological information from Megalonchos mandleyi, as well as his two new species. However, it now seems clear that Prudhoe’s species are much more consistent in morphology with Biloculuncus Nasin, Caira & Euzet, 1997 than they are with M. mandleyi, as both of Prudhoe’s species possess post-vaginal testes and bothridia that bear two pairs of uni-pronged hooks rather than one pair of bi-pronged hooks. As a consequence, Prudhoe’s species are formally transferred below to Biloculuncus.
The decision to transfer Prudhoe’s species out of Megalonchos was influenced to a large part by our recent examination of a second species of hemigaleid shark, Hemipristis elongatus (Klunzinger), from off northern Australia, which resulted in the collection of two new species of the genus. These species are consistent with M. mandleyi in a number of respects, but most conspicuously in their possession of bi-pronged hooks that lack talons but exhibit pores on their axial prongs, and also in their lack of post-vaginal testes. Although both species are bordering on being hyperapolytic in that no fully mature attached proglottids were seen, the terminal proglottids were sufficiently developed as to provide additional information on the proglottid anatomy of the genus. These two new species are described below and the diagnosis of Megalonchos is amended to include information on proglottid anatomy available from the new species, as well as to exclude Prudhoe’s two species.
Materials and methods
Sharks were caught using a bottom trawl on the FV ‘Ocean Harvest’ in the Arafura Sea, northeast of the Wessel islands off northern Australia in November, 1999. Two males (117 and 162 cm in total length) and two females (106 and 132 cm in total length) of the snaggletooth shark Hemipristis elongatus were dissected. Spiral intestines were removed and opened with a longitudinal incision and a sample of cestodes was transferred to seawater-buffered formalin and shaken relatively vigorously for approximately one minute. Cestodes were subsequently transferred to fresh seawater-buffered 10% formalin for approximately one week and then to 70% ethanol for storage. Spiral intestines were fixed in seawater-buffered formalin for several weeks and then transferred to 70% ethanol for storage.
Cestodes prepared as whole-mounts were hydrated in a graded ethanol series, stained in Delafield’s haematoxylin, dehydrated in a graded ethanol series, cleared in methyl salicylate and mounted on glass slides in Canada balsam. Measurements were taken using an ocular reticle or a Hitachi HV-C20 video camera mounted on a Zeiss Axioskop using the image analysis software ImagePro® Express. Measurements in micrometres (except where indicated) are given in the text as ranges followed in parentheses by the mean, standard deviation, number of specimens examined and number of measurements taken. Owing to the somewhat immature state of attached proglottids, measurements of the ovary and cirrus-sac are not presented. Illustrations were made with the aid of a drawing tube. Hook measurements taken are illustrated in Fig. 1. It should be noted that the length of the axial hook prong anterior to the pore is designated as B1, and B1’ in the lateral and medial hooks, respectively, and the length of the axial hook prong posterior to the pore is designated as B2 and B2’, in the lateral and medial hooks, respectively.
Histological sections were prepared from the terminal proglottid of two specimens of Megalonchos sumansinghai n. sp. from H. elongatus. The remainder of the strobila of each specimen was prepared as a whole-mount. Sections were prepared according to the following conventional techniques. Proglottids were embedded in paraffin and sectioned at 8 μm intervals using an Olympus CUT4060 retracting rotary microtome. Sections were mounted on glass slides flooded with 2.5% sodium silicate and dried on a slide warmer for 4–8 hr. Sections were stained with Gill’s haematoxylin and eosin, cleared in xylene and mounted with coverslips on glass slides in Canada balsam.
Scoleces of two specimens of both new species were prepared for examination using scanning electron microscopy (SEM). These specimens were hydrated in a graded ethanol series, transferred to 1.5% osmium tetroxide overnight, dehydrated in a graded ethanol series and placed in hexamethyldisilizane (HMDS, Ted Pella Inc., Redding, CA) for 15 min. They were allowed to air dry and were subsequently mounted on carbon tape and grounded with carbon paint on aluminum stubs. Specimens were sputter-coated with c.40 nm of gold/palladium and examined with a LEO/Zeiss DSM 982 Gemini Field Emission Scanning Electron Microscope.
