Abstract
A convenient, rapid, and highly efficient procedure for synthesis of 5-substituted-1H-tetrazoles was developed via multicomponent domino Knoevenagel condensation/1,3-dipolar cycloaddition reaction between aromatic aldehydes, malononitrile, and sodium azide in presence of Fe3O4 magnetic nanoparticles, using microwave irradiation and conventional heating, under solvent-free conditions. The procedure is efficient due to the low cost and nontoxicity of the catalyst, elimination of volatile and toxic solvents, very short reaction time, excellent product yield, easy methodology, and simple workup. The magnetite catalyst was recycled using an external magnet and could be reused in at least five consecutive runs, delivering high product yield.
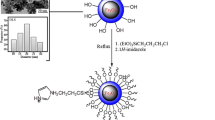
Graphical abstract
We illustrate that green, efficient, and recoverable Fe3O4 magnetic nanoparticles can catalyze Knoevenagel condensation/1,3-dipolar cycloaddition of aromatic aldehydes, malononitrile, and NaN3 to synthesize 5-substituted-1H-tetrazoles using microwave irradiation and conventional heating.

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the context of green chemistry, design and development of organic syntheses performed via multicomponent domino reactions (MDRs) have become a significant area of research in organic chemistry due to their improved atom economy, efficiency, and convergence [1]. In addition, MDRs reduce reaction time, minimize reaction and purification steps, and save raw materials, without complex product isolation [2]. In a condensation reaction, on the other hand, two, three, or four molecules or moieties, often functional groups, combine to form a larger molecule, together with loss of a small molecule [3]. Because of these advantages, multicomponent coupling reactions are preferred to tune reaction steps in combinatorial synthesis [4]. Various heterocyclic compounds such as benzopyrans [5], benzoxanthene [6], polyhydroquinoline [2], and benzochromene [7] can be developed by applying this one-pot multicomponent reaction strategy. Huisgen et al. [8] were the first to fully recognize the general concept and scope of 1,3-dipolar cycloaddition, a reaction with considerable scope for synthesis of five-membered heterocyclic rings. Also, the 1,3-dipolar cycloaddition reaction, which can be used to synthesize tetrazole ring, is classified as (2 + 3) cycloaddition, in which two atoms of the first component (imine or nitrile groups as 1,3-dipolarophiles) react with three atoms of the second component (azide group as a 1,3-dipolar molecule) [9]. Tetrazoles are an interesting and useful class of heterocyclic compounds with a five-membered ring containing one carbon and four nitrogen atoms, possessing the highest nitrogen content among known stable heterocycles. Such heterocyclic systems rarely occur in nature and are well known for their pharmacological properties such as antifungal, antiinflammatory, antibacterial, antihypertensive, and anticancer activities [10,11,12]. Tetrazoles have a wide range of applications in coordination and organic chemistry, information recording systems, photographic industry, organometallic and high-energy chemistry, and in particular, medicinal chemistry [13,14,15]; For example, in drug design, 5-monosubstituted tetrazoles are nonclassical bioisosteres of carboxylic acids, and 1,5-disubstituted tetrazoles are bioisosteres of the cis-amide bond of peptides. These bioisosteres have displayed similar types of biological activities, such as enhancing metabolic stability [16]. Recently, antiproliferative properties of tetrazole derivatives with antitumor applications have also been reported [17]. Also, a tetrazole-based peptidomimetic was discovered as a human growth hormone secretagogue [18]. Lately, tetrazole moieties have been widely used to bind aryl thiotetrazolylacetanilides with human immunodeficiency virus (HIV)-1 reverse transcriptase [19]. Also, amino-substituted tetrazoles exhibit receptor modulator activities [20]. Tetrazoles can be used to synthesize drugs of the sartan family, including losartan and valsartan [21]. They have also been shown to be tumor necrosis factor (TNF)-α inhibitors [22], potential P2X7 antagonists [23], and inhibitors of anandamide cellular uptake [24]. Because of their usefulness, research on synthesis of tetrazoles has attracted great attention over recent years. Various protocols for synthesis of tetrazoles from oximes, nitriles, amines, isocyanides, acyl cyanides, amides, thioamides, imidoyl chlorides, or