Abstract
The low Mg 2p value (47.0 eV) of MgO reported in Res Chem Intermed 44(2018)829 is carefully analyzed and discussed based on a series of X-ray photoelectron spectroscopy (XPS) experiments and literature results. Presence of carbon or ferric oxide did not change the binding energy (BE) of Mg 2p of MgO, and the Mg 2p peak of MgO should be above 49.0 eV instead of at 47.0 eV.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Dear Editor,
We recently read the paper entitled “Enhanced physical and chemical adsorption of carbon dioxide using bimetallic copper–magnesium oxide/carbon nanocomposite.” Its authors synthesized CuO–MgO/activated carbon (AC) nanocomposite adsorbents for CO2 capture, and the effect of the MgO content was studied comprehensively [1]. However, they reported an Mg 2p binding energy of the MgO component of 47.0 eV in their Table 2 and Fig. 1 [1], and the spectra were referenced to the C 1s line at 284.5 eV (p. 831). In comparison with literature, where the BE of Mg 2p of MgO ranges between 49.9 and 50.2 eV [2], this value of 47.0 eV is strangely low. Nonetheless, the authors studied mixed Cu and Mg oxides loaded on nitrogen-rich activated carbon (AC) made from Nypha fruticans biomass, rather than ordinary magnesia, hence it is necessary to explore whether carbon or other metal oxides affected the value of MgO in the XPS measurements.
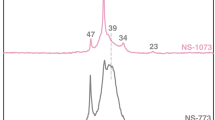
In this respect, we prepared two MgO samples from magnesium acetate tetrahydrate [analytical reagent (AR) purity, Xilong Chemical, China]; one was calcined from the salt at 823 K in air and named MgO, while the other was carbonized at 823 K and is thus called MgO-C, because it contained about 2 % extrafine carbon particles [3]. Also, commercial MgO (AR purity, Shanghai Zhenxin Reagent Factory, China) and its mixture with FeO (3 wt%, TCI), named 3FM-mix, were used. XPS measurements were performed on a PHI 5000 VersaProbe instrument (ULVAC-PHI) using monochromatic Al Kα radiation (produced in Chigasaki, Kanagawa, Japan) [4], and the spectra were referenced to the C 1s line at 284.6 eV. The Mg 2p peak for the four samples appeared in the range of 48.4–49.4 eV (Table 1), rather than at 47.0 eV as reported in Ref. [1], and all of them contained three components: hydroxide, carbonate, and oxide (Table 1), but none located below 48.0 eV (Fig. 1). In contrast, Ref. [1] reported an Mg 2p BE of the MgO component of 46.8 eV, with an Mg 2p BE of MgCO3 of 47.3 eV [1]. Clearly the choice of the raw material of MgO, either magnesium acetate tetrahydrate or commercial magnesia, has a minor influence on the Mg 2p spectrum of MgO samples, within the experimental error of ±0.2 eV. This is consistent with literature [2], where the Mg II spectra of MgO is not influenced by either the nature of the oxide precursor salts or the temperature used during oxide preparation. Also, the presence of small amounts of carbon or ferric oxide dopant in MgO has a similar minor influence (Table 1). Thus, the strange data for Mg 2p (47.0 eV) in Ref. [1] cannot be attributed to sample preparation or presence of carbon.
This result coincides with literature [5,6,7,8,9,10,11,12,13,14], where the Mg 2p value of MgO film was found to be 50.5–50.8 eV [4], while that of metal Mg was 49.6 eV. In Ref. [7], the Mg 2p BE of MgO on melt-spun Mg was 50.7–51.6 eV, and that of metal Mg was 49.5 eV. These values were confirmed as 50.8 eV for MgO or Mg(OH)2, 51.9 eV for MgCO3, and 49.8 eV for metal Mg [11]. Loading of MgO on porous carbon is not the reason for the low Mg 2p value of 47.0 eV, because two literature studies [9, 14] reported that the Mg 2p BE of MgO loaded in carbon lies in the range of 49.8–50.5 eV. Consequently, the low Mg 2p value of 47.0 eV [1] is incorrect, possibly being caused by the XPS measurements or data processing; For example, the authors analyzed their samples at analysis chamber pressure of approximately 1 × 10−10 Pa, which is very unlikely since most XPS tests are probably performed at 10−8 or 10−10 mbar.
