Abstract
Over the past two of decades, iron oxide nanoparticles (IONPs) have attracted significant attention for a wide range of biomedical applications. For the successful use of IONPs in nano-biotechnology, surface coating and specific functionalization is critical. Many types of materials can be used in the surface coating of IONPs for nano-bio applications, including organic compounds and inorganic materials. This review focuses on recent developments and various strategies for surface coating of IONPs. In addition, the different materials used for the functionalization of IONPs are classified and discussed in detail. The design of IONPs with multifunctional coatings for bio-applications is an area of considerable interest. Surface functionalization of IONPs allows them to attach to various biomolecules, making them a promising candidate for bio-applications.
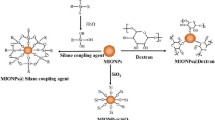
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nanoscale systems have received significant attention due to their biological, medicinal, and industrial applications. Surface modification of nanoparticles enables their use in nano-biomedical applications—for instance, as contrast agents for magnetic resonance imaging (MRI) or targeted drug delivery in tumor therapy [1,2,3,4,5]. Iron oxide nanoparticles (IONPs) have been of particular interest, especially for biomedical and pharmaceutical applications, because of their physical properties, magnetic susceptibility, biocompatibility, low toxicity, stability, and availability for surface modification. Further, their ability to be easily controlled by the application of an external magnetic field allows the release of pharmaceutical agents at an exact rate and a specific site for diagnosis and therapy, or theranostics [6,7,8,9,10]. The ferrite colloids magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the primary forms of IONPs, and have been used for a wide range of biomedical applications, including as contrast agents for vascular and tumor imaging, drug delivery, gene therapy, hyperthermia treatment, and magnetic separation of cells or molecules [3, 11,12,13,14,15]. Recent developments in the synthesis, characterization, and—most importantly—the surface modification of the IONPs has enabled researchers to answer important questions related to their clinical application [16]. It is well known that molecules comprising the surface coating of IONPs act as the primary interface between IONPs and the body’s immune system [11]. Thus, the surface chemistry, desired application, and method of administration, as well as the pharmacokinetics and biodistribution behavior of the IONPs, differ according to the method used for their synthesis [17]. The design and development of a unified multifunctional biomedical platform for individual nanoparticles that is effective in both the diagnosis and treatment of disease poses a significant challenge [18]. Efforts toward the development of magnetic nanocarriers continues, with the goal of (i) reducing the associated side effects by decreasing the systemic distribution of cytotoxic drugs, and (ii) reducing the required dose through more efficient, localized drug targeting.
Synthesis strategies
The main pathways for the synthesis of Fe3O4 NPs are as follows: (i) physical methods, consisting of gas-phase deposition and electron beam lithography, although control of particle size down to the nanometer scale is difficult [19,20,21]; (ii) chemical preparation methods, such as sol–gel, oxidation, chemical co-precipitation, hydrothermal reactions, flow injection, electrochemical, aerosol/vapor phase, sonochemical decomposition reactions, supercritical fluid, and syntheses using nanoreactors [22,23,24,25,26,27,28,29,30,31]; (iii) microbial methods, which ensure high yields, good reproducibility and scalability, low cost and moderate temperatures [32, 33].
In Synthesis of Metal-Doped Iron Oxide Nanoparticles, Ferrites are complex magnetic oxides derived from iron oxides, such as magnetite (Fe3O4) and maghemite (γ-Fe2O3), that are chemically combined with one or more metallic elements. Various methods have been proposed for the synthesis of these spinel metal ferrites. The ferrites have a common component, MFe2O4, where M can be Co2+, Mn2+, Ni2+, Zn2+, and so on. [34, 35]. Iron oxide nanoparticles are synthesized using one of two main chemical methods: the well-established conventional method involving co-precipitation of Fe2+ (ferrous) and Fe3+ (ferric) ions in a basic solution [36], and thermal decomposition of organic complexes of iron (e.g., iron pentacarbonyl, iron oleate, or FeOOH) in the presence of capping agents (e.g., oleic acid and oleyl amine) [37].
Surface modification
The stability of IONPs is important for their storage and various applications. IONPs are reactive toward oxidizing agents and moisture. Unfortunately, bare IONPs are usually unstable and tend to agglomerate. Therefore, surface coating and functionalization are necessary to impart colloidal stability and prevent agglomeration. Due to the interactions between IONPs and the surrounding media, efficient surface coating methods are needed.
In addition to preventing aggregation and enhancing colloidal stability, the functionalization of IONPs gives rise to higher water compatibility and better magnetic controllability, while protecting and stabilizing the surface of IONPs, rendering them biocompatible by lowering or eliminating their toxicity. In addition, functionalization installs reactive handles for conjugation of biologically active substances, an important process for nano-bio applications, and non-immunogenicity, non-antigenicity, and protection from opsonization by plasma proteins [38, 39]. Also, coating of IONPs with different materials can affect the magnetic and physicochemical properties, which can decrease the saturation magnetization (Ms), size, charge, hydrophobicity, and hydrophilicity of the nanoparticles. The Ms of bare and functionalized IONPs is an important parameter that describes the magnetic response of IONPs. Although the Ms is improved by agglomeration of IONPs, it has been shown to reduce if the IONPs are stabilized. The anchor chemistry can also affect the Ms via strong interactions with the ions in the surface layer of the magnetic core [40].
Surface modification strategies for IONPs
This section focuses on surface coating strategies for the stabilization, protection, and functionalization of IONPs. The coating method is dependent on the nature of the coating materials and the intended application. Surface modification of IONPs is generally achieved via ligand addition, ligand exchange, or encapsulation. A diverse group of materials can be used in these coating processes, including small molecule organic ligands, polymeric ligands, dense polymer matrix, and inorganic materials.
In situ surface modification
In this one-pot synthesis method, abridged particle size and narrow particle size distributions can be achieved, enabling both the synthesis and surface functionalization of IONPs to be carried out in a single step. During IONP synthesis, the coating process starts as soon as nucleation occurs, preventing further particle growth. For direct functionalization, carboxylates, phosphonates, thiol, and hydroxyl groups have commonly been used (Fig. 1).
Ligand addition
This method involves the addition of a ligand to the external surface of the IONPs. Carboxylates, phosphonates, thiol, and hydroxyl are unique among functional groups because of their strong binding to IONPs. Citrate has been used extensively for the colloidal stabilization of IONPs. This acid may be adsorbed on the surface of the IONPs via coordination through one or more carboxylate functionalities, depending upon the steric nature and the curvature of the surface [41]. Amino acids interact with the IONP's surface through their carboxyl groups [42, 43]. The possible structures involved in the coordination between carboxyl anions and IONPs is depicted in Fig. 2 [44]. Molecules with thiol groups can also be used for the in situ stabilization of IONPs. For example, a one-step sonochemical synthesis of amorphous Fe3O4 colloids covered with cysteine molecules was reported by Cohen et al. [45].
