Abstract
A two-dimensional coordination polymer {Ag(PMK)(OTf)·MeCN} n (1) based on multi-modal bridging ligand, namely N,N′-bis[1-(pyrazin-2-yl)ethylidene]-hydrazine or 2-pyrazyl methyl ketazine (PMK), and AgOTf salt has been synthesized and characterized by ESI-MS, 1H-NMR, ATR-IR, and single crystal X-ray diffraction. The PMK shows distinct binding sites, both chelating and monodentate, and bridging modes in 1 where each silver(I) centre is five coordinate, and bound to one bidentate pyrazylketimine and a monodentate pyrazine through the peripheral N atom from another ligand, and also a bridging pyrazine through the peripheral N atom of the adjacent chelating unit from another ligand, and to triflate anion to feature one-dimensional infinite chain. The triflate anions have effectively increased the 1D coordination polymers to a 2D network via H-bonding interactions. These 2D planes are stacked together building up channels (1D tube) in which the acetonitrile solvent molecules reside and form very weak contacts with the triflates and the pyrazylketimine units via C–H···O and C–H···N, respectively. In addition, the fluorescent spectrum of 1 in the solid state exhibits two emission maxima at 496 and 522 nm. The ESI-MS, IR, and 1H-NMR confirm the structure.
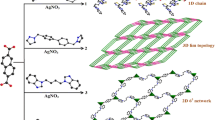
Graphical Abstract
Self-assembly reaction of multimodal ligand (PMK = N,N′-bis[1-(pyrazin-2-yl)ethylidene]-hydrazine) and AgOTf gives unprecedented one-dimensional coordination polymer {Ag(PMK)(OTf)·MeCN} n (1) with distinct coordination modes of the ligand and the solid state fluorescence spectrum. The triflate anions which are weakly coordinated to silver(I) centres in 1, cross-link the chains by intermolecular hydrogen bonding interactions into a 2D network. These 2D planes are stacked together building up square channels (1D tubes) in which the acetonitrile solvent molecules reside.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past decades, pronounced interest has been focused on the construction of polymeric coordination complexes owing to their structural topologies and interesting catalytic, electronic, magnetic, and spectroscopic properties [1]. The building-block methodology is the most useful and flexible strategy for the crystal engineering design of novel extended networks. The construction of coordination networks relies upon utilizing the specific geometries of both metal ions and ligands [2]. In this context, multi-modal bridging ligands have recently gained considerable interest since they contain distinct binding sites, both chelating and monodentate, and bridging modes [2–4]. An assembly process containing multidentate ligands is usually very complicated and is influenced by various factors such as solvent system, templates, counter ions, valences and the geometrical preference for the metal ions [5, 6]. Therefore, it is difficult to design and synthesize supramolecular architectures with predicted structures and properties when the number of donor atoms in the ligands is more than two. This has been exemplified by the novel organic–inorganic composite coordination polymers generated from PMK having additional pyrazine nitrogen atoms in comparison to pyridylketimine ligand and Ag(I) salts [3, 4]. Depending on the anions, the ligand shows distinct coordination modes as illustrated in Fig. 1. It has also been demonstrated that changes in the counter ion can have influence upon the solid state structures of a series of compounds, often with surprising results [3].
We have previously reported the single crystal X-ray structure of PMK which confirmed the expected ligand structure [7]. In the solid state structure, PMK assumes a planar structure and adopts a trans-configuration about the central N–N bond, with the methyl groups on the opposite sides, suggesting conjugation throughout the π systems. The crystal structures of complexes {[Ag2(PMK)2][PF6]2·MeCN} n (2), and [Ag2(PMK)2](SbF6)2·CH2Cl2 (3) [Ag5(PMK)3(NO3)3][Ag(NO3)3]·3CHCl3 (4) reveal that the coordination behavior of the ligand in all complexes are distinctly different from each other featuring specific architectures. First two are aggregates of double-helical architectures in which different connectivity of the helical units led to a quite different network, whereas the latter one is a trinuclear circular helicate formed from three ligands wrapping around the three silver(I) centres. Comparison of these silver(I) complexes was described in detail by Hannon [4]. Herein, we wish to report the synthesis and spectroscopic properties, luminescent and X-ray single crystal structure of the novel coordination polymer {Ag2(PMK)(OTf)·CH3CN} n (1) in comparison to previously reported silver(I) complex salts of PMK with different anions, namely PF6 −, SbF6 −, and NO3 −, respectively. In the present case, CF3SO3 − was chosen as the counter anion since it can act as a non-coordinating anion or as a labile ligand through the sulfonate group, and also its ability to form hydrogen bonds.
