Abstract
Three 2,3-bis(hydroxymethyl)-2,3-dinitro-1,4-butanediol tetranitrate (SMX)–based propellants were firstly reported, then the specific impulses of SMX-based propellants were calculated by the Energy Calculation Star program. Meanwhile, the migration property of the plasticizers and SMX was investigated by molecular dynamic method, and the main results as follows: the theoretical specific impulses of three SMX-based propellants all overpass 280 s, which suggests that they have the potential to be high-energy propellants. The migrating property of plasticizers in SMX-based propellants and ethylene propylene diene monomer (EPDM) insulation all decrease in the order Bu-NENA> BTTN> TMETN. Meanwhile, the plasticizers much easier migrate in EPDM insulation than in SMX-based propellants, and TMETN is significantly more difficult to migrate than the other. The glass transition temperatures (Tg) of GAP/Bu-NENA/Al/SMX, GAP/BTTN/Al/SMX, and GAP/TMETN/Al/SMX systems are 282.3 K, 278.1 K, and 287.6 K, respectively. Due to lower Tg of EPDM, the EPDM/plasticizer systems have no obvious glass transition between 233 and 323 K. The SMX is almost more difficult to migrate than plasticizers in SMX-based propellants while temperature is above 273 K, whereas it is contrary under 273 K.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pursuit of high-energy propellant is an eternal target for solid rocket, which can increase the range and load of rocket and realize more powerful missions [1]. Therefore, it is important to explore novel high-energy propellant formations which show good stability and safety. Therein, the migration of component is an important influence factor for stability of propellant and safety of the solid rocket [2,3,4]. The migration of plasticizer in solid rocket propellants is dangerous for their application, which may result in the inhomogeneous distribution of components in propellant grain. Ultimately, the inhomogeneous distribution may introduce the change of ballistic property and even unnormal explosion.
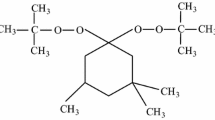
Owing to high density and heats of formation, 2,3-bis(hydroxymethyl)-2,3-dinitro-1,4-butanediol tetranitrate (SMX, as presented in Fig. 1), whose energy approximates that of cyclotetramethylene tetranitramine (HMX) and decomposition temperature exceeds 150 °C, has attracted interests of experts and has the potential to be an novel oxidant of high-energy propellants [5,6,7]. Reese et al. [8] found that SMX-based propellants exhibit good performance and high density, and may offer a promising perchlorate-free alternative to existing ammonium perchlorate (AP)-based formulations. Hou et al. [9] investigated the compatibilities of SMX with components of hydroxyl-terminated polybutadiene (HTPB) propellants, and results showed that SMX was mainly compatible with HMX, cyclotrimethylenetrinitramine (RDX), and Al powder. Sizov et al. [10] discovered that SMX can be used as partial substitution of nitroglycerin (NG) in catalyzed double-base propellants and increase the ballistic properties of propellants. However, current investigations of SMX mainly focus on that SMX is used as a substituent or additive of propellant system, and there are few comprehensive studies on SMX serving as main oxidant in solid propellants and its migrating ability in SMX-based propellant.
Molecular dynamic (MD) method is an effective way to predict the migration of plasticizers in solid rocket propellant. And the diffusion coefficient of plasticizers is often used to predict their migrating ability [11, 12]. Li et al. [13] calculated the diffusion coefficient of DOS in HTPB-based propellants based on MD method and found that the magnitude order of its calculated diffusion coefficient is in accordance with its experiment value. Qu et al. [14] predicted the diffusion coefficient of nitric ester (NG/BTTN) by applying MD simulation, and results showed that BTTN easier migrate than NG in the system at 328 K and 338 K. And their magnitude order also approximates experimental values.
In this work, three novel SMX-based propellants are designed and investigated. Firstly, their specific impulse and related parameters were calculated by the Energy Calculated Star (ECS) program; secondly, the migration of plasticizers in SMX-based propellant was predicted by MD method; thirdly, the migration of plasticizers in insulation was estimated; finally, the migrating ability of SMX in propellant was also calculated. It is hoped that our works can help guide the preparation and application of SMX-based propellants.