Encapsulation
Encapsulation is another method for the functionalization and stabilization of IONPs. In this protocol, IONP synthesis occurs through consecutive linking with an encapsulating agent present in solution. In situ encapsulation with polymeric materials and metals such as gold has been described. Using a combination of a pre-gel method and co-precipitation in aqueous solution, Liao and et al. prepared a core–shell nanostructure consisting of Fe3O4 nanoparticles as the core and organic alginate as the shell, with cell-targeting ligands (i.e., D-galactosamine) ornamented on the outer surface (denoted as Fe3O4@Alg-GA nanoparticles) [46]. Superparamagnetic Au-Fe3O4 bio-functional nanoparticles were synthesized using a one-step hot-injection precipitation method, as reported by Pariti et al. [47]. Basuki et al. [48] synthesized a library of magnetic nanoparticles through the in situ co-precipitation of ferrous (Fe2+) and ferric (Fe3+) ions from aqueous solutions in the presence of functional block copolymers.
Post -synthesis surface modification
In this type of surface modification, the protocol is divided into two parts: the synthesis of IONPs and their surface modification. One advantage of this method is that a large number of coupling agents are commercially available. Post-synthesis surface functionalization of magnetic nanoparticles is performed mainly via three mechanisms [49], which are described below.
Ligand addition
Ligand addition involves the addition of a ligand to the external surface of the synthesized IONPs, without the removal of any pre-existing ligands. Carboxylate, phosphonate, thiol, and hydroxyl functional groups can bind to the surface of IONPs. Similar to in situ ligand addition, citrate and other small molecules have been applied for colloidal stabilization of the IONPs by post-synthesis surface functionalization strategies [41, 50, 51] (Fig. 3).
Ligand exchange
Among small molecules, dopamine and its derivatives are unique. The catechol unit of dopamine can effectively coordinate with the IONP surface by the formation of stable five-membered rings [52]—for example, carboxylates such as citric acid and 2,3-dimercaptosuccinic acid (DMSA), which contain two carboxyl groups and two sulfhydryl groups [53,54,55] (Fig. 4).
Encapsulation
The encapsulation of IONPs in a biocompatible polymer or inorganic compounds is another stabilization and modification strategy. Also, biocompatible hydrophilic shell encapsulation could be used as a promising method for IONP modifications. There are several encapsulation methods, which can be categorized according to the shell materials and encapsulation technique (Fig. 5). Amphiphilic ligands, water-soluble polymer matrixes, and hydrophilic inorganic materials are typical shell materials. In this method, a large number of natural and synthetic biodegradable polymers—e.g., polyaspartate [56], polysaccharides [57], alginate [58], PEG [58], chitosan [59], co-polymers such as poly(maleic anhydridealt-1-octadecene)−PEG [60], polystyrene-co-PEG (PS-co-PEG) [61], poly(lactic acid)-co-PEG (PLA-co-PEG) [62], and inorganic material such as silica [63] and gold [3]—can be used.
Materials used for the modification of IONPs
Many types of materials can be used to coat the surface of IONPs and thus tailor their use for nano-bio applications. This section focuses on materials used for the stabilization, protection, and functionalization of IONPs through surface coating. A diverse set of materials, including organic compounds and inorganic materials, are utilized in such processes, which are summarized in Fig. 6 and briefly described in the following sections.
Organic compounds
Organic compounds possess good biocompatibility and biodegradability, and can provide functional groups such as aldehyde, amino, carboxyl, thiol, and hydroxyl groups. These groups enable linkage with active bio-substances such as drugs, antibodies, DNA, enzymes, and proteins for further nano-bio applications. A diverse group of organic compounds, such as small molecule organic and polymeric ligands, can be applied in such coating processes.
Small molecules
Functionalization of IONPs using small molecules is a simple process. Since bio-applications require a small hydrodynamic size, the use of small molecules for functionalization is helpful. Among various small molecules, compounds with thiol, carboxyl, and hydroxyl functional groups possess higher binding affinity toward IONPs. Dopamine is the most common high-affinity binding group used for the stabilization of IONPs in water and physiologic buffers [52, 64, 65]. The catechol unit of dopamine can coordinate with the IONP surface. Amstad et al. [66] identified catechol-derived anchor groups which possess inherent binding affinity to iron oxide and thus can optimally disperse superparamagnetic nanoparticles under physiologic conditions. Hashemi et al. [67] recently reported the synthesis of magnetic molecularly imprinted polymers using polydopamine to coat magnetic nanoparticles. 2,3-Dimercaptosuccinic acid (DMSA), which contains two carboxyl groups and two sulfhydryl groups, is another typical small molecule ligand [55, 68]. Carboxylates, including citric acid, constitute an additional class of small molecule ligands. Citric acid binds to the surface of Fe atoms by coordinating one or two carboxylic acid groups. Thus, at least one carboxylic acid group is exposed to the aqueous solvent, endowing the nanoparticle surface with negative charge, thereby enhancing its water-solubility [53, 69, 70]. The short-chain amines and amino silanes are typically used as stabilizing agents in IONPs [71]. Finally, various amino acids and peptides have been used as stabilizers for coating and functionalization of IONPs [42, 43, 51, 72, 73].
Table 1 provides a list of small organic molecules that are used for the functionalization of IONPs.
Macromolecules
Organic polymers are used extensively as coating ligands due to their unique features, including multidentate binding capability and steric repulsion effects. In contrast to most small molecules, organic polymers attach to nanoparticles via multiple functional groups, resulting in a stronger steric repulsive force. Owing to the excellent colloidal stability of IONPs, polymer-functionalized IONPs are receiving greater attention. Polymer coating typically requires the use of active terminal groups. Reactive monomers that have been used to promote the attachment of polymer coatings to the surface of MNPs include alkoxysilanes, citric acid, bisphosphonates, and DMSA [66, 70, 84]. In some cases, two or more polymers must be employed to achieve efficient surface coatings [85,86,87]. Polymer functionalizing materials can be classified into two groups, natural and synthetic polymers, which are discussed below.
Table 2 provides a summary of synthetic and natural polymers used for the functionalization of IONPs.
