We present the regularities of hydrogen degradation of 03Kh12N10MT, 15Kh12N2MFAV and 13Kh11N2V2MF steels under a pressure of 30 MPa within the temperature range of 293–673 K. The minimum values for plasticity, low-cycle fatigue, and static crack resistance, which do not decrease with an increase in pressure of hydrogen atmosphere and content of the absorbed hydrogen, are found. The difference between temperature dependences of the coefficients of influence of hydrogen on static and cyclic crack resistance of martensitic steels with various content of austenite is established. The main fractographic features of the influence of hydrogen on the micromechanism of fracture of steels under different types of loading and temperatures are determined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction. Trouble-free operation of power plants, which is characterized by high level of pressure and temperature of gaseous hydrogen in units and systems with exacting requirements for specific weight coefficients, is determined by correct selection of the structural materials. Therefore, the problem of hydrogen embrittlement in structural materials is critical for modern power engineering [1, 2]. At present, there is a lack of reliable procedures for evaluating the influence of the hydrogen-containing media on the material state under elevated pressure and temperatures as well as the only test method, which makes it possible to obtain enough information for designing and calculating the lifetime of structural elements [3–5]. There is a need to carry on additional investigations on the influence of hydrogen on the material properties. Moreover, it is very important to assess the role of such underlying factors as test temperature, structure and chemical composition of materials, type and rate of loading, working medium pressure and content of the preabsorbed hydrogen. The serviceability assessment of the material under particular operating conditions is required to be performed on the basis of complex experimental investigations involving determination of not only standard mechanical characteristics of strength, plasticity of materials and low-cycle fatigue, but also crack resistance. The use of crack resistance characteristics – stress intensity factors (SIF) K 1c (K c ) under static loading and threshold ΔK th and critical K fc values under cyclic loading – to estimate serviceability under particular operating conditions for gaseous hydrogen under elevated pressures and temperatures, is of crucial importance for developing structural materials with high hydrogen resistance and determining the lifetime of structural elements [1, 5–7].
This paper will review the research conducted on the integral assessment of the influence of high-pressure and high-temperature gaseous hydrogen on strength, durability, crack resistance and microstructural features of fracture for corrosion-resistant martensitic steels.
Materials and Procedure of Investigations. The authors tested 03Kh12N10MT (ÉP810), 15Kh12N2MFAV (ÉP517) and 13Kh11N2V2MF (ÉI961) martensitic steels. These types of steel are used in the aerospace and power machine building [3, 4, 7]. Their chemical composition, modes of thermal treatment, and mechanical properties in air at room temperature are presented in Tables 1 and 2. The martensitic steels differ mainly by the contents of carbon and nickel (Table 1). The structure of the steels consists of tempered martensite, a small amount of residual austenite (up to 10%), alloyed carbides Me23C6, (W, Nb, Ni)C and small amount of intermetallic compounds. Martensite in carbon steels manifests itself as a mixture of lath (packet) and plate (needled) martensite. The content of the lath martensite in 15Kh12N2MFAV and 13Kh11N2V2MF steels is 75–85 and 60–70%, respectively, length of the laths in a packet is 20–28 and 10–23 μm, their width is 2–8 and 1–3 μm, i.e., the structure of 13Kh11N2V2MF steel is more dispersed than 15Kh12N2MFAV steel (Fig. 1a and b). Martensite of the nickel-alloyed carbon-free steel 03Kh12N10MT is a packet (lath) martensite with the austenitic grain size of 50–80 μm, width of the packets of 30–70 μm and width of the laths in a packet of 3–10 μm (Fig. 1). The high nickel content in 03Kh12N10MT steel enhances its plasticity and toughness, and reduces the stress concentration sensitivity [8]. The optimal thermal treatment (TT1) of this steel ensures a high dispersion of phases and about 10% of residual and secondary austenite in the form of thin layers (< 1 μm) between the plates of martensite (Fig. 1c). A coarser structure with austenite content of up to 30% is formed as a result of the additional heating of this steel to 1373 K (TT2), moreover, secondary austenite distributes nonuniformly with the formation of “islets.” It is know that the presence of a soft structural component of finely divided residual austenite in the structure of stainless low-carbon martensitic steel facilitates enhancement of strength, plasticity, impact toughness and crack resistance with its optimum content from 10 to 25% [8].