Computational details
Construction of SMX-based propellants
The amorphous cell of three SMX-based propellants was constructed by Amorphous Cell Module in Material Studio 8.0 [15] based on the mass ratio of GAP: plasticizer (Bu-NENA, BTTN, or TMETN): Al: SMX (12: 8: 15: 65). The number of molecules in three systems is listed in Table 1, and the mole mass of GAP is 3700 g/mol. The structure optimization was performed by COMPASS force field (Forcite Module in MS 8.0), which is effective in investigating properties of condense phase [16, 17], and their balanced structures are presented in Figs. 2 and 3. And the COMPASS force field was applied in all the next operations. Van der Waals force was calculated by atom-based method, and Ewald method was calculated by electrostatic interaction [18, 19].
Simulation of migration in SMX-based propellants
After structure optimization, the cell of SMX-based propellants was relaxed by 50 ps constant particle number, pressure, and temperature (NPT) ensemble; 50 ps constant particle number, volume, and temperature (NVT) ensemble; and annealing simulation (temperature from 300 to 500 K). Afterwards, the relaxed structures were used to perform MD simulation. The total calculated time was 1000 ps, and the step time was 1 fs. The given temperature and pressure were 298 K and 100 kPa, respectively. The Anderson method and Berendsen method were used to control temperature and pressure, respectively [20, 21]. Initial velocity was sampled by Maxwell distribution, and velocity Verlet arithmetic was utilized [22]. Van der Waals force and electrostatic interaction were calculated by atom-based method and Ewald method, respectively.
Based on balanced structures, three systems of high-energy propellants were performed 500 ps MD calculation (NVT ensemble), and the computing method and parameters were as above. The temperature was successively set from 323 to 233 K, and the interval is 10 K. The ultimate consequences were used to analyze mean square displacement (MSD) of different plasticizers.
Construction of EPDM insulation and migration in EPDM insulation
The molecular chain of ethylene propylene diene monomer (EPDM) was built by ethylene (55% mass ratio), propylene (34% mass ratio), and 5-ethylidene-2-norbornene (ENB, 11% mass ration), and the mole mass of chain is 5600 g/mol. Meanwhile, the chain was optimized. Next, the amorphous cell of EPDM prepolymer was constructed by Amorphous Cell Module in MS 8.0, which contained 8 EPDM chains. After structure optimization and relaxation, the EPDM prepolymer cell was performed 1000 ps NPT ensemble simulation and its balanced structure was obtained (the method is the same as the above “Simulation of migration in SMX-based propellants” section).
Based on balanced structure of EPDM prepolymer cell, the plasticizers (Bu-NENA, BTTN, and TMETN) are randomly put into EPDM prepolymer cell, and the molecular number of plasticizer is 20. The new cells were optimized and then preformed 1000 ps NVT ensemble simulations. Afterward, the balanced EPDM/plasticizer structures were successively performed 500 ps MD calculations (NVT ensemble) form 323 to 233 K(the temperature interval is 10 K).
Calculation of migration
The migration of plasticizer is related to its diffusion coefficient (D), which depends on the MSD and time (t) of MD simulation. Therefore, D is described in Eq. 1 based on the relation of Einstein [23, 24]:
where r(t) is coordinate of plasticize in t, and r(0) is the original coordinate.
The relationship of MSD is calculated by Eq. 2 [25]:
The D is ultimately estimated by Eq. 1 and Eq. 2 [26]:
where m is the slope of MSD-t curve.
Results and discussion
Energy of SMX-based propellant
The energy characteristics of propellants are predicted by ECS program based on heats of formation (ΔHf) and chemical formula of components, which are contained in ECS program. Therein, the specific impulse (Isp) is used to compare performances of solid rocket propellant, and its value is significant for the propellant, which determinates whether the propellant meets the ballistic requirements. The specific impulse (Isp), characteristic velocity (C*), combustion temperature (Tc), the average relative molecular mass of combustion gas (\( \overline{M} \)), and oxygen balance (OB) of SMX-based propellants are presented in Table 2.
It is easily seen that the specific impulse of three novel SMX-based propellant all overpass 280 s, which exhibit high energy and have the potential to be high-energy propellant. Otherwise, the relationship between Isp, Tc, and \( \overline{M} \) is presented in Eq. (4), and it is obvious that Isp is in direct proportion to Tc and \( \overline{M} \). Otherwise, it is easy to find that the Tc and \( \overline{M} \) values of GAP/Bu-NENA/Al/SMX, GAP/BTTN/Al/SMX, and GAP/TMETN/Al/SMX system are close to each other, and the results of Isp values suggest that their sizes are consistent with the size of the \( \overline{M} \) values, which indicates that the \( \overline{M} \) values determine the Isp values of the systems when they own approximating Tc values. In the end, the GAP/BTTN/Al/SMX and GAP/TMETN/Al/SMX systems exhibit similar Isp values because they are almost equal with the \( \overline{M} \) values. The GAP/Bu-NENA/Al/SMX system exhibits larger Isp value than the other for more than 2 s, which signifies that the same solid rocket can fly much further distance or apply much lesser weight propellant for the same distance by using the GAP/Bu-NENA/Al/SMX system.
