Abstract
In this paper, a rapid, easy and very efficient method for the synthesis of bis(pyrazolyl)methanes has been reported in the presence of nickel–guanidine complex immobilized on MCM-41 (MCM-41@Gu@Ni) as a recyclable nanocatalyst. This mesoporous silica catalyst was effective for the one-pot multi-component reaction of arylaldehydes, phenylhydrazine and ethyl acetoacetate in ethanol solvent. The current synthesis shows attractive features, for example the use of recoverable and reusable nanocatalyst (5 times), easy workup, suitable one-pot procedure, short reaction times, good to excellent yields and moderate reaction conditions.
Graphic abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Today, the evolution of technology has become directly linked to the smaller dimensions of particles, so nanoscience has attracted considerable attention [1, 2]. Nanocatalysts are very important tools in this field, because they have different chemical and physical properties compared to bulk materials [3, 4]. In fact, with the transition of microparticles to nanoparticles we confront two fundamental changes: (1) increase the surface-to-volume ratio [5]; (2) enter the particle sizes into the realm of mechanical effects and view the unusual properties resulting from the nanoscale (magnetic, optical, etc.) [6]. Some others of the characteristics of nanoparticles which make them attractive as a catalyst include: improved energy efficiency and economy, minimum chemical waste, easy functionalization, high mechanical stability, low density and high permeability [7,8,9,10,11,12].
A domino reaction is a method including at least two bond-forming transformations under identical conditions without increasing excess catalysts and reagents [13, 14]. In this process, the subsequent reactions are the result of the formation of functional groups in the previous stages, so these reactions are an important tool in the modern total synthesis to produce macromolecules [15, 16]. Domino reactions have remarkable advantages compared to conventional reactions, such as saving resources by minimizing waste and reducing the reaction time by minimizing the steps to reach the target molecule [16, 17].
One of the most active classes of compounds with a wide range of approved biological activities are the heterocycles containing pyrazole core [18]. For example, pyrazole derivatives such as bis(pyrazolyl)methanes being used as antiviral [19], anti-inflammatory [20], antihypertensive [21], antioxidant [21], antidepressant [22], antimicrobial [23], gastric secretion stimulatory [24], antibacterial [25], anticancer [26], anti-HIV [27], antipyretic [28], antimalarial [29] and antifilarial activities [30]. Additionally, these derivatives are applied as pesticides [31], dyestuffs [32], extracting reagents and chelating for diverse metal ions [32] and important intermediates in organic synthesis [33]. The common method for the synthesis of bis(pyrazolyl)methanes is pseudo-three-component condensation reaction of arylaldehydes with 3-methyl-1-phenyl-1H-pyrazol-5(4H)-one. This protocol has been reported in the attendance of various catalysts, for example alpha-casein [34], Cu–ZnO [35], morpholinium glycolate [36], silica-bonded n-propyl-4-aza-1-azoniabicyclo[2.2.2]octane hydrogen sulfate ((SB-DABCO)HSO4) [37], N,2-dibromo-6-chloro-3,4-dihydro-2H-benzo[e][1,2,4]thiadiazine-7-sulfonamide 1,1-dioxide (DCDBTSD) [38], N,N,N′,N′-tetramethylethylenediaminium-N,N′-disulfonic acid hydrogen sulfate ([TMEDSA][HSO4]2) [39], 1,3-disulfonic acid imidazolium trifluoroacetate ([Dsim][TFA]) [40], ʟ-proline [41] and silica-bonded N-propylpiperazine sulfamic acid (SBPPSA) [42]. Anyway, some of these reported procedures suffer from defects such as tedious work-up methods, strongly acidic or basic conditions, longer reaction time, low yield, corrosive and toxic solvent, expensive catalysts or reagents, large amounts of catalysts, non-recyclable catalysts and poor selectivity or commercial unavailability. However, restricted ways have been reported for the production of these compounds by one-pot pseudo-five-component condensation reaction of ethyl acetoacetate, phenylhydrazine derivatives and arylaldehydes. So, due to of the significance of these derivatives, the presentation of a quicker, easier and more environmentally friendly method joined with higher efficiency is quite required. We report here effectual protocol for the synthesis of bis(pyrazolyl)methanes via one-pot pseudo-five-component domino reaction of ethyl acetoacetate (2 eq.) and phenyl hydrazine (2 eq.) with aromatic aldehydes (1 eq.) at 60 °C in ethanol solvent.