ketones have been reported [25,26,27,28]. Recently, tetrazole derivatives were synthesized based on click reactions using various homogeneous and heterogeneous catalysts or reagents [29, 30]. Although synthesis of tetrazole derivatives from nitriles and sodium azide has been carried out using Fe3O4-based nanocatalyst [31,32,33,34], no reports on synthesis of 2-(1H-tetrazole-5-yl) acrylonitrile derivatives by multicomponent domino reaction between aldehydes, malononitrile, and sodium azide using Fe3O4-based catalyst were found. To the best of the authors’ knowledge, there are only a limited number of reports on use of multicomponent domino reaction between aldehydes, malononitrile, and sodium azide for synthesis of 2-(1H-tetrazole-5-yl) acrylonitrile derivatives [35,36,37,38,39]. Although these methods are beneficial, a number of them have several deficiencies such as use of expensive and toxic reagents, long reaction time, tedious workup, and difficulty in separation and reusability of the catalyst. Moreover, many catalysts require use of dimethylformamide (DMF) as solvent during the reaction, while from a green chemistry viewpoint, it is highly desirable to develop solvent-free processes.
The choice of an appropriate catalyst for each reaction is of great importance to achieve excellent results. Recently, application of solid acids as heterogeneous catalysts has attracted particular attention in different areas of organic synthesis [40]. On the other hand, magnetic nanoparticles such as Fe3O4 have emerged as one of the useful, high-efficiency, and green heterogeneous solid-acid catalysts, because of their high surface-to-volume ratio, good thermal stability, and ready availability [41]. Moreover, their magnetic properties enable perfect recovery of the catalyst using an external applied magnetic field, eliminating the need for filtration, tedious centrifugation, or membrane separation steps. Various strategies have successfully demonstrated the applications of Fe3O4 nanoparticle-supported or nanoparticle-immobilized catalysts [42, 43]. However, investigation of Fe3O4 nanoparticles without modification as a magnetically retrievable catalyst for organic synthesis is rare [44,45,46,47]. It is clear that development of “free” nanoparticles with tunable catalytic activity is of great significance for both industry and academia [48].
In addition, it is well established that the combination of microwaves and a heterogeneous catalyst can lead to development of fast, efficient, and environmentally benign synthetic procedures [49]. Interestingly, few protocols on preparation of 5-substituted-1H-tetrazole derivatives in presence of heterogeneous catalysts using microwave irradiation have been reported [50]. Regarding the advantages and green aspects of application of microwaves in organic reactions, it was thought worthwhile to apply microwave irradiation in the current research [51, 52]. As part of our ongoing investigation into development of multicomponent reactions for synthesis of heterocyclic compounds using heterogeneous catalytic systems [48, 53,54,55], we therefore report herein an easy and efficient method for preparation of 2-(1H-tetrazole-5-yl) acrylonitrile derivatives through three-component domino Knoevenagel condensation/1,3-dipolar cycloaddition reaction of aldehydes, malononitrile, and sodium azide using Fe3O4 magnetic nanoparticles (MNPs) as catalyst under both conventional heating and microwave irradiation.
Experimental
Materials and instrumentation
All chemicals were obtained from Merck and Sigma chemical companies and used without further purification. An LG MG 3017S microwave oven was used. Product formation was confirmed by thin-layer chromatography (TLC) on Merck silica gel 60 F254 plates. The melting point of all products was determined using a Thermo Scientific 9100 apparatus. The mass spectrum of the newly synthesized compounds was recorded on an Agilent 6410 triple-quadrupole liquid chromatography (LC)/mass spectrometry (MS) device. Fourier-transform infrared (FT-IR) spectra were obtained in the range from 4000 to 400 cm−1 using a Shimadzu 8400 spectrometer. X-ray powder diffraction (XRD) analysis was performed using a Philips device with Cu Kα radiation at wavelength of 1.54 Å. Transmission electron microscopy (TEM) was carried out using a Philips CM120 microscope. Nuclear magnetic resonance (NMR) spectra were recorded in deuterated dimethylsulfoxide (DMSO) on a Bruker Advance 400 MHz spectrometer with tetramethylsilane (TMS) as internal reference.