Migration in propellants
Afterwards, all MSD curves and their linear fitting curves are presented in Fig. 4. Meanwhile, the slopes (m) of all linear fitting curves are obtained. In the end, the diffusion coefficients (D) of three plasticizers in SMX-based propellants are calculated by Eq. 3.
As presented in Table 3, it is obvious that the D values of three plasticizers in SMX-based propellants all gradually decrease when temperature is reduced. When the temperature is 323 K (the applying temperature of most propellants), the D values of three plasticizers are close to each other. The D values of Bu-NENA significantly reduce from 323 to 293 K, but change few and fluctuate around 0.40 × 10−11 m2/s between 293 and 253 K. The D values of BTTN dramatically decrease when temperature is 313 K and the range of decline is larger than that of Bu-NENA; however, it shows few changes after 283 K. It is worth noting that the D values of TMETN exhibit more decline than the former at 293 K, but it has no clear change after temperature is 293 K. The above phenomenon suggests that all systems of SMX-based propellants may have glass transition between 293 and 263 K. In the end, the D values of Bu-NENA, BTTN, and TMETN are respectively 0.14 × 10−11 and 0.14 × 10−11 m2/s when temperature is 233 K. In conclusion, the migrating ability of the three plasticizers in SMX-based propellants decreases in the order Bu-NENA > BTTN > TMETN.
In order to obtain the glass transition temperature (Tg) of the three SMX-based propellants, the relationships between diffusion coefficient (D) of plasticizers and temperature (T) are showed in Fig. 5. It is easy to see that all systems exhibit glass transition and the Tg of GAP/Bu-NENA/Al/SMX, GAP/BTTN/Al/SMX, and GAP/TMETN/Al/SMX propellants are 282.3 K, 278.1 K, and 287.6 K, respectively, which are close to each other. Moreover, the Tg of all systems are slightly lower than the room temperature (298.15 K), which suggests that three SMX-based propellants may possess good plasticity and are easy to process at normal temperature. Otherwise, the D values of Bu-NENA and BTTN show better linear relationship with temperature compared with that of TMETN when temperature is above their own Tg values. However, when the temperature is lower than their own Tg values, the D values of BTTN and TMETN exhibit better linear relationship with temperature by contrast with that of Bu-NENA.
Migration in EPDM
EPDM insulation is widely used in solid rocket motor owning to its low density, excellent aging performance, and long life storage. Therefore, the migration of Bu-NENA, BTTN, and TMETN in EPDM insulation should be payed more attention, which may impair performance of solid rocket. The MSD curves of plasticizers at different temperatures and their linear fitting curves are presented in Fig. 6. Meanwhile, the slopes of linear fitting curves and diffusion coefficients of three plasticizers are obtained and listed in Table 4.
It is easily seen that the D values of three plasticizers show a trend of gradual decrease when temperature reduces, which indicates that their migration in EPDM decreases. Otherwise, the D value of BTTN is particularly larger than those of Bu-NENA and TMETN when temperature is 323 K. However, the D value of BTTN drastically decreases when temperature reduces to 313 K. Afterwards, the D value of BTTN has no significant change between 313 and 293 K, and obviously reduces at 283 K. The D value of Bu-NENA has no obvious change when temperature reduces from 323 to 293 K (except 313 K). While the temperature is 283 K, the D value of Bu-NENA obviously decreases, and that gradually reduces after 283 K. TEMTN exhibits relatively lower D value compared with the other. And the D value of TMETN presents twice obvious decrease when the temperatures are 303 K and 273 K. Ultimately, the D values of Bu-NENA, BTTN, and TMETN at 233 K are 0.29 × 10−11, 0.12 × 10−11, and 0.05 × 10−11 m2/s. The migrating ability of the three plasticizers in EPDM insulation decreases in the order Bu-NENA > BTTN > TMETN, which is the same as in the corresponding SMX-based propellants, And TMETN is more difficult to migrate in EPDM compared with Bu-NENA and BTTN.
