Abstract
Supported oxides of some transition metals have been prepared via incipient wetness impregnation of a mesoporous silica. Obtained compositions have been studied using DTA-TG and XRD analysis, FTIR, Raman, UV–Vis and XPS spectroscopy as well as low-temperature nitrogen adsorption–desorption. Deposited oxides have amorphous or low-crystalline structure and exhibit electronic properties different from those for bulk oxides. Red shift of adsorption edge is observed for most supported samples compared with corresponding bulk oxides. All tested samples regardless of the band gap showed photocatalytic activity under visible irradiation in process of rhodamine B degradation. The prepared supported oxides were more active compared with bulk oxides. The dispersion of oxides on the surface of the support and the greater accessibility of dye molecules to the active sites of the surface are the main reason for this. As a result, Ag/SiO2 and TiO2/SiO2 samples exhibited maximal photocatalytic activity. Three mechanisms of initiation for photocatalytic process were considered.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transition metal oxides are versatile materials since they have numerous applications in the bulk and deposited states: as catalysts, ion exchangers, materials for gas sensors [1,2,3,4]. Being a semiconductor by their nature, they are promising photocatalysts for the processes of water splitting as well as organic pollutants degradation [5,6,7,8]. The efficiency of these applications is determined by crystal, porous and surface structure, as well as electronic characteristics of the oxides. The main disadvantage of “white” oxides as a photocatalysts is large band gap (3.3–4.0 eV), the consequence of which is their activity only under UV irradiation. The latter one limits their practical use, since UV radiation is only about 4% in sunlight.
In principle, photocatalytic activity will be enhanced if the absorption of light is higher or if the recombination losses for photogenerated charge carriers are lower. Based on this, there are several ways to overcome these shortcomings. Generally, photocatalytic activity of these bulk oxides under visible irradiation could be achieved by defect engineering or doping [6, 9]. Defect engineering implies controlled introduction of native defects, such as vacancies, interstitials and antisites, while doping implies controlled introduction of impurities into the crystal lattice. As a result, the band gap narrowing and manifestation of activity under visible irradiation take place [6, 9, 10].
Another approach can be the deposition of an active phase onto the surface of mesoporous supports [2, 4] as it was shown for simple [11,12,13,14] and mixed oxides [15,16,17]. This method also allows to overcome the general lack of all bulk oxide photocatalysts characterized by the limited accessibility of large molecules of organic pollutants to active surface sites. In contrast to bulk semiconductor metal oxides, highly dispersed (isolated) metal oxide species loaded on inorganic supports such as silica and alumina have attracted a lot of attention. Particularly, it has been reported that supporting the semiconductors onto a suitable support, such as zeolites, significantly increases the photocatalytic efficiency of the supported semiconductors compared to unsupported ones [18,19,20]. For example, NiO/clinoptilolite-supported composition showed significant increase in the photocatalytic degradation of cephalexin in aqueous medium compared to bulk nickel oxide [21].
The best studied supported photocatalysts are the ones based on titanium dioxide [11,12,13], when the various oxide supports were used [22]. There are quite a lot of studies concerning the deposition of other semiconductor oxides on supports, but they are not systematic. It is noteworthy that often used supports are also semiconductors (for example, ZrO2, CeO2, SnO2, Nb2O5, etc.) and not inert substrates. As a result, it is very difficult to distinguish the effect of photocatalyst deposition on such semiconductor supports. In other words, there is an unobvious answer to the question whether this is deposition on a support or doping (coupling) a semiconductor support.
On this basis, we chose silica gel as a support, which is a dielectric and, therefore, is not photocatalytically active. The oxides of transition metals with a different band gap and valence were used as deposited active phases. It should be noted that selected oxides are often used as dopants for TiO2 and others semiconductor photocatalysts [4, 10, 23, 24]. From this point of view, it will allow to determine the net effect as the result of deposition of these oxides on the support. The changes in the physicochemical characteristics and photocatalytic activity of the compositions obtained under visible irradiation compared with those ones for bulk oxides can be a measure of this effect.
The aim of this work was to investigate crystal and porous structure, electronic characteristics of transition metal oxides supported onto silica as well as the influence of these physicochemical properties on their photocatalytic activity under visible irradiation.