Results and discussion
Nickel–guanidine complex immobilized on MCM-41 (MCM-41@Gu@Ni) was synthesized according to Scheme 1 [43]. The FE-SEM (field emission scanning electron microscopy) and TEM (transmission electron microscopy) micrographs of the nanocatalyst are shown in Figs. 1 and 2. The exact amount of nickel in catalyst which was obtained by inductively coupled plasma (ICP) technique was found to be 1.6 × 10−3 mol g−1 [43].
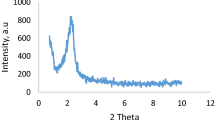
The low-angle powder X-ray diffraction patterns of both MCM-41 and MCM-41@Gu@Ni samples are shown in Fig. 3. The pattern of the MCM-41 shows three reflection peaks corresponding to d100, d110 and d200 which are typical confirming the presence of the ordered hexagonal mesoporous structure of MCM-41. However, upon post-synthetic grafting, the d110 and d200 reflections are no longer observed and an overall decrease in intensity of d100 is observed. This is probably due to the difference in scattering contrast of the pores and the walls, and to the irregular immobilization of nickle complex on nano-channels.
N2 adsorption–desorption isotherms of MCM-41 and MCM-41@Gu@Ni are shown in Fig. 4. Based on the IUPAC classification, the samples exhibit the type IV curves, which is corresponded to mesoporous materials. The nitrogen adsorption–desorption isotherms exhibit the surface area of MCM-41 and MCM-41@Gu@Ni which is 902.23 and 428.25 m2 g−1, respectively. The pore diameter and pore volume of the mesoporous materials indicate a marked decrease as a result of grafting organic layers and nickel complex on the surface of the MCM-41. These results indicate that due to the presence of the large organic moieties within the pore channels of mesoporous silica, the nickel complex loading appears as a thin layer on the inner surface of MCM-41. The surface properties of the synthesized materials are summarized in Table 1.
Catalytic activity of MCM-41@Gu@Ni was investigated in the synthesis of bis(pyrazolyl)methanes (Scheme 2). At first, the condensation of arylaldehydes, phenylhydrazine and ethyl acetoacetate for the synthesis of bis(pyrazolyl)methanes has been selected as the model reaction (Scheme 2). Afterward, the reaction conditions for the synthesis of bis(pyrazolyl)methanes have been optimized for diverse factors (Table 2). For this purpose, the effect of diverse amounts of MCM-41@Gu@Ni catalyst, reaction temperature and solvent type, in the model reaction, was examined (Table 2). The best results were obtained in 2 mL ethanol as solvent using 0.02 g of MCM-41@Gu@Ni at 60 °C (Table 2, entry 4). Furthermore, performing the reaction under solvent-free conditions did not afford good results (Table 2, entries 1–2). The reaction was also checked in the presence of the components for the synthesis of MCM-41@Gu@Ni, i.e., MCM-41, guanidine, MCM-41@guanidine and nickel(II) nitrate hexahydrate (Table 2, entries 9–12). As it is shown in Table 2, the starting substances could not effectively catalyze the reaction; these results confirmed that our design for immobilization of guanidine and nickel metal on MCM-41 surface in order to prepare MCM-41 was reasonable and successful.
After determining the optimal conditions, in order to identify performance and generality of MCM-41@Gu@Ni, synthesis of diverse derivatives such as bis(pyrazolyl)methanes was tested by various arylaldehydes (including benzaldehyde and arylaldehydes having electron-acceptor, halogen and electron–donor substituents); the corresponding results are displayed in Table 3. As it can be observed in this table, all arylaldehydes worked well in the reaction and obtained the related derivatives in short times and with excellent yields.