The curves of D vs temperature of three plasticizers are presented in Fig. 7. It is obvious that the curves of BTTN and TMETN are good linear, which indicates that the EPDM systems have no glass transition. Due to the lower Tg values (243–233 K) of EPDM [], it is normal that the curves have no apparent point of glass transition. However, that of Bu-NENA is chaotic, and it needs further studies. Otherwise, it is worth to note that the D values of plasticizers in EPDM are mainly larger than those in the corresponding SMX-based propellants, which suggest that plasticizers are easy to migrate in EPDM.
Migration of SMX in propellants
The migration of SMX in three SMX-based propellants is analyzed, and their MSD curves at different temperature and corresponding linear fitting curves are presented in Fig. 8. Meanwhile, the slopes of linear fitting curves and diffusion coefficients of SMX in three SMX-based propellants are obtained and listed in Table 5.
It is obvious that the diffusion coefficients of SMX at 323 K are particularly lower than those of the three plasticizers, which indicates that the migration of SMX (0.39–0.71 × 10−11 m2/s) is weaker than that of the three plasticizers (1.01–1.13 × 10−11 m2/s) in SMX-based propellants. Meanwhile, the D values (0.39 × 10−11 m2/s) SMX in GAP/TMETN/Al/SMX system are significantly smaller than the others (0.70 × 10−11 and 0.71 × 10−11 m2/s) at 323 K. Otherwise, the D values of SMX in GAP/BTTN/Al/SMX and GAP/TMETN/Al/SMX systems decrease below 0.2 × 10−11 m2/s and have no significant changes when temperature reduces below 293 K. However, the D values of SMX in GAP/Bu-NENA/Al/SMX system show few changes while temperature decreases from 313 to 283 K, have an obvious decrease at 273 K, and change a few below 273 K. Ultimately, the D values of SMX at 233 K are 0.10 × 10−11, 0.07 × 10−11, and 0.04 × 10−11 m2/s. In conclusion, the migrating ability of SMX in the three SMX-based propellants reduces in the order GAP/Bu-NENA/Al/SMX > GAP/BTTN/Al/SMX > GAP/TMETN/Al/SMX. And the migrating ability of SMX is lower than those of Bu-NENA, BTTN, and TMETN, which may result from phase morphology of SMX, because SMX is solid at normal temperature. However, the plasticizers are difficult to migrate than SMX when temperature is less than 273 K, which may result from the glass transition of propellants. In conclusion, compared with the migration of plasticizers in SMX-based propellants, SMX is much weaker to migrate when temperature exceeds 273 K, but stronger under 273 K.
Conclusion
Three novel SMX-based propellants are firstly designed, and their energetic properties are predicted. Meanwhile, the migration of plasticizers in propellants and EPDM insulation and the migrating ability of SMX in propellant are estimated based on MD stimulation. The main results are as follows:
-
(1)
The Isp values of three SMX-based propellants all overpass 280 s, and it is impressive that they have the potential to be high-energy propellants.
-
(2)
The glass transition temperatures (Tg) of GAP/Bu-NENA/Al/SMX, GAP/BTTN/Al/SMX, and GAP/TMETN/Al/SMX systems are 282.3 K, 278.1 K, and 287.6 K, respectively. The migrating property of plasticizers in SMX-based propellants is Bu-NENA> BTTN> TMETN. And TMETN is significantly more difficult to migrate than the other.
-
(3)
The migrating property of plasticizers in EPDM insulation is also Bu-NENA> BTTN> TMETN. And the EPDM/plasticizer systems have no obvious glass transition between 233 and 323 K due to lower Tg values of EPDM. Otherwise, the plasticizers are difficult to migrate in corresponding SMX-based propellants than in EPDM.
-
(4)
The migration of SMX is much weaker than that of plasticizers in SMX-based propellants when temperature exceeds 273 K, which may own to the solid morphology of SMX at that temperature. But the migration of former is stronger than that of the latter under 273 K, which may result from the glass transition of propellants.