Natural polymers
-
a. Dextran
Dextran, a polysaccharide polymer consisting of R-D glucopyranosyl units, is an interesting material for coating of IONPs, and has been used in diverse applications such as MRI and cancer imaging and treatment [115]. IONPs coated with dextran have been clinically approved for use in MRI of the liver [116]. Most clinical MNPs have used dextran as a surface stabilizer (Combidex, dextran; Feraheme, carboxymethyl dextran; Feridex, dextran; and Resovist, carboxy dextran) [117,118,119,120]. One of the main drawbacks to the use of dextran coating is the weak bonding between dextran and the IONP surface [38]. Several published studies have investigated dextran coating of IONPs. Creixell and co-workers [121], for example, used dextran to coat the surface of peptized iron oxide nanoparticles. Jafari et al. [122] described dextran-coated IONPs that were conjugated with bombesin to produce a targeting contrast agent for MRI application.
-
b. Chitosan
Chitosan is a linear polysaccharide composed of randomly distributed β-(1-4)-linked D-glucosamine (deacetylated) and N-acetyl-D-glucosamine (acetylated) units. Chitosan is an alkaline, biocompatible, biodegradable, hydrophilic, and safe polymer [123]. In addition, it has antimicrobial properties and is able to absorb toxic material. Chitosan-coated IONPs have received considerable attention due to their easy synthesis and numerous applications [124,125,126,127]. Because of the presence of primary amine groups, chitosan is commonly preferred in pharmaceutical applications. It is also frequently chosen for drug and gene delivery applications due to its mucoadhesive property and positive charge. Castello et al. [128] reported the use of chitosan for the synthesis of IONPs and their functionalization. Arias et al. reported an Fe3O4/chitosan nanocomposite for magnetic drug targeting cancer therapy [104]. The application of Fe3O4–chitosan nanoparticles for hyperthermia treatment was described by Jingmiao et al. [103].
Synthetic polymers
-
a. Polyethylene glycol (PEG)
Polyethylene glycol (PEG) is a synthetic, biocompatible, flexible, and hydrophilic polymer, devoid of antigenicity and immunogenicity, which is frequently used in the functionalization of nanoparticles for biomedical applications [129]. The PEG-coated IONPs exhibit excellent solubility and stability in both aqueous solution and physiological media. The biocompatibility of PEG has been recognized by the US Food and Drug Administration (FDA) [130]. The presence of PEG on the surface of IONPs prevents opsonization and reduces the uptake of the IONPs by macrophages [131]. This leads to nanoparticles with increased blood circulation time, which may be very beneficial in drug release applications. The only disadvantage of functionalizing with PEG is that it is not biodegradable in the human body [132]. Anbarasu et al. reported a facile method for the synthesis of PEG-coated IONPs by way of chemical co-precipitation, in which polymerized polyethylene glycosylated bilayers were used to prepare the novel IONPs [133]. Several approaches have been suggested for attaching PEG to IONPs, including polymerization at the NP surface [134], modification through the sol–gel approach [135], and silane grafting onto the oxide surfaces [136, 137]. In addition to PEG, the polysaccharide dextran has been widely used in the design of IONP surface coatings for in vivo imaging applications [138,139,140].
-
b. Polyvinyl alcohol (PVA)
Polyvinyl alcohol (PVA) is a synthetic, biocompatible, hydrophilic polymer with low toxicity, which prevents agglomeration of nanoparticles in biological media [141, 142]. The multiple hydroxyl group of PVA give rise to its enhanced crystallinity, which results in high elastic modulus and tensile strength for bio-related applications [143, 144]. A key drawback of PVA-coated IONPs is their limited tissue distribution and penetration [145]. Pardoe et al. [110] reported the coating of IONPs with PVA using in situ strategies. Mahmoudi et al., on the other hand, used a post-synthesis strategy, in which they first synthesized iron oxide nanoparticles and then coated them by adding the PVA solution [146]. Albornoz et al. [147] reported the synthesis of an aqueous ferrofluid and the preparation of a magnetic gel with PVA and glutaraldehyde.
-
c. Alginate
Alginate, an anionic polysaccharide, is typically extracted from brown algae. This water-soluble biopolymer consists of two monomeric units: alpha-(1-4)-L-glucuronic acid and beta-(1-4)-D mannuronic acid. The carboxyl groups of alginate and iron ions interact, resulting in electrostatic repulsion. Alginate gels are widely used in encapsulation and controlled drug release [148,149,150,151]. Ma et al. [112] developed a novel modified two-step co-precipitation method for the synthesis of alginate-coated IONPs. Additional work by Morales et al. [152] demonstrated that the use of a polymer limited particle size. Magnetic microcapsules based on alginate and synthesized through a completely green route have also been described [87].
-
d. Co-polymers
PEG−phospholipid copolymers are archetypal co-polymer IONP coatings. Lee et al. synthesized encapsulating magnetite nanoparticles functionalized with PEG−phospholipid, which demonstrated high colloidal stability and good biocompatibility [153]. Many other copolymers can be used as well, including polylactide−PEG [154], poly(maleic anhydridealt-1-octadecene)−PEG [60], and polystyrene−poly(acrylic acid) (PS−PAA) [155]. The incorporation of IONPs in a hydrophilic, dense polymeric matrix is an additional method for generating water-soluble IONPs. Numerous copolymers, such as polystyrene-co-PEG (PS-co PEG) [61], poly(lactic-co-glycolic acid)-co-PEG (PLGA-co-PEG) [156], polystyrene-co poly-(acrylic acid) (PS-co-PAA) [157], and poly(lactic acid)-co-PEG (PLA-co-PEG) [62], have been used as matrixes.
Inorganic compounds
To provide colloidal stability and to prevent agglomeration of IONPs, inorganic compounds such as silica, metal, nonmetal, metal oxides, and sulfides, as well as silica (SiO2), gold (Au), and silver (Ag) coating materials, have been used. The functionalization of IONPs with inorganic compounds can significantly increase their antioxidant properties when compared to bare IONPs [39].
Table 3 provides a list of inorganic materials that are used for coating of IONPs.
Silica
Silica is one of the most extensively used coating materials due to its efficiency, hydrophilicity, reduced toxicity of the derived nanoparticles, and its ability to prevent nanoparticle aggregation. Additional advantages include the high density of surface functional groups that silica provides, and its readily tuned shell thickness [63, 167, 168]. The surface of silica is negatively charged as a result of the deprotonation of terminal silanol groups. Thus, electrostatic repulsive forces stabilize IONPs encapsulated in silica. Furthermore, silica-coated IONPs are both colloidally and photochemically stable, robust, and water-soluble [169]. A common method for encapsulating IONPs in silica is the sol–gel reaction (also known as the Stöber process), in which silica is synthesized via the hydrolysis and condensation of silicon orthoester (Si(OR)4) (e.g., tetraethyl orthosilicate [TEOS] and tetramethyl orthosilicate [TMOS]) [168, 170]. Im et al. [171] used the Stöber process to prepare silica colloids loaded with superparamagnetic IONPs. In this process, silica is formed in situ through the hydrolysis of a sol–gel precursor. The surface silanol groups can be modified with amine and sulfhydryl functional groups by employing the respective aminoethoxy silane and mercapto ethoxy silane. Carboxylic acid functional groups can also be introduced by reaction with an aminoethoxy silane followed by succinic anhydride [172]. Submicronic silica-coated magnetic sphere aerosols have been prepared by Tartaj et al. [173].