An admissible mechanism, considered by the literature [35], is shown in Scheme 3. Initially, the carbonyl group in the ethyl acetoacetate was activated by the nanocatalyst nickel for attack of lone pair of nitrogen form phenylhydrazine to form intermediate pyrazolone I. In the next step, the activated aromatic aldehyde by nanocatalyst nickel undergone a tandem reaction with intermediate II (which is the tautomer of intermediate I) leads to intermediate III after removal of a H2O molecule (the nanocatalyst also helps removing H2O). The next step is a Michael addition of another intermediate of III to II to form intermediate IV. In the last step, after the tautomeric proton shift, afford bis(pyrazolyl)methanes.
To display the superiority of our catalyst in comparison with other catalysts used in the synthesis of bis(pyrazolyl)methanes via one-pot pseudo-five-component, these catalysts were compared in factors of the turnover frequency (TOF) and turnover number (TON), reaction yield, time and temperature, in the synthesis of compound 10; the results are illustrated in Table 4. According to the table data, MCM-41@Gu@Ni was superior to other catalysts at least in four items of comparison items.
The recovery capability of MCM-41@Gu@Ni was considered for the reaction of phenylhydrazine, ethyl acetoacetate and 4-chlorobenzaldehyde to produce compound 10. Recycling the nanocatalyst was obtained by the stated method in the experimental section; it was reusable for 5 times with insignificant loss of its activity (Fig. 5). The recycled MCM-41@Gu@Ni was also characterized by low-angle powder X-ray diffraction. The XRD pattern of recycled MCM-41@Gu@Ni (Fig. 6) was slightly different relative to the fresh catalyst (the sharp peaks have decreased); this can attributed to aggregation of the nanoparticles and increment of their sizes, during recycling and reusing.
For the purpose of catalyst leaching, hot filtration experiment has been performed in the reaction of phenylhydrazine, ethyl acetoacetate and 4-chlorobenzaldehyde. In this test, 55% of the product was obtained after half-time of the reaction. Moreover, the same reaction was repeated and after the half-time of the reaction (after 12 min), the catalyst was removed and filtrated mixture was allowed to run until the 25 min. In this step, 57% of the product was obtained. These experiments verify that leaching of nickel does not occur.
Conclusions
In summary, we have reported the synthesis of bis(pyrazolyl)methanes using MCM-41@Gu@Ni as a heterogeneous and reusable catalyst in a recyclable media. The advantages of this protocol are the use of a commercially available materials, efficiency, high yield, relatively short reaction time, low cost, cleaner reaction profile, ease of product isolation and simplicity. Also, all products were obtained in high yields without formation of by-product.
Experimental
Materials and apparatuses
All chemicals were purchased from Merck or Fluka Chemical Companies. All known compounds were identified by comparison of their melting points and/or spectral data with those reported in the literature. Reaction progress was monitored by thin-layer chromatography (TLC) on silica gel SIL G/UV 254 plates. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes. 1H NMR (500 MHz) and 13C NMR (125 MHz) were run on a Bruker Avance DPX, FT-NMR spectrometers. The morphologies and particles sizes of samples were characterized by field emission scanning electron microscopy (FE-SEM), model TESCAN MIRA3 LMH. A transmission electron microscope (TEM), model Philips CM200, was used to measure the size and shape of particles.
Procedure for the production of MCM-41@Gu@Ni
Initially, nanosized MCM-41 was prepared using the sol–gel method with CTAB (cetyltrimethylammonium bromide) as the template, TEOS (tetraethyl orthosilicate) as the silicon source and sodium hydroxide as pH control agent. In a typical procedure, CTAB (2.74 mmol, 0.1 g) was added to a solution of deionized water (480 ml) and NaOH (2 M, 3.5 ml) at 80 °C. When the solution became homogeneous (after complete dissolution), TEOS (5 ml) was added dropwise to the solution under continuous stirring at 80 °C, and the obtained gel was aged under reflux for 2 h. The mixture was allowed to cool to room temperature, and the resulting product was filtered and washed with distilled water. The obtained material was calcined at 550 °C in air for 5 h and is designated as MCM-41.