Experimental
Preparation of 1
An acetonitrile solution of PMK (0.240 g, 0.10 mmol) was added AgOTf (0.026 g, 0.1 mmol) in acetonitrile (15 ml) under dry nitrogen, and set to reflux for 6 h with vigorous stirring. The yellowish homogenous solution was concentrated under vacuum and left for crystallization by slow ether diffusion at room temperature to afford yellow needles, 1, suitable for X-ray diffraction. Yield: 82%. Anal. calcd. for C15H15AgF3N7O3S: C, 33.47; H, 2.81; N, 18.22%. Found: C, 33.50; H, 2.78; N, 18.31%. IR data (ATR, cm−1) 1: 3354 (w), 3183 (w), 2924 (vw), 1660 (vw), 1594 (m), 1519 (m), 1478 (m), 1403 (w), 1378 (vw), 1270 (s), 1250 (vs), 1223 (s), 1102 (m), 1029 (vs), 973 (vw), 848 (s), 800 (br, m), 755 (s), 692 (s).1H NMR (ppm, 400 MHz, DMSO-d 6): δ 9.38 (m, J = 8.6 Hz, 5H), 8.75 (m, J = 8.6 Hz, 5H), 2.51 (s, 3H), 2.33 (s, 3H). Mass spectrum (ESI) m/z (%) 538.3 (5) [Ag(PMK)(OTf)(MeCN)]+, 605.8 (25) [Ag4(PMK)2(OTf)2]2+, 1177.0 (100) [Ag5(PMK)5(OTf)3(MeCN)4]2+, 1425.0 (65) [Ag6(PMK)6(OTf)4(MeCN)4]2+, 1694.5 (50) [Ag7(PMK)7(OTf)5(MeCN)5]2+ and 1946.5 (60) [Ag8(PMK)7(OTf)6(MeCN)11]2+.
X-ray crystallography
Diffraction experiments were carried out at 296 K on a Stoe IPDS diffractometer. The structures were solved by direct methods and refined using the programs SHELXS-97 [8] and SHELXL-97 [9]. All non-hydrogen atoms were refined anisotropically by full-matrix least-squares methods. The hydrogen atoms were placed in geometrically idealized positions and refined as riding atoms. Data collection: X-AREA, cell refinement: X-AREA, data reduction: X-RED32 [10]; program(s) used for molecular graphics: ORTEP-3 for Windows [11]; software used to prepare material for publication: WinGX [12] and Mercury 2.3 [13]. Crystal data and structure refinements are summarized in Table 1. Selected bond lengths and bond angles are given in Table 2.
Results and discussion
The coordination geometry around the silver(I) is depicted in Fig. S1 in which each silver(I) centre is five coordinate, bound to one bidentate pyrazylketimine binding unit from one ligand and two a monodentate pyrazine nitrogen donors from two symmetry related ligands, and to triflate anion. The ligand uses only four of six nitrogen donors to bind silver(I) centres and one terminal Npyrazinyl and one Nimine are free, which is distinctly different from the ligation modes of PMK in 2, 3, and 4 as depicted in Fig. 1. However, it resembles the silver(I) complexes of pyridylmethylketazine ligand (L1) in a dinuclear triple helical complex [Ag2(L1)3][PF6]2 [14], where one ligand uses all four donor atoms to coordinate as a bis(bidentate) ligand to the two metal centres, whereas the other two ligands use only three donors and coordinate as a bidentate to one metal centre and a monodentate to the other leaving the imine residue free that acts as a spacer unit between the binding sites. However, with the difference of PMK having additional pyrazine nitrogen donors onto pyrazylketimine binding units also coordinates to another silver(I) centre to form a one-dimensional infinite chain (Fig. 2a). The ligand spans three silver(I) ions, but does not wrap around the metal–metal axis. The twisting of the ligand takes place about the N–N bond between the chelating pyrazylketimine unit (which is slightly deviating from planarity, N(3)–C(10)–C(11)–N(4) 6.9(5)°) and the monodentate part [C(11)–N(4)–N(5)–C(5) = −91.8(4)°]. The weak coordination bonds between the silver(I) atoms and CF3SO3 − anions [Ag(1)–O(3) 2.666(3) Å] seem to stabilize the heavily distorted tetrahedron of the silver(I) atoms to form a distorted trigonal–bipyramidal AgN4O environment in 1. The triflate anions which point out from the 1D chain make weak intermolecular contacts with the adjacent units through C(3)–H(3)···O(2) 2.712(4) Å (Table 3) to form a two-dimensional network (Fig. S2). In the 1D chain, the repeating silver(I) ions are separated by 7.276 Å, whereas in the dinuclear units the Ag···Ag contact is 8.123 Å. The distance between silver(I) ions in the two parallel chains is 10.031 Å. In this structure, the CH3CN molecules are stacked in channels (1D tube) and are stabilized by intermolecular contacts to triflate ions and also to methyl groups onto imine moieties of the ligand strand as shown in Fig. 2b. It is very well known from crystal-engineered coordination polymers that the coordinated anions can result switching between different coordination polymer motifs owing to the ability of the anions to form C–H···X contacts [4, 15]. The CF3SO3 − which seems to have a greater propensity for coordination than NO3 −, PF6 −, or SbF6 − and ability to take part in hydrogen bonding have resulted distinct coordination mode of PMK and very different packing motif in 1 featuring a two-dimensional polymer in comparison to silver(I) complexes of PMK with different anions in 2, 3, and 4, respectively.