Metals
Metallic elements, when used for surface coating, act as a protective layer and are relatively inert. Among the many types of metallic coating materials reported in the literature, we have focused on coatings with gold, as these inorganic materials have been most commonly used in previous studies. Gold-coated magnetic nanoparticles were first reported in 2001 by Lin et al. [174]. Gold (Au) is biocompatible and has a great capacity for functionalization, making it particularly attractive for surface modification of IONPs. Disadvantages include the attenuation of the magnetic properties of IONPs functionalized with gold coatings, and difficulties in the maintenance of the coating arising from interaction between two dissimilar surfaces. Nevertheless, gold-coated IONPs have been found to be stable under neutral and acidic pH [175]. Wang et al. [176] prepared gold-coated IONPs by reducing the gold precursors on them. Xu et al. [177] reported the synthesis of core–shell-structured Fe3O4/Au by reducing HAuCl4 on the IONP surface. Kheiri et al. [158] reported the use of iron-gold core–shell magnetic nanoparticles as contrast agents in radiation therapy for treatment of breast cancer. In addition, this group designed and investigated a novel PEGylated gold-coated IONP as a potential drug delivery system [3, 15].
Conclusion
The ability to tailor the physicochemical properties of IONPs to applications in the pharmaceutical and biomedical fields continues to drive the development of new strategies for the synthesis of IONPs. IONPs have consistently demonstrated their therapeutic and diagnostic potential. Eventually, their unique ability to interface with organic and inorganic materials may lead to their use in various clinical therapies. Optimized and scalable IONP synthesis is critical for the development of pre-clinical and clinical applications. Further research exploring fabrication techniques for nanomaterials, especially applications of IONPs with diverse features, will contribute to innovation in a variety of areas [178]. The design of IONPs with multifunctional coatings for bio-applications has attracted significant attention. Surface functionalization of IONPs allows the attachment of various biomolecules, rendering them a promising candidate for bio-applications. Different chemical materials (synthetic and natural) have been studied and have been found to be efficient for the functionalization of IONPs. This review summarizes the preparation of IONPs through various synthetic techniques, and functionalized with a diverse range of coating molecules.
References
H. Danafar, A. Sharafi, H. Kheiri Manjili, S. Andalib, Pharm. Dev. Technol. 22, 642 (2016)
H.K. Manjili, A. Sharafi, H. Danafar, M. Hosseini, A. Ramazani, M.H. Ghasemi, RSC Adv. 6, 14403 (2016)
H.K. Manjili, L. Ma’mani, S. Tavaddod, M. Mashhadikhan, A. Shafiee, H. Naderi-Manesh, PLoS ONE 11, e0151344 (2016)
S. Al-Musawi, H. Naderi-Manesh, Z. Mohammad Hassan, H. Yeganeh, S. Nikzad, H. Kheiri Manjili, Modares J. Med. Sci: Pathobiol. 17, 25 (2014)
L. Ma’mani, S. Nikzad, H. Kheiri-Manjili, S. Al-Musawi, M. Saeedi, S. Askarlou, A. Foroumadi, A. Shafiee, Eur. J. Med. Chem. 83, 646 (2014)
Z. Wu, S. Yang, W. Wu, Nanoscale 8, 1237 (2016)
T.K. Jain, M.K. Reddy, M.A. Morales, D.L. Leslie-Pelecky, V. Labhasetwar, Mol. Pharm. 5, 316 (2008)
S. Naqvi, M. Samim, M. Abdin, F.J. Ahmed, A. Maitra, C. Prashant, A.K. Dinda, Int. J. Nanomed. 5, 983 (2009)
A. Petri-Fink, B. Steitz, A. Finka, J. Salaklang, H. Hofmann, Eur. J. Pharm. Biopharm. 68, 129 (2008)
J.L. Arias, L.H. Reddy, M. Othman, B. Gillet, D. Desmaele, F. Zouhiri, F. Dosio, R. Gref, P. Couvreur, ACS Nano 5, 1513 (2011)
K.M. Krishnan, IEEE Trans. Magn. 46, 2523 (2010)
S. Laurent, S. Dutz, U.O. Häfeli, M. Mahmoudi, Adv. Coll. Interface. Sci. 166, 8 (2011)
A. Singh, S.K. Sahoo, Drug Discovery Today 19, 474 (2014)
X. He, X. Wu, X. Cai, S. Lin, M. Xie, X. Zhu, D. Yan, Langmuir 28, 11929 (2012)
A. Izadi, H.K. Manjili, L. Mamani, E. Moslemi, M. Mashhadikhan, Am. Int. J. Contemp. Sci. Res. 2, 84 (2015)
K.E. Sapsford, W.R. Algar, L. Berti, K.B. Gemmill, B.J. Casey, E. Oh, M.H. Stewart, I.L. Medintz, Chem. Rev. 2013, 113 (1904)