Then, mesoporous MCM-41 modified by 3-chloropropyltrimethoxysilane (MCM-41@nPrCl) was synthesized according to our previous reported method [41]. For immobilization of the guanidine on the surface of MCM-41@nPrCl, guanidine nitrate (2 mmol) and Na2CO3 (2 mmol) were added to mixture of MCM-41@nPrCl (1 g) in toluene (10 ml), and the reaction mixture was stirred for 24 h at 100 °C. The obtained MCM-41@nPr-guanidine was filtered and washed with ethanol for several times and then dried under air atmosphere. In the final step, immobilization of nickel onto MCM-41@nPr-guanidine was performed by mixing the MCM-41@nPr-guanidine (1 g) and Ni(NO3)2·6H2O (2 mmol) in ethanol. The resulted mixture was stirred for 20 h under reflux conditions. The obtained MCM-41@nPr-guanidine@Ni was filtered and washed with ethanol for several times and, then, dried under air atmosphere (Scheme 1) [43]. Moreover, the exact amount of nickel in catalyst which was obtained by ICP technique was found to be 1.6 × 10−3 mol g−1. The FE-SEM and TEM micrographs of the nanocatalyst are shown in Figs. 1 and 2.
General procedure for the production of bis(pyrazolyl)methanes
A mixture of phenylhydrazine (2 mmol), ethyl acetoacetate (2 mmol) and MCM-41@Gu@Ni (0.02 g, 3.24 mol%) was stirred for 2 min at 60 °C. After this time, the arylaldehydes (1 mmol) and EtOH (2 mL) were added and stirred at 60 °C. The progress of the reaction was monitored by TLC. After the completion of reaction, EtOH (7 mL) was added, and the resulting mixture was stirred for 2 min at 60 °C, followed by centrifugation and decanting to separate the nanocatalyst. The recovered catalyst was washed with EtOH (2 × 2 mL), dried (at 90 °C under vacuum condition) and reused for the same reaction. The EtOH resulted from the decanting was evaporated, and the solid crude product was recrystallized from hot ethanol (95%) to afford the bis(pyrazolyl)methane as a pure product.
Selected spectral data of the products
Product 5
1H NMR (250 MHz, DMSO-d6): δ (ppm) 2.10 (s, 3H, –CH3), 3.16 (s, 6H, 2CH3), 4.78 (s, 1H, CH, methine), 7.07 (d, J =8.1, 2H, HAr), 7.15 (d, J =8.1, 2H, HAr), 7.22 (t, J =8.1, 2H, HAr), 7.43 (t, J =7.6, 4H, HAr), 7.73 (d, J =7.7, 4H, HAr), 11.97 (br, 1H, OH), 14.44 (s, 1H, OH) (Supplementary Material, Fig. S1 and S2); 13C NMR (62.50 MHz, DMSO-d6): δ (ppm) 22.9, 34.4, 117.9, 123.0, 128.4, 131.2, 131.3, 137.3, 139.3, 141.6, 146.5 (Supplementary Material, Fig. S3).
Product 10
1H NMR (500 MHz, DMSO-d6): δ (ppm) 2.28 (s, 6H, 2-CH3), 4.93 (s, 1H, CH, methine), 7.17–7.23 (m, 4H, HAr), 7.29 (d, J =8.6, 2H, HAr), 7.39 (t, J =8.0, 4H, HAr), 7.66 (d, J =7.9, 4H, HAr), 12.48 (br, 1H, OH), 13.85 (s, 1H, OH) (Supplementary Material, Fig. S4 and S5); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 12.3, 33.1, 121.1, 126.2, 128.6, 129.5, 129.7, 131.1, 141.7, 146.8 (Supplementary Material, Fig. S6).