Museum abbreviations used are as follows: BMNH, Natural History Museum, London, UK; LRP, Lawrence R. Penner Parasitology Collection, University of Connecticut, Storrs, Connecticut, USA; QM, Queensland Museum, Brisbane, Australia; SAMA, South Australian Museum, Adelaide, Australia; and USNPC, United States National Parasite Collection, Beltsville, Maryland, USA. For comparative purposes, some of Southwell’s material of Uncibilocularis mandleyi (BMNH Nos 1999.2.17–1-10) as well as the syntypes of Megalonchos dubius (SAMA V156-V158) and the syntypes of M. musteli (SAMA V152-V155) were examined. Shark nomenclature follows Compagno (1984).
Results
The current generic placement of Megalonchos dubius and M. musteli requires reassessment in light of the current circumscription of onchobothriid genera. These two species are not alone among onchobothriids in their possession of bothridia with two loculi; in fact, a number of onchobothriid genera exhibit this condition. These are Uncibilocularis Southwell, 1925, Phoreiobothrium Linton, 1889, Megalonchos, Erudituncus Healy, Scholz & Caira, 2001 and Biloculuncus. These genera differ most conspicuously from one another in the morphology of their hooks. Whereas the former three genera possess one pair of hooks per bothridium (e.g. see Southwell, 1925; Caira, Richmond & Swanson, 2005; Baer & Euzet, 1962, respectively), the latter two genera possess two pairs of hooks per bothridium (e.g. see Healy et al., 2001; Caira & Ruhnke, 1990, respectively); although the axial hook in each pair is bi-pronged in Erudituncus, it is uni-pronged in Biloculuncus. Whereas most species of Phoreiobothrium possess tri-pronged hooks, members of Uncibilocularis and Megalonchos possess bi-pronged hooks, but while the bi-pronged hooks of Uncibilocularis possess expanded bases and axial prong talons, those of Megalonchos lack expanded bases and axial prong talons, and in fact, appear to be unique in their possession of pores that are not associated with talons (or tubercles). Both M. dubius and M. musteli clearly possess two pairs of uni-pronged hooks per bothridium and thus, among the onchobothriid genera with two bothridial loculi, are most similar to Biloculuncus. Their possession of a post-vaginal field of testes further supports their closer affinity to the latter genus than to Megalonchos, and they are hereby formally transferred as Biloculuncus dubius (Prudhoe, 1969) n. comb. and B. musteli (Prudhoe, 1969) n. comb. This brings the total number of species of Biloculuncus to three. B. musteli is easily distinguished from B. pritchardae (Caira & Ruhnke, 1990) Nasin, Caira & Euzet, 1997, as described by Caira & Ruhnke (1990), in that it is a much smaller worm (1.5–2.7 vs 27–49 mm) that possesses many fewer proglottids (4–7 vs 51–78) and fewer testes (35–50 vs 88–107). B. dubius most conspicuously differs from B. pritchardae in its possession of a greater number of testes (120–150 vs 88–107). The diagnosis of Biloculuncus of Nasin et al. (1997) is fully consistent with the morphology of the newly transferred species and thus does not require amendation to include these new members.
Megalonchos mandleyi (Southwell, 1927) Baer & Euzet, 1962
Syn. Uncibilocularis mandleyi Southwell, 1927
Type-host: Chaenogaleus macrostoma (Bleeker) (=Hemigaleus balfouri Day), hooktooth shark (Carcharhiniformes: Hemigaleidae).
Additional hosts: None.
Site of infection: Spiral intestine.
Type-locality: Off Sri Lanka.
Additional localities: None.
Material-examined: Southwell’s voucher specimens: BMNH 1999.2.17–1-10.
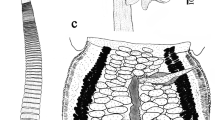
Partial redescription (Fig. 2)
Southwell’s specimens are somewhat macerated, but allow us to provide more specific details of hook measurements including, for the first time, the location of the axial prong pores. This redescription is based on 7 whole-mounts and 4 mounts consisting solely of scoleces.