F.M. Kievit, M. Zhang, Acc. Chem. Res. 44, 853 (2011)
W. Wu, C.Z. Jiang, V.A. Roy, Nanoscale 8, 19421 (2016)
J.G. King, W. Williams, C. Wilkinson, S. McVitie, J.N. Chapman, Geophys. Res. Lett. 23, 2847 (1996)
S. Mathur, S. Barth, U. Werner, F. Hernandez-Ramirez, A. Romano-Rodriguez, Adv. Mater. 20, 1550 (2008)
C. Lee, H. Lee, R. Westervelt, Appl. Phys. Lett. 79, 3308 (2001)
A. Shaabani, H. Nosrati, M. Seyyedhamzeh, Res. Chem. Intermed. 41, 3719 (2015)
F. Vereda, B. Rodríguez-González, J. de Vicente, R. Hidalgo-Álvarez, J. Colloid Interface Sci. 318, 520 (2008)
J.-H. Wu, S.P. Ko, H.-L. Liu, S. Kim, J.-S. Ju, Y.K. Kim, Mater. Lett. 61, 3124 (2007)
Y. Khollam, S. Dhage, H. Potdar, S. Deshpande, P. Bakare, S. Kulkarni, S. Date, Mater. Lett. 56, 571 (2002)
G. Salazar-Alvarez, M. Muhammed, A.A. Zagorodni, Chem. Eng. Sci. 61, 4625 (2006)
L. Cabrera, S. Gutierrez, N. Menendez, M. Morales, P. Herrasti, Electrochim. Acta 53, 3436 (2008)
R. Strobel, S.E. Pratsinis, Adv. Powder Technol. 20, 190 (2009)
F. Dang, N. Enomoto, J. Hojo, K. Enpuku, Ultrason. Sonochem. 16, 649 (2009)
U.T. Lam, R. Mammucari, K. Suzuki, N.R. Foster, Ind. Eng. Chem. Res. 47, 599 (2008)
M. Breulmann, H. Cölfen, H.P. Hentze, M. Antonietti, D. Walsh, S. Mann, Adv. Mater. 10, 237 (1998)
K.B. Narayanan, N. Sakthivel, Adv. Coll. Interface. Sci. 156, 1 (2010)
J.-W. Moon, Y. Roh, R.J. Lauf, H. Vali, L.W. Yeary, T.J. Phelps, J. Microbiol. Methods 70, 150 (2007)
X.-M. Liu, G. Yang, S.-Y. Fu, Mater. Sci. Eng., C 27, 750 (2007)
K.P. Naidek, F. Bianconi, T.C.R. Da Rocha, D. Zanchet, J.A. Bonacin, M.A. Novak, M.D.G.F. Vaz, H. Winnischofer, J. Colloid Interface Sci. 358, 39 (2011)
S. Laurent, J.-L. Bridot, L.V. Elst, R.N. Muller, Future Med. Chem. 2, 427 (2010)
B.H. Kim, N. Lee, H. Kim, K. An, Y.I. Park, Y. Choi, K. Shin, Y. Lee, S.G. Kwon, H.B. Na, J. Am. Chem. Soc. 133, 12624 (2011)
J.R. McCarthy, R. Weissleder, Adv. Drug Deliv. Rev. 60, 1241 (2008)
R.A. Bohara, N.D. Thorat, S.H. Pawar, RSC Adv. 6, 43989 (2016)
W. Wu, Z. Wu, T. Yu, C. Jiang, W.-S. Kim, Sci. Technol. Adv. Mater. 16, 023501 (2015)
L. Li, K. Mak, C. Leung, K. Chan, W. Chan, W. Zhong, P. Pong, Microelectron. Eng. 110, 329 (2013)
A. Ebrahiminezhad, Y. Ghasemi, S. Rasoul-Amini, J. Barar, S. Davaran, Bull. Korean Chem. Soc. 33, 3957 (2012)
Z. Urmus, H. Kavas, M.S. Toprak, A. Baykal, T.G. Altınçekiç, A. Aslan, A. Bozkurt, S. Coşgun, J. Alloy. Compd. 484, 371 (2009)
J.Y. Park, E.S. Choi, M.J. Baek, G.H. Lee, Mater. Lett. 63, 379 (2009)
H. Cohen, A. Gedanken, Z. Zhong, J. Phys. Chem. C 112, 15429 (2008)
S.-H. Liao, C.-H. Liu, B.P. Bastakoti, N. Suzuki, Y. Chang, Y. Yamauchi, F.-H. Lin, K.C. Wu, Int. J. Nanomed. 10, 3315 (2015)
A. Ariti, P. Desai, S. Maddirala, N. Ercal, K. Katti, X. Liang, M. Nath, Mater. Res. Express 1, 035023 (2014)
J.S. Basuki, A. Jacquemin, L. Esser, Y. Li, C. Boyer, T.P. Davis, Polym. Chem. 5, 2611 (2014)
N.T. Thanh, L.A. Green, Nano Today 5, 213 (2010)
R.N. Baig, R.S. Varma, Green Chem. 15, 398 (2013)
K. Pušnik, M. Peterlin, I. Kralj-Cigic, G. Marolt, K. Kogej, A. Mertelj, S. Gyergyek, D. Makovec, J. Phys. Chem. C 120, 1472 (2016)
C. Xu, K. Xu, H. Gu, R. Zheng, H. Liu, X. Zhang, Z. Guo, B. Xu, J. Am. Chem. Soc. 126, 9938 (2004)
M. Taupitz, S. Wagner, J. Schnorr, I. Kravec, H. Pilgrimm, H. Bergmann-Fritsch, B. Hamm, Invest. Radiol. 39, 394 (2004)
J.-H. Lee, Y.-M. Huh, Y.-W. Jun, J.-W. Seo, J.-T. Jang, H.-T. Song, S. Kim, E.-J. Cho, H.-G. Yoon, J.-S. Suh, Nat. Med. 13, 95 (2007)
Y.-M. Huh, Y.-W. Jun, H.-T. Song, S. Kim, J.-S. Choi, J.-H. Lee, S. Yoon, K.-S. Kim, J.-S. Shin, J.-S. Suh, J. Am. Chem. Soc. 127, 12387 (2005)
K. Aurich, M. Schwalbe, J.H. Clement, W. Weitschies, N. Buske, J. Magn. Magn. Mater. 311, 1 (2007)
Y.Y. Liang, L.M. Zhang, W. Jiang, W. Li, ChemPhysChem 8, 2367 (2007)
P.C. Lin, P.H. Chou, S.H. Chen, H.K. Liao, K.Y. Wang, Y.J. Chen, C.C. Lin, Small 2, 485 (2006)
J.L. Arias, M. López-Viota, E. Sáez-Fernández, M.A. Ruiz, Á.V. Delgado, Colloids Surf., A 384, 157 (2011)
W.Y. William, E. Chang, C.M. Sayes, R. Drezek, V.L. Colvin, Nanotechnology 17, 4483 (2006)