Experimental
Reagents and materials
Silica gel KSKG (China) was used as a support. Salts precursors presented in Table 1 were used as a source of metals. For comparison, fumed titanium–silica containing 7% w/w of TiO2 which was prepared at 950 °C [25] as well as a sample with 10% w/w of H2TiO3, modified with titanium peroxide groups, on silica was tested. The peculiarity of the first sample (and its difference from the supported samples) is that the Ti atoms are incorporated into the system of siloxane bonds without the formation of a separate (even amorphous) TiO2 phase. The second sample was prepared according to work [26] from H2TiO3 and fumed silica (aerosil A-200). As well known, chosen oxides are photocatalytically active only under UV (TiO2, SnO2, ZnO) or also visible irradiation [14].
Preparation of the supported compositions
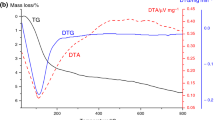
The supported samples with different oxides content, designated as XM, where X = 5 or 10 (the number in the sample designation corresponds to metal oxide content in wt %) were synthesized by deposition of salts on silica gel granules using incipient wetness impregnation (Table 1). For this purpose, a fraction 0.5–2 mm of silica gel was impregnated with an appropriate amount of corresponding salt aqueous solution, held for 1 h, then dried at 110 °C and calcined in air at different temperatures. The temperature of calcination was determined using DTA-TG data. It is the temperature of complete decomposition of corresponding salt as in [14].
Characterization
The crystal structure of the supported samples was studied by X-ray powder diffraction (XRD) using Philips PW 1830 diffractometer with CuKα radiation. The curves of DTA and TG were recorded using the Derivatograph-C apparatus (F. Paulik, J. Paulik, L. Erdey) in the temperature range of 20–1000 °C at a heating rate of 10° min−1. The initial sample mass was about 200 mg, and the sensitivity was 50 mg. Raman spectra were recorded for the selected samples using the spectrograph of Renishaw system (Ar laser, 514 nm). The FTIR spectra in the range of 4000–400 cm−1 were registered using the “Spectrum-One” spectrometer (PerkinElmer). The ratio of the sample and KBr powders was 1:20. The porous structure of the initial silica gel and supported samples was studied using nitrogen adsorption–desorption technique. The isotherms were obtained using an automatic gas adsorption analyzer ASAP 2405 N (“Micromeritics Instrument Corp”) after outgassing the catalysts at 150 °C for 2 h. The specific surface area S, mesopore volume Vme and micropore volume Vmi were calculated from these isotherms using BET, BJH and t-plot methods, respectively. The total pore volume VΣ was determined by the impregnation of the samples, which were preliminary dried at 150 °C, with liquid water (so-called incipient wetness method [27]). Macropore volume Vma was calculated as the difference between VΣ and sorption pore volume Vs. The latter one was determined from the isotherms at nitrogen relative pressure close to 1.0. Mesopore size dme was calculated from the pore size distribution (PSD) curves plotted using the desorption branches of the isotherms. Diffuse reflectance UV–Vis spectra of the powders were registered using Lambda 35 UV–Vis spectrometer (PerkinElmer Instruments). The band gap was determined using the Plank’s formula.
Photocatalytic testing
Photocatalytic degradation was performed in a glass reactor under visible irradiation. LED Cool daylight lamp, Philips (100 W), emits exclusively in the visible range. Its emission spectrum has a broad maximum in the region of 500–700 nm, and a local maximum around 440 nm was used as an irradiation source. The dye rhodamine B (RhB) in the form of 1.5·10−5 mol L−1 solution was used as a model pollutant. The main absorption band λmax in the spectra of the RhB is 553 nm. The catalyst dose was 1 g L−1 (80 mg of a catalyst and 80 ml of solution). Duration of dark adsorption to establish equilibrium was 60 min. The initial solutions and solutions after dye adsorption and degradation for 30–600 min were analyzed spectrophotometrically at λmax (Lambda 35, PerkinElmer Instruments) after centrifugation of the reaction mixture (10 min at 8000 rpm). The calculation of photodegradation rate Kd was based on the temporal changes of the dye concentration after reaching the adsorption equilibrium. The degradation degree was estimated by reducing the intensity of the band at 553 nm (bleaching degree). The total organic carbon (TOC), which is a measure of dye mineralization [28], in the solutions after irradiation was determined using a Shimadzu TOC analyzer (model 5050A).