General procedure for synthesis of Fe3O4
Magnetic nanoparticles were prepared by a reduction–precipitation procedure according to previous literature [56]. Briefly, initially, 1.5 mL ferric chloride (2 M) was added to 5.1 mL ultrapure water. Then, 1 mL sodium sulfite (1 M) was added dropwise to the prior solution with vigorous stirring for 1 min. As soon as Fe3+ and SO 2−3 were mixed, the color of the solution changed from yellow to red, indicating complex ion formation between the two ions. When the color of this solution altered to yellow again, the solution was mixed in 40 mL 0.85 M ammonia solution under magnetic stirring. After 30 min, black precipitate of MNPs formed, which was washed several times with distilled water by magnetic decantation (to pH < 7) and dried at 60 °C under vacuum.
General procedure for synthesis of tetrazole derivatives
Heating method (method A)
A mixture of aromatic aldehyde (1 mmol), malononitrile (1 mmol), sodium azide (1.5 mmol), and nano-Fe3O4 (20 mol%) was heated at 80 °C for appropriate time (Table 3). Upon consumption of the starting material, as indicated by TLC, the reaction mixture was cooled and a small amount of water (5 mL) was added, then the catalyst was separated through magnetic absorption with a permanent magnet and the mixture was filtered. To the filtered solution, 15 mL 2 N HCl was added, followed by stirring to afford the tetrazole in powder form. The product was filtered and dried in an oven to give pure product.
Microwave irradiation method (method B)
A mixture of aromatic aldehyde (1 mmol), malononitrile (1 mmol), sodium azide (1 mmol), and nano-Fe3O4 (20 mol%) was placed in a microwave reaction vial for appropriate time (Table 3). The LG MG 3017S microwave oven was programmed to 250 W. Reaction completion was monitored by TLC. Upon consumption of the starting material, the reaction mixture was allowed to cool and water was added (5 mL), then the catalyst was separated magnetically and the mixture was filtered. To the filtered solution, 15 mL 2 N HCl was added, followed by stirring to afford the tetrazole in powder form. The precipitate was filtered and dried in a drying oven to give pure product. The products were identified using melting point, 1H NMR, 13C NMR, FT-IR, and mass spectroscopy techniques.
Spectroscopic data of synthesized compounds
3-Phenyl-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 1)
Powder. IR (KBr) (υmax/cm−1): 3157 (NH), 2229 (CN), 1585 (C=C). 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.64 (br s, NH, overlap with solvent), 7.59–7.62 (3H, m, CH-Ar), 8.00–8.03 (2H, m, CH-Ar), 8.39 (1H, s, CH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 97.1, 115.5, 129.4, 129.5, 129.9, 132.2, 132.4, 148.5.
3-(2-Chlorophenyl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 3) (new compound)
Powder. IR (KBr) (υmax/cm−1): 3157 (NH), 2235 (CN), 1587 (C=C). MS, m/z: 232 (M + H)+, 254 (M + Na)+. 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.64 (br s, NH, overlap with solvent), 7.56–7.63 (2H, d, CH-Ar), 7.68–7.70 (2H, d, CH-Ar), 8.13–8.14 (1H, d, CH-Ar), 8.54(s, 1H, CH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 101.4, 114.7, 127.9, 129.7, 130.1, 130.5, 133.3, 134.0, 144.1, 155.3.