The five-fold cylindrical specimens with a diameter of the working part of 5 mm were subjected to short-term static tension in a special chamber under the pressure of hydrogen of up to 30 MPa for strain rates equal to 0.1 mm/min. The low-cycle fatigue for pure strain-controlled sigh-preserving bending was found under pressures of up to 30 MPa for the strain amplitude equal to 1.6% and a loading frequency 0.5 Hz on polished plane specimens with a working part of 3 × 6 × 20 mm. The stress intensity factor under static loading K c was calculated complying with the standard [9]. In a high-pressure chamber rectangular compact specimens of 50 × 60 × 20 mm in size were tested for eccentric tension under pressures of 0.4–30 MPa at a strain rate of 0.1 mm/min. The values were calculated using the Srawley–Gross formula [10]. Kinetic fatigue fracture diagrams (KFFD) were plotted using three-point bending of beam specimens with an edge crack 160 × 40 × 20 mm in size at the loading frequency of 20 Hz and coefficient of cycle asymmetry R = 0.22 by the procedure described in [2].
To determine the indicated mechanical characteristics in hydrogen, the working chambers were preliminary evacuated, blown-out with hydrogen, again evacuated, and filled with hydrogen up to a given pressure. At high temperatures, the specimens were held under testing conditions for 0.5 h to the attainment of thermal equilibrium. In order to compare the action of the so-called external and internal hydrogen [2–4, 6], and study the influence of hydrogen dissolved as a result of long-term operation of hydrogen structures on the properties of steel, a part of specimens were preliminary held in the hydrogen atmosphere for 10 h under the pressure of 30 MPa and temperature of 623 K to the hydrogen concentration of about 3.2⋅10−6. The hydrogenated and nonhydrogenated specimens were tested under various pressures in hydrogen, vacuum, helium, or air. The sensitivity of steels to hydrogen degradation was compared by using the coefficient β defined as the ratio of values of the corresponding characteristics measured in hydrogen and in a neutral medium (e.g., the coefficient of influence of hydrogen on the low-cycle fatigue β N = N H/N He).
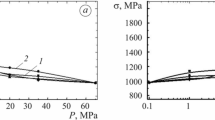
Hydrogen Pressure and Test Temperature Dependencies of Mechanical Properties of Steels. The dependencies of the influence of hydrogen pressure on the fracture toughness, low-cycle fatigue and plasticity characteristics of martensitic steels are quantitatively similar (Fig. 2). The values for crack resistance parameter K c , number of cycles to failure N, relative elongation δ, and transverse narrowing ψ for specimens made of martensitic steels are greatly reduced to a minimum with an increase in the pressure of external hydrogen up to 10 MPa and are slightly changed with its subsequent increase up to 30 MPa (Fig. 2). The additional influence of the preabsorbed hydrogen on the abovementioned properties of martensitic steels at the temperature of 293 K was determined only at the pressure of P H < 10 MPa. Thus, the maximum hydrogen degradation of martensitic steels in the course of static crack resistance, short-term static tension and low-cycle fatigue testing is attained by the pressure of hydrogen atmosphere of 7.5–10 MPa independent on the dissolved hydrogen content. Therefore, temperature dependencies of fracture toughness of the steels were studied on the nonhydrogenated specimens in helium and hydrogen under the pressure of 10 MPa.
If in neutral media the values for plastic characteristics and parameter K c of the steels are quite similar (Table 2, Fig. 2), then with the maximum hydrogen degradation they are significantly different. So, relative elongation of the specimens δ in hydrogen is 3 (steel 15Kh12N2MFAV) and 14% [steel 03Kh12N10MT (TT1)]. In hydrogen under the pressure of 30 MPa, 03Kh12N10MT steel (TT1) with packet martensite and homogeneous distribution of the small amount (up to 10%) of finely divided austenite (Fig. 1c) exhibits the highest fracture toughness value (112 MPa\( \sqrt {\text{m}} \)), 15Kh12N2MFAV steel with plate martensite has the lowest fracture toughness value (49 MPa\( \sqrt {\text{m}} \)) (Table 2, Fig. 2). 03X12N10MT steel (TT2) with elevated content of (30%) islandlike austenite and 13Kh11N2V2MF steel with the values K c in hydrogen 74 and 77 MPa\( \sqrt {\text{m}} \), respectively, are found to take an in-between position. Thus, finer structure of the carbon martensite in 13Kh11N2V2MF steel ensures its higher resistance to hydrogen degradation compared to 15Kh12N2MFAV steel, while optimization of the content, size and distribution character of residual austenite improves hydrogen resistance of the carbon-free nickel martensite in 03Kh12N10MT steel.