Product 12
1H NMR (500 MHz, DMSO-d6): δ (ppm) 2.33 (s, 6H, 2CH3), 5.16 (s, 1H, CH, methine), 7.14 (t, J =7.3 Hz, 1H, HAr), 7.24 (t, J = 7.4 Hz, 2H, HAr), 7.35 (t, J = 7.3 Hz, 1H, HAr), 7.44 (t, J = 8.0 Hz, 4H, HAr), 7.57 (d, J = 7.6 Hz, 1H, HAr), 7.73 (d, J = 8.0 Hz, 4H, HAr), 7.87 (d, J = 7.2 Hz, 1H, HAr), 12.72 (br, 1H, OH), 13.81 (s, 1H, OH) (Supplementary Material, Fig. S7 and S8); 13C NMR (125 MHz, DMSO-d6): δ (ppm) 12.5, 34.9, 104.4, 121.1, 123.4, 126.0, 128.0, 128.8, 129. 3, 131.1, 133.2, 137.7, 141.6, 146.6 (Supplementary Material, Fig. S9).
References
V. Madhavan, P.K. Gangadharan, A. Ajayan, S. Chandran, P. Raveendran, Nano-Struct. Nano-Objects 17, 218 (2019)
M. Nasrollahzadeh, S.M. Sajadi, M. Sajjadi, Z. Issaabadi, Interface Sci. Technol. 28, 1 (2019)
A. Zare, A. Kohzadian, Z. Abshirini, S.S. Sajadikhah, J. Phipps, M. Benamara, M.H. Beyzavi, New J. Chem. 43, 2247 (2019)
M. Nikoorazm, M. Ghobadi, Silicon 11, 983 (2019)
H. Filian, A. Ghorbani-Choghamarani, E. Tahanpesar, J. Porous Mater. 26, 1091 (2019)
L. Chacko, P.K. Rastogi, T.N. Narayanan, M.K. Jayaraj, P.M. Aneesh, RSC Adv. 9, 13465 (2019)
H. Mohammadi, H.R. Shaterian, Monatsh. Chem. 150, 327 (2019)
E. Korani, K. Ghodrati, M. Asnaashari, Silicon. 10, 1433 (2018)
H. Mohammadi, H.R. Shaterian, J. Iran. Chem. Soc. 16, 479 (2019)
A. Zare, M. Sadeghi-Takallo, M. Karami, A. Kohzadian, Res. Chem. Intermed. 45, 2999 (2019)
A. Mondal, B. Banerjee, A. Bhaumik, C. Mukhopadhyay, ChemCatChem 8, 1185 (2016)
S. Ray, A. Bhaumik, A. Duttab, C. Mukhopadhyay, ChemistrySelect 3, 1267 (2013)
Q. Xia, C. Li, Y. Zhang, C. Qi, F. Zhang, ChemistrySelect 31, 9232 (2018)
S. Poursan, S. Ahadi, S. Balalaie, F. Rominger, H.R. Bijanzadeh, ChemistrySelect 4, 6403 (2019)
C.P. Haas, U. Tallarek, ChemistryOpen 8, 606 (2019)
L.J. Sebren, J.J. Devery, C.R. Stephenson, ACS Catal. 4, 703 (2014)
G.L. Wu, Q.P. Wu, ChemistrySelect 3, 5212 (2018)
A. Tanitame, Y. Oyamada, K. Ofuji, M. Fujimoto, N. Iwai, Y. Hiyama, K. Suzuki, H. Ito, H. Terauchi, M. Kawasaki, K. Nagai, M. Wachi, J.-I. Yamagishi, J. Med. Chem. 47, 3693 (2004)
K. Sujatha, G. Shanthi, N.P. Selvam, S. Manoharan, P.T. Perumal, M. Rajendran, Bioorg. Med. Chem. Lett. 19, 4501 (2009)
S. Sugiura, S. Ohno, O. Ohtani, K. Izumi, T. Kitamikado, H. Asai, K. Kato, M. Hori, H. Fujimura, J. Med. Chem. 20, 80 (1977)
X. Yang, P. Zhang, Y. Zhou, J. Wang, H. Liu, Chin. J. Chem. 30, 670 (2012)
D.M. Bailey, P.E. Hansen, A.G. Hlavac, E.R. Baizman, J. Pearl, A.F. DeFelice, M.E. Feigenson, J. Med. Chem. 28, 256 (1985)