Results and discussion
XRD
As for supported tin dioxide [14], the temperature of salts destruction and oxides formation was determined using DTA-TG measurements. Therefore, the calcination temperature of the precursors prepared after impregnation and drying was within 250–500 °C (Table 1, column 5).
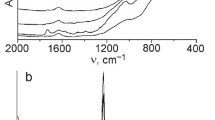
According to XRD data, the obtained supported samples after calcination are X-ray amorphous or weakly crystalline. It is understandable since the deposited oxide content is 5–10%, and the support is also amorphous. This is also evidence of uniform dispersion of the oxides on the support surface which results in the impossibility of aggregation of oxides species in large crystallites and formation of a separate crystal phase. Particularly, it is characteristic for deposited silver, zinc and titanium dioxide (5–10%) as well as fumed titanosilica even after calcination at 300–500 °C which was earlier established for such loading of a deposited phase [11, 12, 22, 23, 29, 30]. For example, the XRD patterns of titanium-containing samples 5Ti and 10Ti are presented in Fig. 1a. However, Raman spectra recorded for these samples contain low-intensity bands characteristic for various crystalline modifications of TiO2 (Fig. 1b). Similar to work [22], broadbands with maxima about 148 cm−1 and 400 cm−1, which can be attributed to anatase [2, 31, 32], are present on the spectra obtained for samples 5Ti and 10Ti. More intense bands are contained in a spectrum of TAS-7 prepared at a higher temperature: 154 cm−1 and 610 cm−1. They are assigned to high-temperature modifications of titania brookite and rutile, respectively. The band at 983 cm−1 (for 5Ti and 10Ti) as well as 936 cm−1 (for TAS-7) can be attributed to Si–O–Ti bonds.
At the same time, XRD patterns obtained for the sample containing deposited tin and titanium dioxides mixture in the total amount of only 5% (Fig. 1a) show only peaks, attributed to rutile phase of SnO2 (JCPDS No. 41-1445). The latter one is agreed with the results obtained for SnO2/SiO2 compositions with 3–10% of SnO2 [14]. XRD pattern of the sample containing surface titanium peroxide groups has only peaks inherent to initial H2TiO3 although they are slightly shifted toward higher values of 2θ. This is obviously associated with embedding the peroxide groups into the structure of H2TiO3, as well as with its deposition on the silica surface.
Moreover, the supported samples with additives of Mo, Co, Mo–Co and Cu oxides also contain low-crystalline phases (Fig. 1c). Using the sample 10%Co/SiO2 as an example, the broadening of the peaks for the deposited cobalt oxide Co3O4 compared to the bulk ones can be seen (JCPDS No. 76-1802). As a result, estimation of the crystallite size using the Scherrer’s formula (where it is possible) indicates that the deposited oxides are in nano-state. The calculated values are 5–10 nm.
Another feature is that deposition is accompanied by partial re-orientation of main crystallographic planes. This strategy of photocatalysts structure modification is sometimes called “facet engineering,” i.e., morphology optimization for enhancing the proportion of active facets [9, 33]. It can be expressed in terms of intensities ratio for different planes from XRD patterns. These changes are most pronounced for the samples containing molybdenum trioxide. Therefore, the I040/I021 value for rhombohedral MoO3 is 1.15 and 1.10 for Mo/SiO2 and Mo–Co/SiO2 compositions, respectively, while this parameter is about 14 for bulk MoO3 (JCPDS No. 05-0508). Similar results were also obtained for MoO3 mechanochemically deposited onto fumed silica, titania and alumina [34].
FTIR
In general, the obtained FTIR spectra are the superposition of the spectra of the components. In addition to the absorption bands characteristic for silica, the spectra contain low-intensity bands related to the deposited oxide phases. The bands with the maxima at about 1100 and 800 cm−1 are attributed to stretching frequencies of SiO4, –Si–O–Si– bending modes and –O–Si–O– bending [35, 36]. The absorption bands belonging to the components overlap to a large extent [34], but these spectra allow to clarify the structure of the titanium-containing samples (Fig. 2a). Thus, the presence of bands at 945 cm−1 and 960 cm−1 indicates the bonds Si–O–Ti in the structure of 5Ti and TAS-7 [25, 37].