M. Muthiah, S.J. Lee, M. Moon, H.J. Lee, W.K. Bae, I.J. Chung, Y.Y. Jeong, I.-K. Park, J. Nanosci. Nanotechnol. 13, 1626 (2013)
J. Ren, H. Hong, T. Ren, X. Teng, React. Funct. Polym. 66, 944 (2006)
Y. Lu, Y. Yin, B.T. Mayers, Y. Xia, Nano Lett. 2, 183 (2002)
J. Xie, C. Xu, N. Kohler, Y. Hou, S. Sun, Adv. Mater. 19, 3163 (2007)
H. Gu, Z. Yang, J. Gao, C. Chang, B. Xu, J. Am. Chem. Soc. 127, 34 (2005)
E. Amstad, T. Gillich, I. Bilecka, M. Textor, E. Reimhult, Nano Lett. 9, 4042 (2009)
H. Hashemi-Moghaddam, S. Kazemi-Bagsangani, M. Jamili, S. Zavareh, Int. J. Pharm. 497, 228 (2016)
Y.-W. Jun, Y.-M. Huh, J.-S. Choi, J.-H. Lee, H.-T. Song, S. Kim, S. Kim, S. Yoon, K.-S. Kim, J.-S. Shin, J. Am. Chem. Soc. 127, 5732 (2005)
K. Nawara, J. Romiszewski, K. Kijewska, J. Szczytko, A. Twardowski, M. Mazur, P. Krysinski, J. Phys. Chem C 116, 5598 (2012)
R. Lakshmanan, M. Sanchez-Dominguez, J.A. Matutes-Aquino, S. Wennmalm, G. Kuttuva Rajarao, Langmuir 2014, 30 (1036)
N. Kohler, C. Sun, J. Wang, M. Zhang, Langmuir 21, 8858 (2005)
J. Xie, K. Chen, H.-Y. Lee, C. Xu, A.R. Hsu, S. Peng, X. Chen, S. Sun, J. Am. Chem. Soc. 130, 7542 (2008)
Y. Lai, W. Yin, J. Liu, R. Xi, J. Zhan, Nanoscale Res. Lett. 5, 302 (2009)
M. Martín, P. Salazar, R. Villalonga, S. Campuzano, J.M. Pingarrón, J.L. González-Mora, J. Mater. Chem. B 2, 739 (2014)
R.N. Baig, R.S. Varma, Chem. Commun. 48, 2582 (2012)
P. Brillet, F. Gazeau, A. Luciani, B. Bessoud, C.-A. Cuénod, N. Siauve, J.-N. Pons, J. Poupon, O. Clément, Eur. Radiol. 15, 1369 (2005)
A. Ruiz, Y. Hernandez, C. Cabal, E. González, S. Veintemillas-Verdaguer, E. Martinez, M. Morales, Nanoscale 5, 11400 (2013)
S. Kossatz, J. Grandke, P. Couleaud, A. Latorre, A. Aires, K. Crosbie-Staunton, R. Ludwig, H. Dähring, V. Ettelt, A. Lazaro-Carrillo, Breast Cancer Res. 17, 1 (2015)
R. Mejías, S. Pérez-Yagüe, L. Gutiérrez, L.I. Cabrera, R. Spada, P. Acedo, C.J. Serna, F.J. Lázaro, Á. Villanueva, M. del Puerto Morales, Biomaterials 32, 2938 (2011)
S. Wagner, J. Schnorr, H. Pilgrimm, B. Hamm, M. Taupitz, Invest. Radiol. 37, 167 (2002)
L. Sandiford, A. Phinikaridou, A. Protti, L.K. Meszaros, X. Cui, Y. Yan, G. Frodsham, P.A. Williamson, N. Gaddum, R.M. Botnar, ACS Nano 7, 500 (2012)
A. Ebrahiminezhad, S. Davaran, S. Rasoul-Amini, J. Barar, M. Moghadam, Y. Ghasemi, Current Nanosci. 8, 868 (2012)
L. Xiao, J. Li, D.F. Brougham, E.K. Fox, N. Feliu, A. Bushmelev, A. Schmidt, N. Mertens, F. Kiessling, M. Valldor, ACS Nano 5, 6315 (2011)
L. Zhu, D. Wang, X. Wei, X. Zhu, J. Li, C. Tu, Y. Su, J. Wu, B. Zhu, D. Yan, J. Controlled Release 169, 228 (2013)
M.J.-E. Lee, O. Veiseh, N. Bhattarai, C. Sun, S.J. Hansen, S. Ditzler, S. Knoblaugh, D. Lee, R. Ellenbogen, M. Zhang, PLoS ONE 5, e9536 (2010)
R. Bachmann, R. Conrad, B. Kreft, O. Luzar, W. Block, S. Flacke, D. Pauleit, F. Träber, J. Gieseke, K. Saebo, J. Magn. Reson. Imaging 16, 190 (2002)
A.A. Rafi, M. Mahkam, RSC Adv. 5, 4628 (2015)
D.L. Thorek, A.K. Chen, J. Czupryna, A. Tsourkas, Ann. Biomed. Eng. 34, 23 (2006)
R. Weissleder, G. Elizondo, J. Wittenberg, C. Rabito, H. Bengele, L. Josephson, Radiology 175, 489 (1990)
C. Chambon, O. Clement, A. Le Blanche, E. Schouman-Claeys, G. Frija, Magn. Reson. Imaging 11, 509 (1993)
F. Zhao, M. Williams, X. Meng, D.C. Welsh, A. Coimbra, E.D. Crown, J.J. Cook, M.O. Urban, R. Hargreaves, D.S. Williams, Neuroimage 40, 133 (2008)
B. Tomanek, U. Iqbal, B. Blasiak, A. Abulrob, H. Albaghdadi, J.R. Matyas, D. Ponjevic, G.R. Sutherland, Neuro-oncology 14, 53 (2011)
M. Sigovan, L. Boussel, A. Sulaiman, D. Sappey-Marinier, H. Alsaid, C. Desbleds-Mansard, D. Ibarrola, D. Gamondès, C. Corot, E. Lancelot, Radiology 252, 401 (2009)
G. Wang, S. Inturi, N.J. Serkova, S. Merkulov, K. McCrae, S.E. Russek, N.K. Banda, D. Simberg, ACS Nano 8, 12437 (2014)
B.B. Frericks, F. Wacker, C. Loddenkemper, S. Valdeig, B. Hotz, K.-J. Wolf, B. Misselwitz, A. Kühl, J.C. Hoffmann, Invest. Radiol. 44, 23 (2009)
G.H. Simon, J. von Vopelius-Feldt, Y. Fu, J. Schlegel, G. Pinotek, M.F. Wendland, M.-H. Chen, H.E. Daldrup-Link, Invest. Radiol. 41, 45 (2006)
W. Li, S. Tutton, A.T. Vu, L. Pierchala, B.S. Li, J.M. Lewis, P.V. Prasad, R.R. Edelman, J. Magn. Reson. Imaging 21, 46 (2005)