Hooks bi-pronged, hollow, lacking tubercles or talons on axial prongs, with inconspicuous tubercle on proximal, posterior margin of medial and lateral hook bases; internal channels of axial and abaxial prongs continuous, smooth; lateral hooks slightly larger than medial hooks; axial prongs of both hooks longer than abaxial prongs. Axial prongs of medial and lateral hooks with pores on proximal surfaces. Base of lateral hook longer than, and slightly overlapping base of medial hook. Lateral hook measurements: A, 285–340 (316.1 ± 16.9; 12; 18); B, 230–290 (263.2 ± 20.1; 12; 17); B1, 140–190 (168.3 ± 16.9; 12; 18); B2, 85–105 (93.2 ± 6.4; 12; 17); C, 150–180 (162.2 ± 9.3; 12; 18); D, 435–475 (450.7 ± 11.6; 12; 15); position of pore on lateral axial prong from posterior tip of prong 32–39% (35.5 ± 2.4; 12; 15). Medial hook measurements: A′, 205–260 (230.3 ± 13.8; 12; 18); B′, 205–230 (215.7 ± 10.0; 12; 15); B1′, 110–140 (127.5 ± 10.5; 12; 16); B2′, 70–95 (86.0 ± 7.6; 12; 15); C′, 170–210 (186.2 ± 11.8; 11; 17); D′, 350–430 (390.6 ± 25.9; 12; 16); position of pore on medial axial prong from posterior tip of prong 33–46% (40 ± 3.3%; 12; 15); ratio of lateral to medial hook base length 1.3–1.6:1 (1.4:1 ± 0.04; 10; 18).
Megalonchos sumansinghai n. sp.
Type-host: Hemipristis elongatus (Klunzinger), snaggletooth shark (Carcharhiniformes: Hemigaleidae).
Additional hosts: None.
Site of infection: Spiral intestine.
Type-locality: Arafura Sea northeast of the Wessel islands, Australia (137.07°E, 10.40°S).
Additional-locality: Arafura Sea north of Wessel islands, Australia (136.38°E, 10.44°S).
Type-material: Holotype (QM G 227479); 4 paratypes (QM G 227480-G 227483); 3 paratypes and 1 set of proglottid cross-sections (LRP 3988–3991), SEM voucher (LRP 3987); 3 paratypes (USNPC 99214).
Etymology: This species honors Dr Suman Singha, the current Vice Provost for Academic Affairs at the University of Connecticut, who, although more veridical than vermiform, has demonstrated a true appreciation of tapeworms.
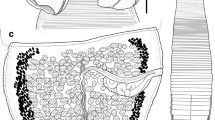
Description (Figs. 3–14)
[Based on 12 whole-mounts, cross-sections of mature proglottids of 2 of these worms and scoleces of 2 worms prepared for SEM.] Worms hyperapolytic, 7.0–19.6 (11.5 ± 3.5; 10) mm long; greatest width at level of scolex. Scolex 1,546–3,256 (2,282.8 ± 560.6; 10) long, consisting of scolex proper and conspicuous cephalic peduncle. Scolex proper 674–802 (740.6 ± 40.8; 10) long by 464–622 (551.5 ± 44.5; 10) wide, with 4 bothridia; bothridial velum not observed. Bothridia tapering posteriorly, with posterior half free. Each bothridium bears specialised anterior region in form of muscular pad bearing apical sucker and 1 pair of hooks, divided into 2 unequal loculi by transverse septum; muscular pad asymmetrical; apical sucker 67–88 (80.5 ± 6.8; 10) wide; anterior loculus 469–559 (528.4 ± 27.3; 10) long; posterior loculus 80–117 (100.2 ± 12.8; 10) long; maximum width of scolex at level of hooks. Cephalic peduncle 1,100–2,901 (1,872.6 ± 580.9; 10) long, 76–137 (101.3 ± 18.7; 10) wide.