Absorption bands centered at 860 and 1414 cm−1 show the presence of peroxide moieties for the sample 10Ti-per. Some shift of these adsorption bands in comparison with the values presented in the literature [38, 39] may be due to the fact that peroxo-groups are only on the surface of the precursor, H2TiO3. The spectrum obtained for 10Co contains the bands at 543 and 677 cm−1 (Fig. 2b) attributed to cobalt oxides [40, 41]. The bands at 826 and 1012 cm−1 related to Mo–O bonds [34] are present in the spectra of Mo-containing samples (Fig. 2b).
Porous structure
Parameters of porous structure for the initial silica gel and supported samples were calculated from nitrogen adsorption–desorption isotherms, examples of which are shown in Fig. 3. All of them (with the exception of the isotherm for TAS-7) belong to type IV according to IUPAC classification. As can be seen, they contain capillary condensation hysteresis with hysteresis loop close in shape to type H1. These isotherm and hysteresis loops are characteristic for mesoporous materials. Pore size distribution (PSD) curves (inset to Fig. 3) also indicate it: Pore diameter is within the range of 4–20 nm although the pore fraction with a size of 6–12 nm is about 70%. The isotherm obtained for TAS-7 is related to type II which is characteristic for non-porous solids. Indeed, TAS-7 is practically non-porous powder. A sharp rise in the isotherm at relative nitrogen pressures close to 1 is associated with the filling of voids between powder particles.
Therefore, the initial silica gel and supported samples mainly contain mesopores with a size in the range of 4–16 nm and maximum on PSD curve at about 7.8–8.0 nm but do not contain micro- and macropores (Table 2). Thus, the coincidence of VƩ and Vme values indicates the absence of macropores. Embedding the oxides into pores of silica gel predictably leads to a decrease in the specific surface area, total pore volume and mesopore volume. This decrease was significantly higher than for tin dioxide deposited on the same silica gel [14]. Only the sample 10Ag has slightly larger pores. It is important to note that the specific surface area for the resulted compositions is significantly higher than for any bulk oxide prepared under the same conditions: The values of specific surface area for bulk oxides are within 5–150 m2 g−1.
The samples based on fumed silica are different. They have smaller specific surface area. Moreover, TAS-7 is practically non-porous. A sharp rise in the isotherm at p/p0 > 0.95 is associated with the filling of voids between freely packed particles (Fig. 3). On the contrary, reference samples 10TiOH and 10Ti-per have maximal values of pore volume and pore size (Table 2). A wider pore size distribution with a diffused maximum at about 18 nm was obtained for 10Ti-per (inset to Fig. 3). Therefore, all prepared supported samples as well as the reference ones possess high specific surface area and developed mesoporous structure accessible for dye molecules.
UV–Vis spectra
Diffuse reflectance UV–Vis spectra for some supported samples are presented in Fig. 4a, b. One can see that some red shift of an absorption edge Δλ is observed as a result of TiO2 deposition: from 330 nm for bulk X-ray amorphous TiO2 (prepared under the same conditions) to 356 and 338 nm for the supported samples with 5 and 10% of TiO2, respectively (Fig. 4a, Table 3). Maximum value of Δλ is observed for the sample containing 5% of TiO2. All the samples also show absorption in the visible region. Such an effect was earlier described for silica-supported SnO2 [14], TiO2 [11, 12] and for sample containing SnO2–TiO2 solid solution phase embedded into SBA-15 structure [42]. As can be seen from Table 3, the absorption edge is in the UV region for the samples containing TiO2, SnO2, ZnO and MoO3. However, an additional absorption edge in visible range is observed for TAS-7 and 10Ti-per containing peroxo-groups. The latter is agreed with the results obtained for bulk titanium peroxide [38, 39]. Moreover, the absorption edge for the samples with deposited copper and cobalt is located in visible region (Fig. 4b) as for bulk oxides. In addition, an observed increased absorption in the visible region compared to that of unsupported oxides can be ascribed to the increased concentration of surface states/defect levels compared to that of bulk oxides.
The position of the absorption edge determines the band gap Eg (Table 3, column 3) and, accordingly, the possibility of using the samples as photocatalysts under visible light.
Photocatalytic properties
It is well known that the photocatalytic properties of semiconductors depend on their electronic characteristics, crystal and porous structure, specific surface area and hydroxylation degree and, therefore, adsorption capacity toward the substrate [10, 28]. However, there are various controversies concerning the relationship between these properties and photocatalytic activity. Nevertheless, we tried to establish the influence of physicochemical characteristics of the supported oxides on their photocatalytic properties. The enhanced activity for supported oxides was attributed to the high dispersion of oxides on silica and also due to the separation of the photoinduced electrons and holes by the dielectric support. Moreover, an increased specific surface area plays role of hole scavengers during photocatalytic process [43].