3-(5-Bromo-2-hydroxyphenyl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 4) (new compound)
Powder. MS, m/z: 292.1 (M + H)+, 314.1 (M + Na)+. 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.54 (br s, NH, overlap with solvent), 7.54–7.63 (1H, CH-Ar), 7.64–7.71(1H, CH-Ar), 8.12–8.14 (1H, CH-Ar), 8.44(s, 1H, CH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 115.9, 119.9, 121.4, 126.1, 128.6, 136.3, 143.9, 146.9, 148.3, 149.6.
3-(4-Fluorophenyl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 5) (new compound)
Powder. IR (KBr) (υmax/cm−1): 3109 (NH), 2228 (CN), 1595 (C=C). MS, m/z: 216 (M + H)+, 238 (M + Na)+. 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.50 (br s, NH, overlap with solvent), 7.45–7.49 (2H, CH-Ar), 8.098–8.120 (2H, CH-Ar), 8.40 (1H, CH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 96.7, 119.8, 119.9, 123.8, 131.8, 132.5, 132.6, 147.0.
3-(2-Hydroxy-3-methoxyphenyl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 6) (new compound)
Powder. IR (KBr) (υmax/cm−1): 3174 (NH), 2201 (CN), 1580 (C=C). MS, m/z: 243 (M + H)+, 266 (M + Na)+. 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.50 (br s, NH, overlap with solvent), 3.81 (3H, OCH3), 7.36–7.44 (2H, CH-Ar), 7.53–7.55 (2H, CH-Ar), 9.00 (1H, CH), 11.00 (1H, OH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 56.2, 112.6, 115.9, 118.9, 120.9, 125.1, 128.6, 142.9, 144.4, 146.3, 149.6.
3-(4-Methylphenyl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 9)
Powder. IR (KBr) (υmax/cm−1): 3124 (NH), 2223 (CN), 1596 (C=C). 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.64 (br s, NH, overlap with solvent), 2.39 (3H, s, CH3), 7.40–7.42 (2H, d, CH-Ar), 7.91–7.93 (2H, d, CH-Ar), 8.33 (1H, s, CH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 21.3, 95.4, 115.7, 129.5, 130.0, 130.0, 130.0, 143.1, 148.5.
3-(4-Methoxyphenyl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 10)
Powder. IR (KBr) (υmax/cm−1): 3135 (NH), 2225 (CN), 1589 (C=C). 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.57 (br s, NH, overlap with solvent), 3.85 (3H, s, OCH3), 7.14–7.16 (2H, d, CH-Ar), 8.01–8.03 (2H, d, CH-Ar), 8.27 (1H, s, CH). 13C NMR (100 MHz, DMSO-d6): δc (ppm) 55.7, 92.9, 114.4, 114.9, 116.1, 124.7, 132.3, 148.1, 155.3.
3-(Thiophene-2-yl)-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 12)
Powder. IR (KBr) (υmax/cm−1): 3196 (NH), 2223 (CN), 160 (C=C). 1H NMR (400 MHz, DMSO-d6): δH (ppm) 3.64 (br s, NH, overlap with solvent), 7.32–7.34 (t, 1H, CH), 7.93–7.94 (d, 1H, CH), 8.11–8.12 (d, 1H, CH), 8.59 (s, 1H, CH). 13C NMR (100 MHz, DMSO-d6): δC (ppm) 99.2, 115.8, 128.6, 134.9, 136.1, 137.8, 141.3, 155.1
Results and discussion
Characterization of catalyst
The XRD pattern of the Fe3O4 nanoparticles is shown in Fig. 1. A number of prominent Bragg reflections with indices of (220), (311), (400), (422), (511), and (440) reveal that the resultant nanoparticles were Fe3O4 with spinel structure. The crystallite diameter (D) of the magnetic nanoparticles was calculated using the Debye–Scherrer formula (D = Kλ/βcosθ), where β is the angular line width at half-maximum intensity in radians, θ is the position of the maximum of the diffraction peak, K is the so-called shape factor, which usually takes a value of about 0.9, and λ is the X-ray wavelength (1.5406 Å for Cu Kα). The average size of the Fe3O4 nanoparticles was estimated to be around 17 nm.