References
Lempert D, Nechiporenko G, Manelis G (2011) Energetic performances of solid composite propellants[J]. Cent Eur J Energetic Mater 8(1):25–38
Gottlieb L, Bar S (2003) Migration of plasticizer between bonded propellant interfaces[J]. Propell Explos Pyrot: An International Journal Dealing with Scientific and Technological Aspects of Energetic Materials 28(1):12–17
Li S, Liu Y, Tuo X et al (2008) Mesoscale dynamic simulation on phase separation between plasticizer and binder in NEPE propellants[J]. Polymer 49(11):2775–2780
Grythe KF, Hansen FK (2007) Diffusion rates and the role of diffusion in solid propellant rocket motor adhesion[J]. J Appl Polym Sci 103(3):1529–1538
Chavez DE, Hiskey MA, Naud DL et al (2008) Synthesis of an energetic nitrate ester[J]. Angew Chem 120(43):8431–8433
Fischer N, Fischer D, Klapötke TM et al (2012) Pushing the limits of energetic materials-the synthesis and characterization of dihydroxylammonium 5,5′-bistetrazole-1,1′-diolate[J]. J Mater Chem 22(38):20418–20422
Fuqiang B, Junliang Y, Bozhou W et al (2011) Synthesis, crystal structure and properties of 2, 3-bis (hydroxymethyl)-2,3-dinitro-1,4-butanedioltetranitrate[J]. Chin J Org Chem 31(11):1893–1900
Reese DA, Son SF, Groven LJ (2014) Composite propellant based on a new nitrate ester[J]. Propellants Explos Pyrotech 39(5):684–688
HUO B, HE J-x, REN X-t, CAO Y-l (2017) Synthesis, crystal morphology control of SEM and its compatibility of HTPB propellant. Chin J of Energetic Mater 25(4):348–352
Sizov VA, Pleshakov DV, Asachenko AF et al (2018) Synthesis and study of the thermal and ballistic properties of SMX [J]. Cent Eur J Energetic Mater 15(1):30–46
Huang Z, Nie H, Zhang Y et al (2012) Migration kinetics and mechanisms of plasticizers, stabilizers at interfaces of NEPE propellant/HTPB liner/EDPM insulation[J]. J Hazard Mater 229:251–257
Reese DA, Groven LJ, Son SF (2014) Formulation and characterization of a new nitroglycerin-free double base propellant[J]. Propellants Explos Pyrotech 39(2):205–210
Li H-X, Qiang H-F, Li X-Q et al (2012) Measurement of diffusion coefficient of plasticizer in HTPB propellant [J]. J Solid Rocket Technol 35(3):387–390
Bei Q, Pan Q, Tang Q-f et al (2018) Molecular dynamics simulation and experimental study on migration of nitric Ester in NEPE propellant[J]. Chin J Energetic Mater 41(3):278–284
Material Studio 8.0[C] //Acceryls Inc.: San Diego, 2014
Ma X, Zhao F, Ji G et al (2008) Computational study of structure and performance of four constituents HMX-based composite material[J]. J Mol Struct THEOCHEM 851(1–3):22–29
Lu Y, Shu Y, Liu N et al (2017) Theoretical simulations on the glass transition temperatures and mechanical properties of modified glycidyl azide polymer[J]. Comput Mater Sci 139:132–139
Ewald PP (1921) Die Berechnung optischer und elektrostatischer Gitterpotentiale[J]. Ann Phys 369(3):253–287
Karasawa N, Goddard III WA (1989) Acceleration of convergence for lattice sums[J]. J Phys Chem 93(21):7320–7327
Andersen HC (1980) Molecular dynamics simulations at constant pressure and/or temperature[J]. J Chem Phys 72(4):2384–2393
Berendsen HJC, Postma JPM, van Gunsteren WF et al (1984) Molecular dynamics with coupling to an external bath[J]. J Chem Phys 81(8):3684–3690
Verlet L (1967) Computer “experiments” on classical fluids. I. Thermodynamical properties of Lennard-Jones molecules[J]. Phys Rev 159(1):98
Liu QL, Huang Y (2006) Transport behavior of oxygen and nitrogen through organasilicon-containing polystyrenes by molecular simulation[J]. J Phys Chem B 110(35):17375–17382
Haesslin HW (1985) Dimethylsiloxane-ethylene oxide block copolymers, 2. Preliminary results on dilute solution properties[J]. Die Makromolekulare Chemie Banner 186(2):357–366
YU Z-f, FU X-l, YU H-j et al (2015) Mesoscopic molecular simulation of migration of NG and BTTN in polyurethane [J]. Chin J Energetic Mater 23(9):858–864
Li M, Zhao FQ, Xu SY et al (2013) Comparison of three kinds of energy calculation programs in formulation design of solid propellants[J]. Chin J Explos Propellants 36(3):73–77
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, K., Liu, N., Li, Jq. et al. Migrating simulation of novel high-energy SMX-based propellants based on molecular dynamics. Struct Chem 30, 1233–1241 (2019). https://doi.org/10.1007/s11224-019-1282-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-1282-x