Scanning electron micrographs of Megalonchos sumansinghai n. sp. 7. Scolex. Small numbers indicate locations of higher magnification images in Figs. 8, 10–14. 8. Detail of anterior margin of muscular pad. 9. Detail of apical sucker on muscular pad. 10. Detail of surface of muscular pad. 11. Detail of microtriches on proximal bothridial surface. 12. Detail of microtriches on cephalic peduncle. 13. Detail of microtriches on distal surface of anterior loculus. 14. Detail of microtriches on distal surface of posterior loculus. Scale-bars: 7, 100 μm; 8, 10–14, 2 μm; 9, 20 μm
Hooks bi-pronged, hollow, lacking tubercles or talons on axial prongs, with inconspicuous tubercle on proximal, posterior margin of medial and lateral hook bases; internal channels of axial and abaxial prongs continuous, smooth; lateral hooks slightly larger than medial hooks; axial prong of lateral hook longer than abaxial prong; axial prong of medial hook generally shorter than abaxial prong; axial prongs of medial and lateral hooks with pores on proximal surfaces. Upper part of base of medial hook slightly overlapping lateral hook; lower part of base of lateral hook slightly overlapping base of medial hook; base of lateral hook longer than base of medial hook. Lateral hook measurements: A, 180–200 (190 ± 7.7; 9; 10); B, 160–185 (172.3 ± 10.3; 10; 13); B1, 50–65 (57.9 ± 5.0; 10; 12); B2, 100–125 (112.9 ± 10.3; 10; 12); C, 120–145 (133.7 ± 9.2; 10; 15); D, 245–345 (307.3 ± 27.5; 10; 11); position of pore on lateral axial prong from posterior tip of prong 62–70% (65 ± 2.5; 10; 15). Medial hook measurements: A′, 140–170 (156.7 ± 9.4; 10; 12); B′, 130–165 (152.0 ± 12.1; 10; 15); B1′, 40–55 (46.7 ± 4.9; 10; 15); B2′, 80–120 (105.7 ± 11.6; 10; 15); C′, 145–175 (160.0 ± 10.8; 10; 15); D′, 245–295 (276.4 ± 16.7; 10; 11); position of pore on medial axial prong from posterior tip of prong 72–73% (69.4 ± 93.2; 10; 15); ratio of lateral to medial hook base length 1.1–1.4:1 (1.2:1 ± 0.1; 10; 11).
Anterior margin of sucker (Fig. 8) covered with blunt blade-like spinitriches and elongate filitriches; all other surfaces of muscular pad and apical sucker covered with elongate filitriches only (Figs. 9, 10). Proximal bothridial surfaces (Fig. 11) covered with densely arranged blade-like spinitriches with slightly elongated distal tips. Distal surface of anterior loculus (Fig. 13) covered with long filitriches interspersed with elongate blade-like spinitriches; distal surface of posterior loculus (Fig. 14) covered with long filitriches and densely arranged smaller blade-like spinitriches with elongated distal tips. Cephalic peduncle (Fig. 12) covered with densely arranged blade-like spinitriches. All spinitriches oriented with points directed posteriorly.
Proglottids acraspedote. Immature proglottids 64–137 (106.4 ± 23.9; 10) in number, initially wider than long, becoming longer than wide with maturity. Fully mature proglottids and gravid proglottids not observed. Terminal 2 immature proglottids: 355–695 (501.4 ± 101.2; 20; 10) long, 209–306 (254.6 ± 25.3; 10; 20) wide; length-to-width ratio 1.2–3.3:1 (2.0:1 ± 0.5; 10; 20). Testes 77–120 (92.1 ± 10.4; 9; 18) in number, arranged in single continuous field anterior to genital pore in 5–6 irregular columns, stopping short of anterior margin of proglottid, irregularly oval in frontal view, 16–28 (20.5 ± 2.6; 9; 18) × 11–20 (15.2 ± 2.0; 9; 18), 1 layer deep in cross-section. Genital pores marginal, irregularly alternating. Cirrus-sac elongate-oval, contains coiled cirrus. Vas deferens coiled, at anteromedial margin of cirrus-sac. Ovary H-shaped in frontal view, bilobed in cross-section, located near posterior end of proglottid. Vagina thin-walled, extending from oötype along medial line of proglottid to anterior margin of cirrus-sac, then laterally along anterior margin of cirrus-sac to common genital atrium; vaginal sphincter and seminal receptacle not observed. Uterus thick walled, sacciform, extending from midway between posterior margin of ovary and ovarian bridge to level of genital atrium. Vitellarium follicular, in 2 lateral bands, each consisting of 1 dorsal and 1 ventral column of relatively small follicles; bands extend from near anterior margin of testicular field to near posterior margin of proglottid, interrupted dorsally and ventrally by cirrus-sac and vagina, not interrupted by ovary; vitelline follicles 8–12 (8.8 ± 1.2; 9; 18) × 4–8 (5.8 ± 1.3; 9; 18). Excretory ducts lateral. Conspicuous group of densely staining cells located posterior to ovary medial to excretory duct. Eggs not observed.