Temperature has the greatest influence on the material properties during plastic strain accumulation, crack initiation and its growth. Since the temperature dependencies of strength, plasticity, and low-cycle fatigue characteristics of the investigated steels in gaseous hydrogen have been already analyzed [11], let us consider temperature and hydrogen influence on the parameters of static K c (K 1c ) and cyclic ΔK th and K fc crack resistance. Fracture toughness of all steels in helium decreases with an increase in the temperature (Fig. 3), which is typical of brittle materials [12, 13]. In hydrogen media, an increase in the temperature leads to attenuation of the action of hydrogen, i.e., the increase of the K c parameter value. At the temperature of 673 K static crack resistance values in hydrogen and helium are identical, the maximum influence of hydrogen on K c was revealed at the temperature of 293 K (Fig. 3). The 15Kh12N2MFAV steel, which is additionally alloyed by nitrogen, niobium and molybdenum, with less dispersed carbon martensite and more heat-resistant structure at the temperature of 673 K shows better resistance to fracture during static crack resistance testing compared to 13Kh11N2V2MF steel. The 03Kh12N10MT steel (TT1) has the highest fracture toughness value within the whole investigated temperature range.
In helium, the steels are characterized by fairly high plasticity in the entire analyzed temperature range (Table 2), therefore, the thickness of the specimens is insufficient to achieve the plane-strain condition. The fracture diagrams are curvilinear (of type III) according to [9] and the fracture surface is ductile-brittle with typical lateral bindings. The work on the propagation of fracture toughness involves plastic strain and retarded crack propagation. Gaseous hydrogen decreases the crack resistance coefficient and affects the fracture behavior. Under conditions of maximum hydrogen embrittlement, the load–displacement diagrams become linear with sharp maximum (as function of the load) and correspond to type I [9], the fracture surfaces of the specimens are covered with cleavage facets and with a small amount of intergranular cracks typical of brittle fracture. Moreover, the values K c attain critical value K 1c , i.e., stress field at the edge of the crack approximates plane-strain conditions. Such conditions are satisfied at temperatures of 293–423 K for 03Kh12N10MT (TT2) and 15Kh12N2MFAV steels at pressures of hydrogen of more than 10 MPa. The obtained static crack resistance parameters represent the invariant characteristics of the materials and they can be used for calculating the residual strength of structural elements in hydrogen using fracture mechanics approaches [1, 12–14].
Under the action of fatigue loading on the beam specimens in helium and hydrogen, the threshold coefficient ΔK th increases as temperature grows from 293 to 673 K (Fig. 4, curves 1 and 2), and the critical stress intensity factor K fc decreases (curves 3 and 4). The percentage reduction of the parameters of cyclic crack resistance in hydrogen at temperature of 293 K is 51 and 31% (13Kh11N2V2MF steel), respectively, and 43 and 46% (15Kh12N2MFAV steel) in helium (Fig. 4). At room temperature, the most sensitive to the action of hydrogen is low-cycle fatigue (Fig. 4). The degree and temperature range of the decrease in the fracture toughness K c and relative transverse narrowing ψ are similar. At the temperature of 293 K hydrogen has the weakest influence on cyclic crack resistance, however with a decrease in the value ΔK th and K fc it remains constant in the entire analyzed temperature range, whereas the degree of hydrogen degradation decreases in the course of the other testing with an increase in the temperature. Thus, at the value of T ≥ 600 K the most sensitive to hydrogen degradation at the temperature of 293 K are exactly the parameters of cyclic crack resistance ΔK th and K fc of the martensitic steels (Fig. 4).
Temperature influence on the cyclic resistance parameters (1, 2) and (3, 4) in helium (1, 3) and in hydrogen under the pressure of 30 MPa after preliminary hydrogenation (623 K, 30 MPa H2, 10 h) (2, 4) of 13Kh11N2V2MF (a) and 15Kh12N2MFAV (b) steels and degree of hydrogen degradation of 15Kh12N2MFAV steel under different types of loading (c).