Theoretically, the band gap < 3.26 eV and the absorption of light with a wavelength > 380 nm are necessary conditions for photocatalytic activity under visible irradiation [10]: In this case, it is possible to form an electron–hole pair on the photocatalyst surface and, accordingly, initiate the process of substrate transformation (conventional photocatalytic process). However, all tested supported samples, regardless of these requirements, showed activity in RhB degradation under visible irradiation. This is clearly confirmed by temporal spectral changes presented in Fig. 5a–d. One can see a sharp decrease in the intensity of the band at 553 nm already after 60 min of irradiation. It is well known that RhB photodegradation in the presence of oxide photocatalysts can occur by two pathways [14, 23, 44]: as de-ethylation process in a stepwise manner (with the formation of three intermediates from RhB to Rh110) or as a direct cleavage of the chromophore rings. A gradual blue shift of the band at 553 nm in the spectrum of the RhB solution is observed in the first case, and the decrease in its intensity without a shift takes place in the second case. The second route is observed for most of the tested samples, for example for 10Zn and 10Cu (Fig. 5b, d, respectively). Similar results were obtained in the works [3, 4]. Mixed mechanism is realized for all Ti-containing samples as well as for 10Ag (Fig. 5a, c) as it was described earlier for doped, milled and supported SnO2 [14, 23, 45]: (1) decrease in the intensity of the band at 553 nm which corresponds to the direct degradation of RhB and (2) simultaneous blue shift of this band which indicates its de-ethylation.
The RhB degradation under visible irradiation (as dye bleaching) using the supported samples proceeds according to the first-order kinetic equation (Fig. 6a, b) with a correlation coefficient R2 = 0.93–0.99, which is consistent with the literature data [11,12,13,14, 42]. The photodegradation rate constants Kd were calculated from the slopes of the plots ln(D/D0) − t (where D and D0 are values of optical density of RhB solution after time t and after dark adsorption, respectively). The Kd values as the measure of photocatalytic activity are presented in Table 3 (column 5). In particular, important is the fact that activity is shown by “white” oxides (TiO2, ZnO) possessing a band gap > 3.26 eV as was previously observed for the supported tin dioxide [14]. Moreover, these samples turned out to be the most active among other supported oxides (with the exception of silver-containing sample). This effect may be due to the fact that the adsorbed dye molecules, but not the surface groups of the photocatalyst, are excited by visible light. This is the so-called photosensitization process [28, 44] characteristic for both TiO2 as a catalyst and RhB as a substrate [46, 47]. This is the second mechanism for initiating the photocatalytic reactions: In this case, excited adsorbed dye molecules inject electrons to conduction band of the catalyst. As a consequence, active species at the catalyst surface are generated. As can be seen from Table 3, sample 5Ti exhibits maximum activity among all Ti-containing samples. This is consistent with the results of the paper [29], where it was shown that the supported photocatalyst with 5% TiO2 was the most active.
Reference samples TAS-7 and 10Ti-per, characterized by an additional absorption edge in visible region, showed lower activity. It can be caused by their much smaller specific surface area compared with 5Ti and 10Ti (Table 2). Besides, as it has been shown by the authors of [48, 49], co-precipitated titanosilicas, which TAS-7 can be related to, have lower photocatalytic activity compared to supported samples due to the lower accessibility of substrate molecules to active sites of the surface. At the same time, sample 10Ti-per is more active than 10TiOH since it contains surface peroxo-groups (Fig. 6a). These groups promote formation of reactive oxidative species facilitating transformation of dye molecules.
Obviously, the contribution of photosensitization to RhB transformations also takes place when photocatalysts with the band gap < 3.26 eV are used. However, the formation of electron–hole pairs under visible irradiation is a main mechanism for initiation of photocatalytic process in this case. The above-mentioned increasing in the surface content of (021) MoO3 plane can cause an increase in activity of Mo-containing supported samples compared to bulk MoO3 as manifestation of facet engineering effect.