Remarks
Megalonchos sumansinghai n. sp. is readily distinguished from M. mandleyi in that the pores of the axial prongs are located much more anteriorly in the former species than they are in the latter species in both the lateral (62–70 vs 32–39% from posterior) and medial (72–73 vs 33–46% from posterior) hooks. In addition, the hooks of M. sumansinghai are smaller than those of M. mandleyi (lateral hook length 245–295 vs 350–430 μm; medial hook total length 245–345 vs 435–475 μm). In addition, while the cephalic peduncle is approximately one-sixth the length of the strobila in M. sumansinghai, it is approximately one-half to one-third the length of the body in M. mandleyi.
Megalonchos shawae n. sp.
Type-host: Hemipristis elongatus (Klunzinger), snaggletooth shark (Carcharhiniformes: Hemigaleidae).
Additional hosts: None.
Site of infection: Spiral intestine.
Type-locality: Arafura Sea northeast of the Wessel islands, Australia (137.03°E, 10.36°S).
Additional-localities: Arafura Sea north of Wessel islands, Australia (136.38°E, 10.44°S; 137.07°E, 10.40°S; 137.04E, 11.02°S).
Type-material: Holotype (QM G 227484) and 2 paratypes (QM G 227485-G227486); 2 paratypes (LRP 3985–3986), 2 paratypes (USNPC No. 99215).
Etymology: This species is named for Judith Humphrey Shaw in thanks for her enthusiastic and remarkably generous support of work on elasmobranch tapeworm systematics at the University of Connecticut over the last decade and a half.
Description (Figs. 15–26)
[Based on 7 whole-mounts and scoleces of 2 worms prepared for SEM.] Worms hyperapolytic, 9.1–13.6 (10.6 ± 1.8; 7) mm long; greatest width at level of scolex. Scolex 1,100–2,276 (1,541 ± 398; 7) long, consisting of scolex proper and conspicuous cephalic peduncle. Scolex proper 633–857 (740 ± 84; 7) long by 576–661 (606 ± 29; 7) wide, with 4 bothridia; bothridial velum not observed. Bothridia taper conspicuously posteriorly, with posterior half free. Each bothridium bears specialised anterior region in form of muscular pad bearing apical sucker and 1 pair of hooks, divided into 2 unequal loculi by transverse septum; muscular pad asymmetrical; apical sucker 55–82 (71 ± 10; 6) wide; anterior loculus 359–512 (455 ± 50; 7) long; posterior loculus 111–166 (134 ± 18; 7) long; maximum width of scolex at the level of hooks. Cephalic peduncle 725–1,713 (1,090 ± 351; 7) long, 76–137 (81 ± 13.1; 7) wide.
Scanning electron micrographs of Megalonchos shawae n. sp. 19. Scolex. Small numbers indicate location of higher magnification images in Figs. 20 & 22–26. 20. Detail of anterior margin of muscular pad. 21. Detail of apical sucker on muscular pad. 22. Detail of surface of muscular pad. 23. Detail of microtriches on proximal bothridial surface. 24. Detail of microtriches on cephalic peduncle. 25. Detail of microtriches on distal surface of anterior loculus. 26. Detail of microtriches on distal surface of posterior loculus. Scale-bars: 19, 200 μm; 20, 22–26, 2 μm; 21, 20 μm
Hooks bi-pronged, hollow, lacking tubercles or talons on axial prongs, with inconspicuous tubercle on proximal, posterior margin of medial and lateral hook bases; internal channels of axial and abaxial prongs continuous, smooth; lateral hooks larger than medial hooks; axial prongs of both hooks conspicuously longer than abaxial prongs; axial prongs of medial and lateral hooks with pores on proximal surfaces. Upper part of base of medial hook slightly overlapping lateral hook; lower part of base of lateral hook slightly overlaps base of medial hook; base of lateral hook conspicuously longer than base of medial hook. Lateral hook measurements: A, 225–270 (241 ± 13; 7; 12); B, 190–230 (209 ± 16; 7; 9); B1, 120–145 (128.1 ± 8.8; 7; 8); B2, 70–95 (81 ± 9; 7; 8); C, 110–140 (122 ± 9; 7; 9); D, 305–405 (350 ± 32; 7; 8); position of pore on lateral axial prong from posterior tip of prong 35–41% (38 ± 3; 7; 9). Medial hook measurements: A′, 150–205 (171 ± 18; 7; 9); B′, 150–185 (165 ± 12; 7; 10); B1′, 90–110 (97 ± 7; 7; 9); B2′, 60–85 (81 ± 9; 7; 9); C′, 130–155 (142 ± 9; 7; 10); D′, 275–340 (306 ± 19.1; 7; 9); position of pore on medial axial prong from posterior tip of prong 36–46% (42 ± 3; 7; 9); ratio of lateral to medial hook base length 1.3–1.6 (1.4± 0.1; 7; 9).