R. Sridhar, P.T. Perumal, S. Etti, G. Shanmugam, M.N. Ponnuswamy, V.R. Prabavathy, N. Mathivanan, Bioorg. Med. Chem. Lett. 14, 6035 (2004)
C.E. Rosiere, M.I. Grossman, Science 113, 651 (1951)
R. Mahajan, F. Havaldar, P. Fernandes, J. Indian Chem. Soc. 68, 245 (1991)
A. Balbi, M. Anzaldi, C. Macciò, C. Aiello, M. Mazzei, R. Gangemi, P. Castagnola, M. Miele, C. Rosano, M. Viale, Eur. J. Med. Chem. 46, 5293 (2011)
R.V. Ragavan, V. Vijayakumar, N.S. Kumari, Eur. J. Med. Chem. 44, 3852 (2009)
R. Sridhar, P.T. Perumal, Synth. Commun. 33, 1483 (2003)
B.N. Acharya, D. Saraswat, M. Tiwari, A.K. Shrivastava, R. Ghorpade, S. Bapna, M.P. Kaushik, Eur. J. Med. Chem. 45, 430 (2010)
P.M.S Chauhan, S. Singh, R.K Chatterjee, Ind. J. Chem. Sect. B. 32, 858 (1993)
M. Londershausen, Pest Manag. Sci. 48, 269 (1996)
A.D. Garnovskii, A.I. Uraev, V.I. Minkin, Arkivoc 3, 29 (2004)
W. Hamama, Synth. Commun. 31, 1335 (2001)
J. Milani, M.T. Maghsoodlou, N. Hazeri, M. Nassiri, J. Iran. Chem. Soc. 16, 1651 (2019)
S. Shinde, B. Karale, D. Bankar, S. Arbuj, M. Moulavi, D. Amalnerkar, T. Kim, J. Nanosci. Nanotechnol. 19, 4623 (2019)
M.A. Shaikha, M. Farooquib, S. Abedc, Iran. J. Catal. 8, 73 (2018)
M. Shekouhy, R. Kordnezhadian, A. Khalafi-Nezhad, J. Iran. Chem. Soc. 15, 2357 (2018)
A. Khazaei, F. Abbasi, A.R. Moosavi-Zare, New J. Chem. 38, 5287 (2014)
Z. Abshirini, A. Zare, Z. Naturforsch, B Chem. Sci. 73, 191 (2018)
M. Karami, A. Zare, Org. Chem. Res. 4, 174 (2018)
P.S. Mahajan, M.D. Nikam, V. Khedkar, P. Jha, P.V. Badadhe, C.H. Gill, J. Heterocycl. Chem. 54, 1109 (2017)
S. Tayebi, K. Niknam, Iran. J. Catal. 2, 69 (2012)
H. Filian, A. Ghorbani-Choghamarani, E. Tahanpesar, J. Iran. Chem. Soc. 16, 2673 (2019)
A. Zare, M. Merajoddin, A.R. Moosavi-Zare, M. Zarei, Chin. J. Catal. 35, 85 (2014)
K. Niknam, S. Mirzaee, Synth. Commun. 41, 2403 (2011)
Acknowledgements
A. Kohzadian thanks IlamFarashimi Company for the support of this work. A. Zare and Z. Kordrostami thank the Research Council of Payame Noor University for supporting this work. H. Filian and A. Ghorbani-Choghamarani gratefully acknowledge the financial support through the start-up funds from the Islamic Azad University, Ahvaz and Ilam University, Iran, respectively.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kohzadian, A., Filian, H., Kordrostami, Z. et al. A simple, rapid and effective protocol for synthesis of bis(pyrazolyl)methanes using nickel–guanidine complex immobilized on MCM-41. Res Chem Intermed 46, 1941–1953 (2020). https://doi.org/10.1007/s11164-019-04073-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-019-04073-y