As indicated above, the magnitude of the substrate adsorption on the photocatalyst surface is important for its activity [9, 11]. Thus, the authors of paper [50] believe that medium adsorption constant is necessary to obtain the highest activity. Indeed, adsorption on the most active samples, as a rule, has an average value, namely 35–45% (Table 3, column 4). Bulk oxides exhibit lower adsorption values due to lower specific surface area. The increment in the substrate adsorption for the deposited samples is provided by the precisely developed support surface. On the other hand, increased adsorption may result in blocking the active sites of the surface and deceleration in photocatalytic processes. The sample 10Cu may be an example of the latter: Despite the low value of the band gap (2.89 eV), it demonstrated an average activity, since the dye adsorption was 65%.
Finally, silver-containing samples exhibited the highest activity (Table 3, Fig. 5c). Based on the literature data, this is an expected result in general [10, 28, 51] and for the degradation of rhodamine B, in particular [23, 30, 52, 53]. High activity of Ag photocatalysts is explained by strong absorption of visible light due to so-called surface plasmon resonance (SPR) effect which may excite interband transition and induce reactions of the molecules adsorbed on the silver particles [51]. Therefore, Ag nanoparticles on the surface of semiconductors and electron-donor substances cause charge separation of photogenerated electron–hole pairs, thus enhancing the overall photocatalytic activity. However, the semiconductor support may contribute to the overall result of photocatalysis in mentioned cases [53, 54]. In this study, Ag species are embedded into the structure of a dielectric silica support—sample 10Ag. Nonetheless, it showed high activity. Obviously, this is the net result of dispersing the silver particles in the pores of the support, which can be attributed to surface plasmon resonance effect. As mentioned above, XRD indicates the absence of silver-containing crystalline phases. XPS spectrum obtained for this sample shows that silver is in a metallic state (Fig. 7a). Binding energies 368.0 and 374.1 eV for 3d electrons, namely Ag 3d5/2 and Ag 3d3/2, respectively, indicate this [23, 54, 55]. Indeed, Ag0 is formed at calcination temperature 500 °C as was shown in [55]. UV–Vis spectrum of this sample (Fig. 7b) contains sharp rise in the wavelength region more than 450 nm which is characteristic for deposited metallic silver and corresponds to surface plasmon resonance [53, 55].
The degradation degree was evaluated by two parameters: as bleaching degree of RhB solution and as mineralization degree (as TOC decrease) for 3 h, in order to compare with the results obtained in [30] where testing was carried out under almost the same conditions. In this study, Ag/SiO2 photocatalyst exhibited degradation degree of RhB about 71%. One can see that some tested samples showed activity comparable with this photocatalyst (Table 3, column 6). At the same time, sample 10Ag was noticeably more efficient demonstrating the bleaching degree of 90%. Even more important is the fact that the degree of total RhB mineralization to inorganic substances (as degree of reduction in TOC) for the most active samples reaches 50–75% for 3 h. It should be noted that this parameter being important from a practical point of view is usually not presented in the studies on the photocatalytic properties of silica deposited oxides.
Conclusions
Impregnation of a mesoporous silica support with salts solutions and following calcination is the simplest technique facilitating the dispersion of oxides on the support surface and accessibility of the dye molecules to active sites. Deposited oxides have amorphous or low-crystalline structure and exhibit electronic properties that differ from those for bulk oxides. Red shift of adsorption edge is observed for most supported samples compared with corresponding bulk oxides. All tested samples regardless of the band gap showed activity under visible irradiation. Three mechanisms of initiation for photocatalytic process are considered: (1) formation of electron–hole pairs for the samples with band gap < 3.26 eV (deposited Co and Cu oxides); (2) photosensitization of the dye molecules for the samples with band gap > 3.26 eV (deposited Ti-, Zn- and Mo-containing samples); and (3) surface plasmon resonance effect (Ag-containing sample). As a result, Ag/SiO2 and TiO2/SiO2 samples exhibited maximal photocatalytic activity. Given the fact that a silica support is dielectric, improvement of the activity for the supported oxides compared to bulk oxides can be considered as net result of the dispersion of the oxide particles on the support surface.