Anterior margin of sucker (Fig. 20) covered with relatively blunt blade-like spinitriches and elongate filitriches; all other surfaces of muscular pad and apical sucker covered with elongate filitriches only (Figs. 21, 22). Proximal bothridial surfaces (Fig. 23) covered with densely arranged blade-like spinitriches with slightly elongated distal tips. Distal surface of anterior loculus (Fig. 25) covered with long filitriches interspersed with elongate blade-like spinitriches; distal surface of posterior loculus (Fig. 26) covered with long filitriches and sparsely arranged smaller blade-like spinitriches. Cephalic peduncle (Fig. 24) covered with densely arranged blade-like spinitriches. All spinitriches oriented with points directed posteriorly.
Proglottids acraspedote. Immature proglottids 70–136 (103.6 ± 21.5; 7) in number, initially wider than long, becoming longer than wide with maturity. Fully mature proglottids and gravid proglottids not observed. Terminal 2 immature proglottids: 361–863 (538 ± 134; 7; 14) long, 149–324 (222 ± 48; 7; 14) wide; length-to-width ratio 1.7–3.8:1 (2.5:1 ± 0.7; 7; 14). Testes 72–114 (87 ± 10; 7; 14) in number, arranged in single continuous field anterior to genital pore in 6–8 irregular columns, stopping short of anterior margin of proglottid, irregularly oval in frontal view, 17–31 (22 ± 4; 7; 14) long by 11–24 (16 ± 4; 7; 14) wide, 1 layer deep in cross-section. Genital pores marginal, irregularly alternating. Cirrus-sac slightly bent up, containing coiled cirrus. Vas deferens at anteromedial margin of cirrus-sac. Ovary H-shaped in frontal view, bilobed in cross-section, located near posterior end of proglottid. Vagina thin-walled, extending from ovarian bridge along medial line of proglottid to anterior margin of cirrus-sac, then laterally along anterior margin of cirrus-sac to common genital atrium; vaginal sphincter and seminal receptacle not observed. Uterus thick-walled, sacciform, extending from ovarian bridge to level of cirrus-sac. Vitellarium follicular, in 2 lateral bands, each consisting of 1 dorsal and 1 ventral column of relatively small follicles; bands extending from near anterior margin of testicular field to near posterior margin of proglottid, interrupted dorsally and ventrally by cirrus-sac and vagina, not interrupted by ovary; vitelline follicles 6–10 (8 ± 1; 7; 14) × 4−8 (6 ± 1; 7; 14). Excretory ducts lateral. Eggs not observed.
Remarks
Megalonchos shawae n. sp. is readily distinguished from M. sumansinghai n. sp. in that the pores of the axial prongs are located much more posteriorly in the former species than they are in the latter species in both the lateral hooks (35–41 vs 62–70% from posterior) and the medial hooks (36–46 vs 72–73% from posterior). In addition, the base of the lateral hook is usually longer relative to that of the medial hook in M. shawae than it is in M. sumansinghai (1.3–1.6:1 vs 1.1–1.4). M. shawae differs from M. mandleyi in its possession of smaller lateral hooks (total length 305–405 vs 435–475 μm) and medial hooks (total length 275–340 vs 350–430 μm). In addition, while the cephalic peduncle is approximately half the length of the strobila in M. mandleyi, it is only approximately one-fifth to one-sixth of the length of the strobila M. shawae.