Figure 2 shows TEM images of the Fe3O4 nanoparticles before and after the catalyst was used five times in the reaction medium. They appear almost unchanged in their size and shape. The observation of spherical Fe3O4 nanoparticles with mean size of 10–20 nm agrees with the average size estimated using the Debye–Scherrer formula.
The FT-IR spectrum of the Fe3O4 nanoparticles is shown in Fig. 3. The characteristic peak at 584.3 cm−1 corresponds to Fe+2O−2. The signals at 584.3 cm−1 and 1400 cm−1 are the basic absorption peaks of Fe3O4 particles. OH stretching vibration at 3000–3600 cm−1 and OH bending vibration at 1629 cm−1 were observed, in good agreement with reported IR spectra for spinel Fe3O4 [57,58,59].
Optimization of reaction conditions
The initial optimization focused on determining the best iron source, using the reaction of benzaldehyde, malononitrile, and sodium azide as model system under thermal conditions. The results (Table 1) showed that Fe3O4 nanoparticles acted as a highly active catalyst for this kind of reaction. The increased catalytic activity of the iron nanoparticles (entries 4–6) over iron(II) and iron(III) salts (entries 1–3) can be attributed to their higher surface area (Fig. 4).
Later, the reaction was performed using different ratios of nano-Fe3O4 and NaN3, and different temperatures to determine their influence under both microwave irradiation and conventional heating (Table 2). When the model reaction was carried without catalyst at 80 °C or 110 °C, the desired product was formed in low yield. Under the same reaction condition, addition of 20 mol % nano-Fe3O4 to the reaction medium resulted in very good product yield. In an effort to develop better reaction conditions, different amounts of nano-Fe3O4 as heterogeneous catalyst were tested under solvent-free condition at temperature of 80 °C. An amount of 20 mol% of catalyst gave the best results in terms of time and yield. Higher mole ratio of catalyst did not further improve the result, while lower catalyst loading decreased the product yield even after longer reaction time. To determine the optimal temperature, we designed reactions at different temperatures (ranging from 50 to 110 °C and room temperature). Temperature of 80 °C resulted in the highest yield. Increasing the temperature to 110°C did not further improve the yield of the reaction. The influence of the amount of sodium azide on the reaction was also investigated, revealing that 1.5 mmol (under conventional heating) or 1 mmol (under microwave irradiation) NaN3 was sufficient to achieve excellent product yield. Therefore, use of microwaves enhanced the greenness of the method by making the reaction more economical. We next studied the effect of solvents in the reaction. The model reaction was investigated in presence of various solvents as well as solvent-free condition. The results showed that the solvent-free condition was the approach of choice in comparison with use of various solvents. Thus, the best result was obtained at 80 °C with 20 mol% nano-Fe3O4 and 1.5 mmol NaN3 after 4.5 h using the conventional heating method and at 80 °C with 20 mol% nano-Fe3O4 and 1 mmol NaN3 after 35 min under microwave irradiation. It was observed that, under similar conditions, the reaction yield was higher under microwave irradiation compared with the thermal reaction condition (Table 2).

As shown in Table 3, the reaction was carried out in presence of aldehydes containing electron-releasing and electron-withdrawing substituents on the aryl ring; the position of similar groups on the aromatic ring played no considerable role in the result of the reaction. All reactions were completed in appropriate time, with pure tetrazoles isolated in good to high yield. Although nano-Fe3O4 offered good yield under conventional heating, giving the products in 4.5–7 h, high product efficiency was obtained in 35 min under microwave irradiation.
In addition, according to stoichiometry calculations, the amount of Fe in the catalyst was determined to be 12.95 mmol g−1. The turnover number (TON) and turnover frequency (TOF) calculated for all products for both microwave and conventional heating experiments are listed in Table 4.