(a) Self-assembly of one-dimensional coordination polymer from AgOTf and PMK. Hydrogen atoms have been omitted for clarity. (b) View of the 2D network structure of the complex {Ag(PMK)(OTf)·MeCN} n (1) along the crystallographic a-axis showing the presence of channels encapsulating acetonitrile solvent molecules. Hydrogen atoms have been omitted for clarity
The solid state luminescence spectra of compound 1 and free ligand PMK at room temperature are studied. For PMK, no emissions were observed in the visible range. Compound 1 exhibits two emission maxima at 496 and 522 nm upon excitation at 257 nm (Fig. S3). The emission can be attributed to the emission of ligand-to-metal charge-transfer (LMCT). The bathocromic shift in the emission spectrum of 1 compare to 4 [3] indicates that the luminescent property of the free ligand is significantly affected by the counter anions and binding modes of PMK in the silver(I) coordination polymers adopting specific geometries or network patterns in the solid state.
The ESI-MS spectrum of 1 (Fig. S4) reveals the presence of number of architectures in solution [14]. The peaks at m/z = 538.3, 605.8, 1177.0, 1425.0, 1694.5 and 1946.5 are observed corresponding to [Ag(PMK)(OTf)(MeCN)]+, [Ag4(PMK)2(OTf)2]2+, [Ag5(PMK)5(OTf)3(MeCN)4]2+, [Ag6(PMK)6(OTf)4(MeCN)4]2+, [Ag7(PMK)7(OTf)5(MeCN)5]2+ and [Ag8(PMK)7(OTf)6(MeCN)11]2+, respectively showing the supramolecular self-assembly of PMK with AgOTf in solution.
The FTIR spectral data for 1 and the free ligand can be easily compared (Fig.S5). The most characteristic bands in 1 are those aroused by the triflate anion. The observed bands at 1270, 1250, 1223, and 1029 cm−1 can be assigned to the monodentate coordination modes of the triflate anion [16].
In the present system, the coordination polymer (1) constructed by AgCF3SO3 and multimodal ligand (PMK) shows that the coordination modes of the ligand along with the coordination geometry around the silver(I) ion, and the network pattern of the silver coordination polymer are distinctly different than those previously reported which nicely reflects the effect of the coordinated anion on the network formation and the significantly different solid state fluorescence spectrum of 1.
References
Robson R (1996) In: Atwood JL, Eric J, Davies D, MacNicol DD, Lehn JM (eds) Comprehensive supramolecular chemistry. Pergamon Press, Oxford
Oxtoby NS, Blake AJ, Champness NR, Wilson C (2003) Dalton Trans 3838–3839
Dong YB, Zhao X, Tang B, Wang HY, Huang RQ, Smith MD, Zur-Loye HC (2004) Chem Commun 220–221
Pascu M, Tuna F, Kolodziejczyk E, Pascu GI, Clarkson G, Hannon MJ (2004) Dalton Trans 1546–1555
Munakata M, Wu LP, Kuroda-Sowa T, Maekawa M, Moriwaki K, Kitagawa S (1997) Inorg Chem 36:5416–5418
Carlucci L, Ciani G, Gudenberg DW, Prosperpio DM, Siraoni A (1997) Chem Commun 631–632
Sengul A, Karadayi N, Buyukgungor O (2004) Acta Crystallogr C60:507–508
Sheldrick GM (1997) SHELXS-97: program for the solution of crystal structures. University of Gottingen, Germany
Sheldrick GM (1997) SHELXL-97: program for refinement of crystal structures. University of Gottingen, Germany
Stoe & Cie (2002) X-AREA (version 1.18) and X-RED32 (version 1.04). Stoe&Cie, Darmstadt
Farrugia LJ (1997) J Appl Crystallogr 30:565–566
Farrugia LJ (1999) J Appl Crystallogr 32:837–838
Edgington PR, McCabe P, Macrae CF, Pidcock E, Shields GP, Taylor R, Towler M, Van de Streek J (2006) J Appl Crystallogr 39:453–457
Hamblin J, Jackson A, Alcock NW, Hannon MJ (2002) J Chem Soc Dalton Trans 1635–1641
Hanton LR, Lee K (2000) J Chem Soc Dalton Trans 1161–1166
Haynes JS, Rettig SJ, Sams JR, Trotter J, Thompson RC (1988) Inorg Chem 27:1237–1241
Acknowledgments
We are grateful for financial support from the Zonguldak Karaelmas University (grant no. 2007/2-13-02-10) and the Faculty of Arts and Sciences Ondokuz Mayis University, for the Stoe IPDS-II diffractometer (purchased under grand F.279 of the University Research Fund).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11224_2011_9778_MOESM1_ESM.doc
CCDC-779996 contains the supplementary crystallographic data for 1. These data can be obtained free of charge from The Cambridge Crystallographic Data centre via www.ccdc.cam.ac.uk/data_request/cif (DOC 604 kb)
Rights and permissions
About this article
Cite this article
Sengul, A., Kurt, O. & Buyukgungor, O. A 2D network silver coordination polymer with the multimodal ligand 2-pyrazyl methyl ketazine. Struct Chem 22, 925–929 (2011). https://doi.org/10.1007/s11224-011-9778-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9778-z