Many mistakes were found in the original paper [1]. The sentence “The spectra were referenced with respect to the C 1s line at 284.5 eV” seems not to be true on pages 831, 833, and 834, or charge referencing was not done for all spectra. In Table 2 of the paper [1], both the C 1s peak of AC and 5%CuO–25%MgO/AC samples were given as 282 eV instead of the correct value of 284.5 eV. Therefore, the Mg 2p spectra in Fig. 1 wrongly show a maximum at around 47 eV. Besides, the peak assignment seems wrong in this figure: Mg 2p for MgO should have a higher BE than that for Mg(OH)2 [2], but in the plot of Fig. 1 the hydroxide phase has a higher BE than the oxide. Moreover, there is a mistake in the caption to Fig. 1, which should read “……and (c) Mg 2p for (i) 30%MgO/AC, (ii)……” instead of “(c) Mg 2p for (i) 30%CuO/AC……” Also, the sentence “Fig. 1 shows the XPS spectra for 5%CuO–25%MgO/AC after saturating with CO2 for 1 h using TPD-CO2” on page 832 is incorrect, as actually Fig. 1 was “Fig. 1 XPS spectra of components (a) O1s for (i) 30%CuO/AC, (ii)……, (b) Cu2p for (i) 30%CuO/AC, (ii) …… and (c) Mg2p for (i) 30%CuO/AC, (ii) ……” on page 834. We recommend that the authors make a correction without delay for the sake of posterity.
The following conclusions can be made: (1) Presence of carbon or ferric oxide did not change the BE of Mg 2p of MgO. (2) The Mg 2p peak of MgO is above 49.0 eV instead of at 47.0 eV. (3) The low value of 47.0 eV resulted from the spectra not being charge corrected.
Sir, Research on Chemical Intermediates is a serious scientific journal, so we should safeguard its prestige, especially concerning the validity of data.
Best wishes,
Yours truly
Mr. J. L. Chen and Prof. Dr. J. H. Zhu
References
W.N.R.W. Isahak, S.Z. Hasan, Z.A.C. Ramli, M.M. Ba-Abbad, M.A. Yarmo, Res. Chem. Intermed. 44, 829 (2018)
S. Ardizzone, C.L. Bianchi, M. Fadoni, B. Vercelli, Appl. Surf. Sci. 119, 253 (1997)
Y.Y. Li, M.M. Wan, X.D. Sun, J. Zhou, Y. Wang, J.H. Zhu, J. Mater. Chem. A3, 18535 (2015)
C.L. Shi, S.H. Li, X.D. Sun, Z.P. Wang, Y.Z. Wang, J.W. Xiong, W.B. Gu, W.M. Wang, H.M. Yao, Y. Wang, J.H. Zhu, Chem. Eng. J. 339, 170 (2018)
J.S. Corneille, J.- He, D.W. Goodman, Surf. Sci. 306, 269 (1994)
Y.-X. Li, H. Li, K.J. Klabunde, Environ. Sci. Technol. 28, 1248 (1994)
H.B. Yao, Y. Li, A.T.S. Wee, Appl. Surf. Sci. 158, 112 (2000)
E. Zhecheva, R. Stoyanova, G. Tyuliev, K. Tenchev, M. Mladenov, S. Vassilev, Solid State Sci. 5, 711 (2003)
R. Chakravarti, A. Mano, H. Iwai, S.S. Aldeyab, R.P. Kumar, M.L. Kantam, A. Vinu, Chem. Eur. J. 17, 6673 (2011)
M. Farooq, A. Ramli, D. Subbarao, J. Chem. Eng. Data 57, 26 (2012)
H. Saleh, T. Weling, J. Seidel, M. Schmidtchen, R. Kawalla, F.O.R.L. Mertens, H.-P. Vogt, Oxid. Met. 81, 529 (2014)
Q. Li, L. Zong, C. Li, J. Yang, Appl. Surf. Sci. 319, 16 (2014)
F.A.J. Al-Doghachi, Y.H. Taufiq-Yap, Chem. Sel. 3, 816 (2018)
X. Zheng, M. Huang, Y. You, X. Fu, Y. Liu, J. Wen, Chem. Eng. J. 334, 1399 (2018)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J.L., Zhu, J.H. A query on the Mg 2p binding energy of MgO. Res Chem Intermed 45, 947–950 (2019). https://doi.org/10.1007/s11164-018-3654-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-018-3654-z