Influence of Hydrogen on Character of Fracture of Steels. Differences in the chemical composition and structure of the studied martensitic steels do not influence the specific features of their fracture in helium and hydrogen. In neutral media, at the temperature of 293 K under static and cyclic loading fracture of the specimens is ductile transgranular with dimples (Fig. 5a, c, and e). In the cone portion of the fracture under shearing stresses dimples exhibit an elongated shape. In the presence of hydrogen the formation of smooth cleavage surfaces is observed and significant amount of the surface is covered with multiple intergranular microcracks (Fig. 5f). Compact type specimens with an initial fatigue crack are subjected to similar fracture. In helium, dimples and some separate intergranular microcracks are detected in the zone of static fracture, whereas in hydrogen, facets of quasicleavage and significant number of intergranular microcracks are found (Fig. 5a and b). During static crack resistance testing intensive cracking along the boundaries of martensite laths and islandlike austenite initiates at the edge of the crack in the region of the transition from preliminary loading in air to static loading in hydrogen (Fig. 5b).
Microfractographs of fracture of specimens made of 15Kh12N2MFAV steel at the temperature of 293 K in air (a, c, e) and in hydrogen, 10 MPa (b, d, f): (a, b) induced cracks under static loading; (c, d) upper region of the KFFD; (e) is the center of the KFFD; (f) is the edge of the specimen under short-term tension.
In helium, the formation of irregular fatigue striations and areas of quasicleavage were observed in the zone of fatigue crack initiation for beam specimens of all steels, whereas in the upper region of the KFFD plastic striations and separate regions with cellular topography and integranular fracture were observed (Fig. 5c). The presence of hydrogen in the zone of fatigue crack initiation increases the number of quasicleavage facets for both steels and in the upper region of the KFFD it causes transition from the typical striated topography to fracture, which is characteristic of quasistatic short-term tests. The brittle facets of translgranular and intergranular cleavage with traces of plastic flow at the transition from one cleavage facet to another were detected (Fig. 5d). Carbide particles often promote crack initiation and their cracking impairs cyclic crack resistance of the steel. Thus, the martensite/austenite interface and boundaries of martensitic plates, where there are mainly carbides and intermetallic compounds, play a dominant role in the hydrogen embrittlement of the steels with martensitic-austenitic structure. This is confirmed by the local distribution of hydrogen in the structure of martensitic steels obtained in [15], its maximum concentration at the phase boundaries with any phase composition and origin of the martensite. These boundaries are concurrently the centers of local peak microstresses, the sites of localization of plastic deformation and microlocal sites of fracture in hydrogen. Thereby, reduction of the susceptibility of steels to hydrogen degradation with an increase in the temperature during static tension, crack resistance and low-cycle fatigue testing can be caused by relaxation of interphase stresses and more homogeneous distribution of hydrogen. The features of high-temperature hydrogen degradation with cyclic crack resistance are associated with the interactions of hydrogen with moving dislocations, which have been analyzed in [2].
Thus, hydrogen affects the micromechanism of fracture under short-term and fatigue loading of smooth specimens and specimens with preliminarily induced fatigue cracks, the number of planes of smooth delamination and regions of intergranular fracture is increased. The influence of hydrogen on the morphology of fracture under static tension, crack resistance and low-cycle fatigue testing is revealed within the temperature range of 293–473 K and under cyclic crack resistance testing over the temperature range of 293–673 K.
Conclusions
-
1.
At room temperature the maximum influence of hydrogen on the characteristics of plasticity, low-cycle fatigue, and crack resistance of martensitic steels was obtained at the pressure of hydrogen environment of 10 MPa, additional influence of the preabsorbed hydrogen was insignificant.
-
2.
The reduction of the amount of residual austenite and increase in the dispersity of the structure lead to an increase in hydrogen resistance of martensitic steels under static loading. In hydrogen, the low-carbon nickel 03Kh12N10MT steel (TT1) with packet martensite and homogeneous distribution of a small amount (up to 10%) of finely divided austenite exhibits the highest plasticity and fracture toughness characteristics at the pressure of 30 MPa. The structure of the carbon martensite in 13Kh11N2V2M steel with smaller martensite needles provided higher resistance to hydrogen degradation compared to 15Kh12N2MFAV steel.
-
3.
The maximum influence of hydrogen on the static crack resistance was revealed at the temperature of 293 K when fracture toughness value K c of all steels decreases in hydrogen by 2–3 orders compared to helium. With an increase in the temperature in helium it decrease and in hydrogen it increases, whereas at the temperature of 673 K in hydrogen and helium it is the same. The 15Kh12N2MFAV steel, which is additionally alloyed by nitrogen, niobium and molybdenum, with less dispersed carbon martensite is more heat-resistant and at the temperature of 673 K shows better resistance to fracture during crack resistance testing compared to 13Kh11N2V2M steel.
-
4.