Megalonchos Baer & Euzet, 1962 (amended diagnosis)
Prudhoe (1969) noted the differences in the armature and also in the arrangement of the testes between M. mandleyi and his two new species, and, since a formal diagnosis was not available for Megalonchos, provided a diagnosis for the genus. Euzet (1994) provided a slightly modified diagnosis. However, the transfer of M. dubius and M. musteli to Biloculuncus, in combination with the availability of additional information on the proglottid anatomy of Megalonchos provided here, necessitates a revision of the diagnosis of this genus. The following revised diagnosis of Megalonchos is proposed:
Onchobothriidae: Scolex with four bothridia; each bothridium bearing one pair of bifid hooks, pre-hook region in form of muscular pad bearing apical sucker, and post-hook region subdivided by single transverse septum into one extensive anterior loculus and one inconspicuous posterior loculus. Hooks in each pair asymmetrical; base of lateral hook longer than base of medial hook; medial and lateral hooks with conspicuous pore on proximal side of axial prong. Strobila acraspedote, hyperapolytic. Genital pores lateral, irregularly alternating. Testes numerous, arranged in single preporal field. Ovary posterior, H-shaped in frontal view, bilobed in cross-section. Vagina opens into genital atrium anterior to cirrus. Vitelline follicles in two lateral bands; each band consisting of one column of dorsal and one column of ventral follicles. Uterus sac-shaped, medioventral, reaching to cirrus-sac. Eggs not known. Parasites of Hemigaleidae (Carcharhiniformes) in the Indo-Pacific.
Type-species: M. mandleyi (Southwell, 1927) Baer & Euzet, 1962.
Additional species: M. sumansinghai n. sp, and M. shawae n. sp.
Discussion
The affinities of Megalonchos remain unclear. Among onchobobothriid genera bearing bothridia in which the post-hook region is subdivided into two loculi (i.e. Biloculuncus, Erudituncus, Phoreiobothrium and Uncibilocularis), Megalonchos resembles Biloculuncus in its possession of a uterus that extends only as far anterior as the cirrus-sac, but resembles Erudituncus in its possession of a conspicuous cephalic peduncle. However, among these genera Megalonchos is very unique in its lack of post-vaginal testes and its possession of a single pair of hooks that are each bi-pronged and that bear a conspicuous pore on the axial prongs of the medial and lateral hooks.
Megalonchos is somewhat unusual among onchobothriids in that proglottids appear to drop from the strobila at a relatively immature stage in their development. The interpretation of the immature nature of all attached proglottids in the specimens examined here as hyperapolytic, rather than merely as immature worms, was confirmed by the observation that, although tens of free proglottids of M. shawae and M. sumansinghai were found in the contents of the spiral intestines of sharks examined, none of these free proglottids were mature. This suggests that proglottids are released from the strobila at a relatively early stage in their development. That the terminal proglottids on all of the specimens of M. mandleyi available to us were also immature, suggests that this species exhibits a similar developmental pattern.
The host associations of Megalonchos appear to be relatively restricted. At present, the genus is known only from sharks of the family Hemigaleidae (i.e. weasel sharks), and within that family from only two species: M. mandleyi from Chaenogaleus macrostoma (reported as Hemigaleus balfouri by Southwell [1927]), and M. shawae and M. sumansinghai from Hemipristis elongatus. According to Compagno et al. (2005), the Hemigaleidae is a small family, consisting of only eight species in four genera. Clearly examination of the other six members of the family is in order as it is likely to yield additional species of Megalonchos, or at least closely related taxa.
Based on results presented here, the current understanding of host associations of the onchobothriid genera parasitising the eight families of carcharhiniform sharks as summarised by Caira & Jensen (2001) should be expanded as follows: the Triakidae host species of Biloculuncus, Erudituncus and Calliobothrium; the Hemigaleidae host Megalonchos; and the Carcharhinidae and Sphyrnidae both host Platybothrium Linton, 1890 (see Healy, 2003 for an expanded list) and Phoreiobothrium. Apart from sporadic reports of Acanthobothrium coronatum (Rudolphi, 1819) van Beneden, 1849 from the schylliorhinid shark Scyliorhinus stellaris (L.) (e.g. see Goldstein, 1967; Williams, 1969), little is known of the onchobothriid cestode faunas of the remaining four families of carcharhiniform sharks (i.e. Scylliorhinidae, Proscylliidae, Pseudotriakidae and Leptochariidae). It seems apparent, however, that the cestode faunas of species in these four families would be particularly interesting to explore and are likely to yield additional new onchobothriid taxa.
References
Baer, J. G., & Euzet, L. (1962). Revision critique des cestodes tétraphyllides décrits par T. Southwell (1re partie). Bulletin de la Société Neuchâteloise des Sciences Naturelles, 85, 143–172.