Reaction mechanism
A plausible mechanism for formation of tetrazole derivatives is illustrated in Scheme 1. Based on the presented experimental results together with some literature data [60, 61], it is supposed that the catalytically active site of Fe3O4 NPs, Fe3+, behaves as a Lewis acid and coordinates to carbonyl group of aldehyde. On the other hand, Fe3+ as a Lewis acid also attaches to nitrogen atom of cyano group of malononitrile and facilitates its deprotonation. This interaction accelerates the conjugation and directs the additions of the nucleophiles to the corresponding substrates. Thus, the first step involves formation of ylidenemalononitrile A by Knoevenagel condensation between malononitrile and aldehyde. The nitrogen atom of this intermediate A, forming a complex with the catalyst, forms complex B, which activates it towards attack of the azide ion. The [3 + 2] cycloaddition reaction between the nitrile group of arylidene malononitrile and azide ion produces intermediate C. Addition of HCl finally generates the tetrazole derivatives.
Comparison of catalytic activity of nano-Fe3O4 with precedents in literature
To illustrate the advantages of the presented method, we compared its results with previously reported methods for synthesis of 3-phenyl-2-(1H-tetrazole-5-yl) acrylonitrile (Table 3, entry 1) as model reaction. The results (Table 4) indicate that these procedures are beneficial, but a number of them have several deficiencies such as use of volatile solvents (Table 5, entries 1, 2, 5) or long reaction time (Table 5, entry 3, 4), while preparation of tetrazoles using nano-Fe3O4 was achieved under solvent-free condition in shorter reaction time with good to high product yield.
Recycling and reuse of catalyst
After reaction completion, the magnetic Fe3O4 nanoparticles were simply retrieved using a permanent magnet. The catalyst was washed several times with water and ethanol, then dried at 80 °C. As shown in Fig. 1, the separated catalyst could be used in five consecutive runs with only small loss in activity.
Conclusions
We introduce a novel, safe, and ecofriendly procedure for synthesis of 2-(1H-tetrazole-5-yl) acrylonitrile derivatives by multicomponent domino Knoevenagel condensation/1,3-dipolar cycloaddition reaction in presence of Fe3O4 magnetic nanoparticles as reusable heterogeneous catalyst. A wide range of aryl aldehydes were used for synthesis of a variety of 5-substituted-1H-tetrazoles in good to high yield. Salient features of the presented protocol include use of microwaves, elimination of toxic solvents, short reaction time, inexpensive and recyclable catalyst, mild reaction condition, and easy workup.
References
M.J. Climent, A. Corma, S. Iborra, RSC Adv. 2, 1 (2012)
S.K. Das, S. Mondal, S. Chatterjee, A. Bhaumik, ChemCatChem 10, 11 (2018)