References
F. Fang, J. Futter, A. Markwitz, J. Kennedy, Nanotechnology 20, 245502 (2009)
K. Kaviyarasu, D. Premanand, J. Kennedy, E. Manikandan, Int. J. Nanosci. 12, 1350033 (2013)
C.M. Magdalane, K. Kaviyarasu, G.M.A. Priyadharsini, A.K.H. Bashir, N. Mayedwa, N. Matinise, A.B. Isaev, N.A. Al-Dhabi, M.V. Arasu, S. Arokiyaraj, J. Kennedy, M. Maaza, J. Mater. Res. Technol. 8, 2898 (2019)
C.M. Magdalane, N. Matinise, N. Mayedwa, N. Mongwaketsi, D. Letsholathebe, G.T. Mola, N.A. Al-Dhabi, M.V. Arasu, M. Henini, J. Kennedy, M. Maaza, B. Jeyaraj, S. Afr. J. Chem. Eng. 26, 49 (2018)
S.H.S. Chan, T.Y. Wu, J.C. Juan, C.Y. Teh, J. Chem. Technol. Biotechnol. 86, 1130 (2011)
A.B. Djurisˇić, Y.H. Leung, A.M.C. Ng, Mater. Horiz. 1, 400 (2014)
E. Pelizzetti, C. Minero, Comments Inorg. Chem. 15, 297 (1994)
T. Hisatomi, J. Kubota, K. Domen, Chem. Soc. Rev. 43, 7520 (2014)
Y. Yang, H. Zhong, C.X. Tian, Res. Chem. Intermed. 37, 91 (2011)
M.R. Hoffmann, S.T. Martin, W. Choi, D.W. Bahneman, Chem. Rev. 95, 69 (1995)
Y. Xu, W. Zheng, W. Liu, J. Photochem. Photobiol. A: Chem. 122, 57 (1999)
Z. Ding, X. Hu, G.Q. Lu, P.L. Yue, P.F. Greenfield, Langmuir 16, 6216 (2000)
R. van Grieken, J. Aguado, M.J. López-Muñoz, J. Marugán, J. Photochem. Photobiol. A: Chem. 148, 315 (2002)
S. Khalameida, V. Sydorchuk, S. Levytska, N. Shcherban, J. Therm. Anal. Calorim. 140, 2131 (2020)
V. Sidorchuk, V. Tertykh, V. Klimenko, A. Ragulya, J. Therm. Anal. Calorim. 101, 729 (2010)
Y. Trach, V. Sydorchuk, O. Makota, S. Khalameida, R. Leboda, J. Skubiszewska-Zięba, V. Zazhigalov, J. Therm. Anal. Calorim. 107, 453 (2011)
R. Liu, H.T. Ou, J. Nanotechnol. 2015, 7 (2015)
S. Yusuf, F. Jiao, ACS Catal. 2, 2753 (2012)
K. Vignesh, R. Hariharan, M. Rajarajan, A. Suganthi, Mater. Sci. Semicond. Process. 16, 1521 (2013)
S. Senthilvelan, V.L. Chandraboss, B. Karthikeyan, L. Natanapatham, M. Murugavelu, Mater. Sci. Semicond. Process. 16, 185 (2013)
N. Ajoudanian, A. Nezamzadeh-Ejhieh, Mater. Sci. Semicond. Process. 36, 162 (2015)
R. Sasikala, A.R. Shirole, V. Sudarsan, V.S. Kamble, C. Sudakar, R. Naik, R. Rao, S.R. Bharadwaj, Appl. Catal. A: Gen. 390, 245 (2010)
S. Khalameida, M. Samsonenko, V. Sydorchuk, V. Starchevskyy, O. Zakutevskyy, O. Khyzhun, Theor. Exp. Chem. 53, 40 (2017)
A.V.P. Rao, A.M. Umabala, P. Suresh, J. Appl. Chem. 4, 1145 (2015)
V.M. Gun’ko, V.I. Zarko, E. Chibowski, V.V. Dudnik, R. Leboda, V.A. Zaets, J. Colloid Interface Sci. 188, 39 (1997)
G.V. Jere, C.C. Patel, Z. Anorg, Allg. Chem. 319, 175 (1962)
G. Leofanti, M. Padovan, G. Tozzola, B. Venturelli, Catal. Today 41, 207 (1998)
M.A. Rauf, S.S. Ashraf, Chem. Eng. J. 151, 10 (2009)
N.U.M. Nor, H.O. Lintang, S. Endud, L. Yuliati, Adv. Mater. Res. 1112, 176 (2015)
X. Chen, Z. Zheng, X. Ke, E. Jaatinen, T. Xie, D. Wang, C. Guo, J. Zhao, H. Zhu, Green Chem. 12, 414 (2010)