Caira, J. N., & Jensen, K. (2001). An investigation of the coevolutionary relationships between onchobothriid tapeworms and their elasmobranch hosts. International Journal for Parasitology, 31, 959–974.
Caira, J. N., Richmond, C., & Swanson, J. (2005). A revision of Phoreiobothrium (Tetraphyllidea: Onchobothriidae) with descriptions of five new species. Journal of Parasitology, 91, 1153–1174.
Caira, J. N., & Ruhnke, T. R. (1990). A new species of Calliobothrium (Tetraphyllidea: Onchobothriidae) from the whiskery shark, Furgaleus macki, in Australia. Journal of Parasitology, 76, 319–324.
Compagno, L. (1984). FAO species catalogue. Vol. 4, Part 2: Sharks of the world. An annotated and illustrated catalogue of shark species known to date (pp. 655). FAO Fisheries Synopsis No. 125. Rome: United Nations Development Programme/Food and Agriculture Organization of the United Nations.
Compagno, L., Dando, M., & Fowler, S. (2005). Sharks of the world (pp. 368). Princeton: Princeton University Press.
Euzet, L. (1994). Order Tetraphyllidea Carus, 1863. In L. F. Khalil, A. Jones, & R. A. Bray (Eds.), Keys to the cestode parasites of vertebrates (pp. 149–194). Wallingford: CAB International.
Goldstein, R. J. (1967). The genus Acanthobothrium van Beneden, 1849 (Cestoda: Tetraphyllidea). Journal of Parasitology, 53, 455–483.
Healy, C. J. (2003). A revision of Platybothrium Linton, 1890 (Tetraphyllidea: Onchobothriidae), with a phylogenetic analysis and comments on host-parasite associations. Systematic Parasitology, 56, 85–139.
Healy, C. J., Scholz, T., & Caira, J. N. (2001). Erudituncus n. gen. (Tetraphyllidea: Onchobothriidae) with a redescription of E. musteli (Yamaguti, 1952) n. comb. and comments on its hook homologies. Journal of Parasitology, 87, 833–837.
Nasin, C. S., Caira, J. N., & Euzet, L. (1997). Analysis of Calliobothrium (Tetraphyllidea: Onchobothriidae) with descriptions of three new species and erection of a new genus. Journal of Parasitology, 83, 714–733.
Prudhoe, S. (1969). Cestodes from fish, birds and whales. B.A.N.Z. Antarctic Research Expedition 1924–1931, Series B, 8, 171–193.
Southwell, T. (1925). A monograph on the Tetraphyllidea with notes on related cestodes. Memoirs of the Liverpool School of Tropical Medicine, 2 (New Series), 1–368.
Southwell, T. (1927). On a collection of cestodes from marine fishes of Ceylon and India. The Annals of Tropical Medicine and Parasitology, 21, 351–373.
Southwell, T. (1930). Cestoda. Vol. I. In J. Stephenson (Ed.), The fauna of British India, including Ceylon and Burma (pp. 391). London: Taylor and Francis.
Williams, H. H. (1969). The genus Acanthobothrium Beneden, 1849 (Cestoda: Tetraphyllidea). Nytt Magasin for Zoologi, 17, 1–56.
Acknowledgements
Our success in collecting cestodes from specimens of the shark Hemipristis elongatus would not have been possible without the arrangements made by Bill Passey for us to work on the FV Ocean Harvest and thus to examine fresh material of this otherwise difficult to obtain shark species. We very much appreciate the full cooperation we received from the crew and most especially from Captain Ray Passey, who went out of his way to accommodate all of our needs during the three weeks we spent on the vessel. Kirsten Jensen was of enormous assistance with the collection of this material. We thank David Stemmer of the South Australian Museum and Eileen Harris of the Natural History Museum in London for their assistance with the location and loan of specimens. This work was supported in part with funds from NSF PEET awards No. DEB 9521943 and DEB 0118882 to JNC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caira, J.N., Reyda, F.B. & Mega, J.D. A revision of Megalonchos Baer & Euzet, 1962 (Tetraphyllidea: Onchobothriidae), with the description of two new species and transfer of two species to Biloculuncus Nasin, Caira & Euzet, 1997. Syst Parasitol 67, 211–223 (2007). https://doi.org/10.1007/s11230-006-9085-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-006-9085-z