A. Ying, S. Liu, Y. Ni, F. Qiu, S. Xu, W. Tang, Catal. Sci. Technol. 4, 7 (2014)
M.R. Naimi-Jamal, S. Mashkouri, A. Sharifi, Mol. Divers. 14, 3 (2010)
P. Das, A. Dutta, A. Bhaumik, C. Mukhopadhyay, Green Chem. 16, 3 (2014)
H.R. Shaterian, K. Azizi, Res. Chem. Intermed. 41, 1 (2015)
S.K. Kundu, J. Mondal, A. Bhaumik, Dalton Trans. 42, 29 (2013)
R. Hiiisgm, Angew. Chem. 2, 11 (1963)
Z.H. Abood, R.T. Haiwal, S.M. Radhi, J. Babylon. Univ. Pure Appl. Sci. 21, 1 (2013)
S.K. Prajapti, A. Nagarsenkar, B.N. Babu, Tetrahedron Lett. 55, 24 (2014)
A. Kudelko, K. Jasiak, K. Ejsmont, Monatsh. Chem. 146, 2 (2015)
F. Abrishami, M. Ebrahimikia, F. Rafiee, Appl. Organometal. Chem. 29, 11 (2015)
J. Roh, K. Vávrová, A. Hrabálek, Eur. J. Org. Chem. 2012, 31 (2012)
V. Rama, K. Kanagaraj, K. Pitchumani, J. Org. Chem. 76, 21 (2011)
G. Aridoss, K.K. Laali, Eur. J. Org. Chem. 2011, 31 (2011)
J. Zabrocki, G.D. Smith, J.B. Dunbar, H. Iijima, G.R. Marshall, J. Am. Chem. Soc. 110, 17 (1988)
R. Romagnoli, P.G. Baraldi, M.K. Salvador, D. Preti, M. Aghazadeh Tabrizi, A. Brancale, R. Bortolozzi, J. Med. Chem. 55, 1 (2011)
J. Li, S.Y. Chen, J.J. Li, H. Wang, A.S. Hernandez, S. Tao, N. Flynn, J. Med. Chem. 50, 24 (2007)
A. Gagnon, S. Landry, R. Coulombe, A. Jakalian, I. Guse, B. Thavonekham, B. Simoneau, Bioorg. Med. Chem. Lett. 19, 4 (2009)
E. Vieira, J. Huwyler, S. Jolidon, F. Knoflach, V. Mutel, J. Wichmann, Bioorg. Med. Chem. Lett. 15, 20 (2005)
S. Kumar, S. Dubey, N. Saxena, S.K. Awasthi, Tetrahedron Lett. 55, 44 (2014)
P. Srihari, P. Dutta, R.S. Rao, J.S. Yadav, S. Chandrasekhar, P. Thombare, J. Mohapatra, A. Chatterjee, M.R. Jain, Bioorg. Med. Chem. Lett. 19, 19 (2009)
D.W. Nelson, R.J. Gregg, M.E. Kort, A. Perez-Medrano, E.A. Voight, Y. Wang, G. Grayson, M.T. Namovic, D.L. Donnelly-Roberts, W. Niforatos, P. Honore, M.F. Jarvis, C.R. Faltynek, W.A. Carroll, J. Med. Chem. 49, 12 (2006)
G. Ortar, A.S. Moriello, M.G. Cascio, L.D. Petrocellis, A. Ligresti, E. Morera, V.D. Marzo, Bioorg. Med. Chem. Lett. 18, 9 (2008)
A. Sarvary, A. Maleki, Mol. Divers. 19, 1 (2015)
A. Sarvary, A. Maleki, RSC Adv. 5, 75 (2015)
S.D. Guggilapu, S.K. Prajapti, A. Nagarsenkar, K.K. Gupta, B.N. Babu, Synlett 27, 8 (2016)
R.D. Padmaja, S. Rej, K. Chanda, Chin. J. Catal. 38, 11 (2017)
J. Roh, K. Vávrová, A. Hrabálek, Eur. J. Org. Chem. 2012, 31 (2012)
M. Parveen, F. Ahmad, A.M. Malla, A. Azaz, N. J. Chem. 39, 3 (2015)
A. Ghorbani-Choghamarani, Z. Moradi, G. Azadi, J. Sulfur Chem. 39, 3 (2018)
T. Tamoradi, B. Mehraban-Esfandiari, M. Ghadermazi, A. Ghorbani-Choghamarani, Res. Chem. Intermed. 44, 2 (2018)
M. Darabi, T. Tamoradi, M. Ghadermazi, A. Ghorbani-Choghamarani, Trans. Met. Chem. 42, 8 (2017)
F. Taghavi, M. Gholizadeh, A.S. Saljooghi, M. Ramezani, MedChemComm 8, 10 (2017)