G.A. Tompsett, G.A. Bowmaker, R.P. Cooney, J.B. Metson, K.A. Rodgers, J.M. Seakins, J. Raman Spectrosc. 26, 57 (1995)
S. Khalameida, E. Skwarek, W. Janusz, V. Sydorchuk, R. Leboda, J. Skubiszewska-Zięba, Cent. Eur. J. Chem. 12, 1194 (2014)
G. Liu, J.C. Yu, G.Q. Lu, H.-M. Cheng, Chem. Commun. 47, 6763 (2011)
V. Sydorchuk, O. Makota, S. Khalameida, L. Bulgakova, J. Skubiszewska-Zięba, R. Leboda, V. Zazhigalov, J. Therm. Anal. Calorim. 108, 1001 (2012)
R.M. Almeida, T.A. Guiton, C.G. Pantano, J. Non-Cryst. Solids 121, 193 (1990)
J. Skubiszewska-Zięba, S. Khalameida, V. Sydorchuk, Colloids Surf. A Physicochem. Eng. Asp. 504, 139 (2016)
S. Rasalingam, R. Peng, R.T. Koodali, J. Nanomater. 2014, 42 (2014)
X. Zhao, J. Huang, B. Wang, Q. Bi, L. Dong, X. Liu, Appl. Surf. Sci. 292, 576 (2014)
V. Etacheri, M.K. Seery, S.I. Hinder, S.C. Pillai, Adv. Funct. Mater. 21, 3744 (2011)
C.W. Tang, C.B. Wang, S.H. Chien, Thermochim. Acta 473, 68 (2008)
V. Sydorchuk, I. Lutsyuk, V. Shved, V. Hreb, A. Kondyr, O. Zakutevskyy, L. Vasylechko, Res. Chem. Intermed. 46, 1909 (2020)
N.R. Srinivasan, R. Bandyopadhyaya, Faraday Discuss. 186, 353 (2016)
Y. Kim, M. Yoon, J. Mol. Catal. A: Chem. 168, 257 (2001)
G. Sangami, N. Dharmaraj, Spectrochim. Acta Part A: Mol. Biomol. Spectr. 97, 847 (2012)
S. Khalameida, M. Samsonenko, J. Skubiszewska-Ziȩba, O. Zakutevskyy, Adsorpt. Sci. Technol. 35, 853 (2017)
T. Wu, G. Liu, J. Zhao, H. Hidaka, N. Serpone, J. Phys. Chem. B 102, 5845 (1998)
F. Chen, J. Zhao, H. Hidaka, Int. J. Photoenergy 5, 209 (2003)
J. Aguado, R. Vangrieken, M. Lopezmunoz, J. Marugan, Appl. Catal. A: Gen. 312, 202 (2006)
E.V. Goncharuk, V.N. Mishchenko, V.I. Zarko, V.M. Gun’ko, Theor. Exp. Chem. 42, 26 (2006)
N. Takeda, T. Torimoto, S. Sampath, S. Kuwabata, H. Yoneyama, J. Phys. Chem. 99, 9986 (1995)
V. Rodríguez-González, A. Hernández-Gordillo, in Nanophotocatalysis and Environmental Applications, ed. by G. Inamuddin Sharma, A. Kumar, E. Lichtfouse, A. Asiri (Springer, Switzerland, 2019)
C. Sun, Y. Fu, Q. Wang, L. Xing, B. Liu, X. Xue, RSC Adv. 6, 87446 (2016)
P. Verma, Y. Kuwahara, K. Mori, H. Yamashita, in Current Developments in Photocatalysis and Photocatalytic Materials, ed. by X. Wang, M. Anpo, X. Fu (Springer, Berlin, 2020), p. 353
I.M. Arabatzis, T. Stergiopoulos, M.C. Bernard, D. Labou, S.G. Neophytides, P. Falaras, Appl. Catal. B: Environ. 42, 187 (2003)
G.N. Salaita, Z.F. Hazos, G.B. Hoflund, J. Electron Spectrosc. Relat. Phenom. 107, 73 (2000)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sydorchuk, V., Levytska, S., Shcherban, N. et al. Transition metal oxides supported onto silica gel as visible light-driven photocatalysts. Res Chem Intermed 46, 3997–4015 (2020). https://doi.org/10.1007/s11164-020-04187-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04187-8