C. Kaittanis, T.M. Shaffer, A. Ogirala, S. Santra, J.M. Perez, G. Chiosis, Y. Li, L. Josephson, J. Grimm, Nat. Commun. 5, 3384 (2014)
M. Peng, H. Li, Z. Luo, J. Kong, Y. Wan, L. Zheng, Q. Zhang, H. Niu, A. Vermorken, W. Van de Ven, Nanoscale 7, 11155 (2015)
M.F. Casula, P. Floris, C. Innocenti, A. Lascialfari, M. Marinone, M. Corti, R.A. Sperling, W.J. Parak, C. Sangregorio, Chem. Mater. 22, 1739 (2010)
Y. Wang, H.-Z. Jia, K. Han, R.-X. Zhuo, X.-Z. Zhang, J. Mater. Chem. B 1, 3344 (2013)
Y. Ding, S.Z. Shen, H. Sun, K. Sun, F. Liu, Y. Qi, J. Yan, Mater. Sci. Eng., C 48, 487 (2015)
J. Qu, G. Liu, Y. Wang, R. Hong, Adv. Powder Technol. 21, 461 (2010)
J.L. Arias, L.H. Reddy, P. Couvreur, J. Mater. Chem. 22, 7622 (2012)
A. Javid, S. Ahmadian, A.A. Saboury, S.M. Kalantar, S. Rezaei-Zarchi, Chem. Biol. Drug Des. 82, 296 (2013)
K.L. Hultman, A.J. Raffo, A.L. Grzenda, P.E. Harris, T.R. Brown, S. O’Brien, ACS Nano 2, 477 (2008)
R. Qiao, Q. Jia, S. Huwel, R. Xia, T. Liu, F. Gao, H.-J. Galla, M. Gao, ACS Nano 6, 3304 (2012)
X. Yang, H. Hong, J.J. Grailer, I.J. Rowland, A. Javadi, S.A. Hurley, Y. Xiao, Y. Yang, Y. Zhang, R.J. Nickles, Biomaterials 32, 4151 (2011)
Z.R. Stephen, C.J. Dayringer, J.J. Lim, R.A. Revia, M.V. Halbert, M. Jeon, A. Bakthavatsalam, R.G. Ellenbogen, M. Zhang, ACS Appl. Mater. Interfaces. 8, 6320 (2016)
H. Pardoe, W. Chua-Anusorn, T.G.S. Pierre, J. Dobson, J. Magn. Magn. Mater. 225, 41 (2001)
S. Kayal, R. Ramanujan, Mater. Sci. Eng., C 30, 484 (2010)
H.L. Ma, Y.F. Xu, X.R. Qi, Y. Maitani, T. Nagai, Int. J. Pharm. 354, 217 (2008)
P.V. Finotelli, D. Da Silva, M. Sola-Penna, A.M. Rossi, M. Farina, L.R. Andrade, A.Y. Takeuchi, M.H. Rocha-Leão, Colloids Surf., B 81, 206 (2010)
X.-L. Tang, B.-L. Lin, S. Cui, X. Zhang, Y. Zhong, Q. Wu, X. Zhang, X.-D. Shen, T.-W. Wang, RSC Adv. 6, 43284 (2016)
A.K. Gupta, M. Gupta, Biomaterials 26, 3995 (2005)
X.-M. Zhu, Y. Wang, K. Leung, S.-F. Lee, F. Zhao, D.-W. Wang, J. Lai, C. Wan, C. Cheng, A.T. Ahuja, Int J Nanomedicine 7, 953 (2012)
J.P. Bullivant, S. Zhao, B.J. Willenberg, B. Kozissnik, C.D. Batich, J. Dobson, Int. J. Mol. Sci. 14, 17501 (2013)
C.W. Jung, P. Jacobs, Magn. Reson. Imaging 13, 661 (1995)
P. Reimer, E.J. Rummeny, H.E. Daldrup, T. Balzer, B. Tombach, T. Berns, P.E. Peters, Radiology 195, 489 (1995)
R.A. Weissleder, D.D. Stark, B.L. Engelstad, B.R. Bacon, C.C. Compton, D.L. White, P. Jacobs, J. Lewis, Am. J. Roentgenol. 152, 167 (1989)
M. Creixell, A.P. Herrera, M. Latorre-Esteves, V. Ayala, M. Torres-Lugo, C. Rinaldi, J. Mater. Chem. 20, 8539 (2010)
A. Jafari, M. Salouti, S.F. Shayesteh, Z. Heidari, A.B. Rajabi, K. Boustani, A. Nahardani, Nanotechnology 26, 075101 (2015)
P. Shete, R. Patil, N. Thorat, A. Prasad, R. Ningthoujam, S. Ghosh, S. Pawar, Appl. Surf. Sci. 288, 149 (2014)
S.R. Bhattarai, R.B. Kc, S. Aryal, M.S. Khil, H.Y. Kim, Carbohyd. Polym. 69, 467 (2007)
E.H. Kim, Y. Ahn, H.S. Lee, J. Alloy. Compd. 434, 633 (2007)
P. Sipos, O. Berkesi, E. Tombacz, T.G.S. Pierre, J. Webb, J. Inorg. Biochem. 95, 55 (2003)
A. Shaabani, M.B. Boroujeni, M.S. Laeini, RSC Adv. 6, 27706 (2016)
J. Castelló, M. Gallardo, M.A. Busquets, J. Estelrich, Colloids Surf., A 468, 151 (2015)
H. Danafar, Cogent Med. 3, 1142411 (2016)
C. Nazli, T.I. Ergenc, Y. Yar, H.Y. Acar, S. Kizilel, Int. J. Nanomed. 2012, 7 (1903)
J.V. Jokerst, T. Lobovkina, R.N. Zare, S.S. Gambhir, Nanomedicine 6, 715 (2011)
T. Neuberger, B. Schöpf, H. Hofmann, M. Hofmann, B. Von Rechenberg, J. Magn. Magn. Mater. 293, 483 (2005)
M. Anbarasu, M. Anandan, E. Chinnasamy, V. Gopinath, K. Balamurugan, Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 135, 536 (2015)
J.-F. Lutz, S. Stiller, A. Hoth, L. Kaufner, U. Pison, R. Cartier, Biomacromol 7, 3132 (2006)
A.K. Gupta, R.R. Naregalkar, V.D. Vaidya, M. Gupta, Nanomedicine 2, 23 (2007)
M. Butterworth, L. Illum, S. Davis, Colloids Surf., A 179, 93 (2001)
M. Pilloni, J. Nicolas, V. Marsaud, K. Bouchemal, F. Frongia, A. Scano, G. Ennas, C. Dubernet, Int. J. Pharm. 401, 103 (2010)
T. Shen, R. Weissleder, M. Papisov, A. Bogdanov, T.J. Brady, Magn. Reson. Med. 29, 599 (1993)