Z.N. Tisseh, M. Dabiri, M. Nobahar, H.R. Khavasi, A. Bazgir, Tetrahedron 68, 6 (2012)
J. Safaei-Ghomi, S. Paymard-Samani, Chem. Heterocycl. Compd. 50, 11 (2015)
J. Safaei-Ghomi, S. Paymard-Samani, S. Zahedi, H. Shahbazi-Alavi, Z. Naturforsch. 70, 11 (2015)
S. Khaghaninejad, M.M. Heravi, T. Hosseinnejad, H.A. Oskooie, M. Bakavoli, Res. Chem. Intermed. 42, 3 (2016)
N. Ahmed, Z.N. Siddiqui, RSC Adv. 5, 22 (2015)
A. Banan, H. Valizadeh, A. Heydari, A. Moghimi, Appl. Organomet. Chem. 5, 22 (2017)
A.R. Faraji, S. Mosazadeh, F. Ashouri, J. Colloid Interface Sci. 15, 506 (2017)
Y. Rangraz, F. Nemati, A. Elhampour, J. Colloid Interface Sci. 509, 1 (2018)
H. Veisi, M. Pirhayati, A. Kakanejadifard, Tetrahedron Lett. 58, 45 (2017)
A. Baeza, G. Guillena, D.J. Ramón, ChemCatChem 8, 1 (2016)
N. Nami, D. Zareyee, M. Ghasemi, A. Asgharzadeh, M. Forouzani, S. Mirzad, S.M. Hashemi, J. Sulfur Chem. 38, 3 (2017)
M. Zarghani, B. Akhlaghinia, RSC Adv. 6, 45 (2016)
N.A. Aslam, S.A. Babu, D.K. Singh, A. Rana, Synlett 25, 15 (2014)
N. Koukabi, E. Kolvari, A. Khazaei, M.A. Zolfigol, B. Shirmardi-Shaghasemi, H.R. Khavasi, Chem. Commun. 47, 32 (2011)
A. Dastan, A. Kulkarni, B. Torok, Green Chem. 14, 1 (2012)
S.M. Joshi, R.B. Mane, K.R. Pulagam, V. Gomez-Vallejo, J. Llop, C. Rode, N. J. Chem. 41, 16 (2017)
M. Zhang, Y.H. Liu, Z.R. Shang, H.C. Hu, Z.H. Zhang, Catal. Commun. 88, 5 (2017)
S.L. Barbosa, M. Ottone, M.C. Santos, G.C. Junior, C.D. Lima, G.C. Glososki, N.P. Lopes, S.I. Klein, Catal. Commun. 68, 5 (2015)
N. Koukabi, E. Kolvari, M.A. Zolfigol, A. Khazaei, B.S. Shaghasemi, B. Fasahati, Adv. Synth. Catal. 354, 10 (2012)
E. Kolvari, N. Koukabi, M.M. Hosseini, M. Vahidian, E. Ghobadi, RSC Adv. 6, 9 (2016)
E. Kolvari, N. Koukabi, M.M. Hosseini, J. Mol. Catal. Chem. 397, 1 (2015)
S. Zolfagharinia, E. Kolvari, N. Koukabi, M.M. Hosseini, Arab. J. Chem. (2017)
B. Karami, S.J. Hoseini, K. Eskandari, A. Ghasemi, H. Nasrabadi, Sci. Technol. 2, 2 (2012)
X. Lu, H. Zhao, C. Feng, Q. Chen, Z. Zhang, C. Yang, X. Wang, RSC Adv. 7, 58 (2017)
M. Ma, Y. Zhang, W. Yu, H.Y. Shen, H.Q. Zhang, N. Gu, Colloid Surf. A Phys. Chem. Eng. Asp. 212, 2 (2003)
A.M. Ghasemzadeh, J. Safaei-Ghomi, S. Zahedi, J. Serb. Chem. Soc. 78, 6 (2013)
L. Jing, J. Wei, L. Zhou, Z. Huang, Z. Li, D. Wu, X. Zhou, Chem. Eur. J. 16, 36 (2010)
Acknowledgements
The authors gratefully acknowledge Semnan University Research Council for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akbarzadeh, P., Koukabi, N. & Kolvari, E. Three-component solvent-free synthesis of 5-substituted-1H-tetrazoles catalyzed by unmodified nanomagnetite with microwave irradiation or conventional heating. Res Chem Intermed 45, 1009–1024 (2019). https://doi.org/10.1007/s11164-018-3657-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3657-9