At room temperature, the most sensitive to the action of hydrogen is low-cycle fatigue, which covers 5% of the values in helium. The degree and temperature range of the decrease in the fracture toughness K c and relative transverse narrowing ψ are similar. At the temperature of 293 K hydrogen has the weakest influence on cyclic crack resistance, however with a decrease in the values ΔK th and K fc it remains constant in the entire analyzed temperature range, whereas the degree of hydrogen degradation decreases in the course of the other testing with an increase in the temperature. Thus, at the value of ≥ 600 K the most sensitive to hydrogen degradation at the temperature of 293 K are exactly the parameters of cyclic crack resistance ΔK th and K fc of the martensitic steels.
-
5.
Under static tension and crack resistance hydrogen facilitates transition from the transgranular fracture with dimples to the cracking along the boundaries of martensite laths and grains. The main mechanism of fracture in the upper region of KFFD in hydrogen is the translgranular and intergranular cleavage with traces of plastic flow at the transition from one cleavage facet to another. The influence of hydrogen on the morphology of fracture under static tension, crack resistance and low-cycle fatigue testing is revealed within the temperature range of 293–473 K and under cyclic crack resistance testing over the temperature range of 293–673 K.
References
A. I. Balitskii and V. V. Panasyuk, “Workability assessment of structural steels of power plant units in hydrogen environments,” Strength Mater., 43, No 1. 52–57 (2009).
V. V. Panasyuk (Ed.), Physico-Mechanical Institute: Progress and Results [in Ukrainian], Karpenko Physico-Mechanical Institute of the National Academy of Sciences of Ukraine, Lviv (2001).
V. I. Tkachev, L. M. Ivas’kevich, and V. I. Vitvitskii, Specific features of determination of susceptibility of steels to hydrogen degradation,” Al’ternat. Énerg., No. 12 (32), 46–51 (2005).
A. I. Belogurov, V. S. Radchuk, M. A. Rudis, et al. “Strength analysis of structural elements of hydrogen power-generating equipment,” Mater. Sci., No. 6, 89–94 (2004).
V. V. Panasyuk, Mechanics of Quasibrittle Fracture of Materials [in Russian], Naukova Dumka, Kiev (1991).
B. A. Kolachev, A. V. Mal’kov, and V. I. Sedov, “Use of linear failure mechanics in the study of the hydrogen brittleness of titanium alloys,” Fiz.-Khim. Mekh. Mater., No. 6, 7–12 (1975).
A. V. Fishgoit and B. A. Kolachev, “Strength tests in hydrogen in the aerospace industry,” Fiz.-Khim. Mekh. Mater., No. 4, 151–154 (1997).
M. I. Gol’dshtein, V. S. Litvinov, and B. M. Bronfin, Physics of Metals of High Strength Alloys [in Russian], Metallurgiya, Moscow (1986).
GOST 25506-85. Methods for Mechanical Testing of Metals. Determination of the Characteristics of Crack Resistance (Fracture Toughness) under Static Loading [in Russian], Izd. Standartov, Moscow (1985).
W. F. Brown and J. E. Srawley, “Plane strain crack toughness testing of high strength metallic materials,” in: ASTM STP 410, Philadelphia (1966).
O. Balyts’kyi and V. Mochul’s’kyi, “High-temperature hydrogen resistance of martensitic steels,” Mashinoznavstvo, No. 7 (122), 23–28 (2009).
O. N. Romaniv and G. N. Nikiforchin, Mechanics of Corrosion Fracture of Structural Alloys [in Russian], Metallurgiya, Moscow (1986).
G. P. Cherepanov, Mechanics of Brittle Fracture [in Russian], Nauka, Moscow (1974).
V. V. Panasyuk, “Some actual problems of strength of materials and lifetime of structures,” Fiz..-Khim. Mekh. Mater., No. 2, 5–22 (2009).
S. Z. Bokstein, S. S. Ginzburg, and S. T. Kishkin, Autoradiography of Interfaces and Structure Stability of Alloys [in Russian], Metallurgiya, Moscow (1987).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Problemy Prochnosti, No. 1, pp. 89 – 99, January – February, 2012.
Rights and permissions
About this article
Cite this article
Balyts’kyi, O.I., Ivas’kevych, L.M. & Mochul’s’kyi, V.M. Mechanical properties of martensitic steels in gaseous hydrogen. Strength Mater 44, 64–71 (2012). https://doi.org/10.1007/s11223-012-9350-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11223-012-9350-0