L. Josephson, C.-H. Tung, A. Moore, R. Weissleder, Bioconjug. Chem. 10, 186 (1999)
C. Corot, P. Robert, J.-M. Idée, M. Port, Adv. Drug Deliv. Rev. 58, 1471 (2006)
S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. Vander Elst, R.N. Muller, Chem. Rev. 108, 2064 (2008)
M. Sairam, B.V.K. Naidu, S.K. Nataraj, B. Sreedhar, T.M. Aminabhavi, J. Membr. Sci. 283, 65 (2006)
M. Mahmoudi, S. Sant, B. Wang, S. Laurent, T. Sen, Adv. Drug Deliv. Rev. 63, 24 (2011)
G.S. Demirer, A.C. Okur, S. Kizilel, J. Mater. Chem. B 3, 7831 (2015)
Y. Zhang, N. Kohler, M. Zhang, Biomaterials 23, 1553 (2002)
M. Mahmoudi, A. Simchi, M. Imani, M.A. Shokrgozar, A.S. Milani, U.O. Häfeli, P. Stroeve, Colloids Surf., B 75, 300 (2010)
C. Albornoz, S.E. Jacobo, J. Magn. Magn. Mater. 305, 12 (2006)
P. de Vos, M.M. Faas, B. Strand, R. Calafiore, Biomaterials 27, 5603 (2006)
P. Finotelli, M. Morales, M. Rocha-Leao, E. Baggio-Saitovitch, A. Rossi, Mater. Sci. Eng., C 24, 625 (2004)
E. Kroll, F.M. Winnik, R.F. Ziolo, Chem. Mater. 8, 1594 (1996)
Y. Nishio, A. Yamada, K. Ezaki, Y. Miyashita, H. Furukawa, K. Horie, Polymer 45, 7129 (2004)
M. Morales, P. Finotelli, J. Coaquira, M. Rocha-Leão, C. Diaz-Aguila, E. Baggio-Saitovitch, A. Rossi, Mater. Sci. Eng., C 28, 253 (2008)
N. Lee, H. Kim, S.H. Choi, M. Park, D. Kim, H.-C. Kim, Y. Choi, S. Lin, B.H. Kim, H.S. Jung, Proc. Natl. Acad. Sci. 108, 2662 (2011)
N. Nasongkla, E. Bey, J. Ren, H. Ai, C. Khemtong, J.S. Guthi, S.-F. Chin, A.D. Sherry, D.A. Boothman, J. Gao, Nano Lett. 6, 2427 (2006)
B.-S. Kim, J.-M. Qiu, J.-P. Wang, T.A. Taton, Nano Lett. 2005, 5 (1987)
J. Cheng, B.A. Teply, I. Sherifi, J. Sung, G. Luther, F.X. Gu, E. Levy-Nissenbaum, A.F. Radovic-Moreno, R. Langer, O.C. Farokhzad, Biomaterials 28, 869 (2007)
L. Charoenmark, D. Polpanich, R. Thiramanas, P. Tangboriboonrat, Macromol. Res. 20, 590 (2012)
H.K. Manjili, H. Naderi-Manesh, M. Mashhadikhan, L. Ma’mani, S. Nikzad, J. Paramed. Sci. 5, 85 (2014)
F. Mohammad, T. Arfin, Adv. Mater. Lett 5, 315 (2014)
L.-Y. Bai, X.-Q. Yang, J. An, L. Zhang, K. Zhao, M.-Y. Qin, B.-Y. Fang, C. Li, Y. Xuan, X.-S. Zhang, Nanotechnology 26, 315701 (2015)
H. Yang, Y. Li, T. Li, M. Xu, Y. Chen, C. Wu, X. Dang, Y. Liu, Scientific reports 4, 7072 (2014)
K. Lee, C. Cheong, K.S. Hong, E.K. Koh, M. Kim, H.S. Shin, Y.-N. Kim, S.H. Lee, J. Korean Phys. Soc. 53, 2535 (2008)
J. Gautier, E. Allard-Vannier, J. Burlaud-Gaillard, J. Domenech, I. Chourpa, J. Biomed. Nanotechnol. 11, 177 (2015)
N. Kohler, C. Sun, A. Fichtenholtz, J. Gunn, C. Fang, M. Zhang, Small 2, 785 (2006)
J. Lee, H. Kim, S. Kim, H. Lee, J. Kim, N. Kim, H.J. Park, E.K. Choi, J.S. Lee, C. Kim, J. Mater. Chem. 22, 14061 (2012)
Y. Wang, P. Su, S. Wang, J. Wu, J. Huang, Y. Yang, J. Mater. Chem. B 1, 5028 (2013)
A. Ulman, Chem. Rev. 96, 1533 (1996)
T. Tago, T. Hatsuta, K. Miyajima, M. Kishida, S. Tashiro, K. Wakabayashi, J. Am. Ceram. Soc. 85, 2188 (2002)
N. Erathodiyil, J.Y. Ying, Acc. Chem. Res. 44, 925 (2011)
C. Graf, D.L. Vossen, A. Imhof, A. van Blaaderen, Langmuir 19, 6693 (2003)
S.H. Im, T. Herricks, Y.T. Lee, Y. Xia, Chem. Phys. Lett. 401, 19 (2005)
H. Lee, T.-H. Shin, J. Cheon, R. Weissleder, Chem. Rev. 115, 10690 (2015)
P. Tartaj, T. Gonzalez-Carreno, C.J. Serna, Adv. Mater. 13, 1620 (2001)
J. Lin, W. Zhou, A. Kumbhar, J. Wiemann, J. Fang, E. Carpenter, C. O’Connor, J. Solid State Chem. 159, 26 (2001)
V.I. Shubayev, T.R. Pisanic, S. Jin, Adv. Drug Deliv. Rev. 61, 467 (2009)
L. Wang, L. Wang, J. Luo, Q. Fan, M. Suzuki, I.S. Suzuki, M.H. Engelhard, Y. Lin, N. Kim, J.Q. Wang, J. Phys. Chem. B 109, 21593 (2005)
Z. Xu, Y. Hou, S. Sun, J. Am. Chem. Soc. 129, 8698 (2007)
J. Liu, Z. Wu, Q. Tian, W. Wu, X. Xiao, CrystEngComm 18, 6303 (2016)
Acknowledgements
This work has been supported financially by the Faculty of Pharmacy, Zanjan University of Medical Sciences, Zanjan, Iran.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Nosrati, H., Salehiabar, M., Davaran, S. et al. New advances strategies for surface functionalization of iron oxide magnetic nano particles (IONPs). Res Chem Intermed 43, 7423–7442 (2017). https://doi.org/10.1007/s11164-017-3084-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3084-3