Abstract
A carbon-based solid acid catalyst was prepared by simultaneous carbonization and sulfonation of distillers’ grain with concentrated sulfuric acid. Response surface methodology was adopted to determine the optimal preparation conditions such as sulfuric acid concentration, preparation temperature and preparation time. This catalyst was characterized by XRD, FT-IR, TG, XPS, elemental analysis, N2 adsorption/desorption and acid-base titration methods. The catalytic performance was evaluated under a certain condition in the esterification of acetic acid and n-butanol as a probe reaction. The conversion of acetic acid was 97.6% when the sulfuric acid concentration of 85 wt%, preparation temperature of 20 °C, and preparation time of 1.4 h. The high catalytic activity of this catalyst may be attributed to the higher acid density of 1.73 mmol/g by NaOH titration. The catalyst performance will weaken with the increase of reuse times, but it could be regenerated by treatment with concentrated sulfuric acid (85 wt%).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acid catalysts play an important role in the petroleum refining and chemical industries. Some solid acid catalysts such as zeolite [1], ZSM-5 [2], WO3/ZrO2 [3], ZrO2–WO3 [4], SO4 2−/ZrO2 [5] Nafion [6], Amberlyst-15 [7] and heteropolyacids [8] have been used in acid catalytic reactions. WO3/ZrO2 is synthesized by impregnating Zr(OH)4 with an ammonium metatungstate solution and calcining at 800 °C [3]. SO4 2−/ZrO2 is prepared by impregnating Zr(OH)4 with H2SO4 and calcining at 500 °C for 2 h [5]. Both of them are efficient solid acid catalysts, but are expensive because zirconium is a rare and costly metal and high temperatures are required both for the calcination and for the reactivation of the catalyst. Metal oxide or zeolite catalysts usually have a low acidic site density and a hydrophilic surface. When water is generated in the reaction system, the activity of the catalyst will be reduced due to the water adsorption onto the surface. Although strong acid ion exchange resins possess very strong acid sites, their actual application is limited due to the low heat stability, which is apt to swell during the reaction, and high costs [7]. The major disadvantages of heteropolyacids as catalystd lie in their low thermal stability, low surface area and separation problems from the reaction mixture [8].
Recently, a new class of sulfonated carbon-based solid acid catalysts was reported as promising catalysts for many reactions. Toda’s group [9] synthesized solid acid catalysts by sulfonation of incompletely carbonized sugar. Incomplete carbonization of d-glucose leads to a rigid carbon material that consists of small polycyclic aromatic carbon sheets in a three-dimensional sp 3-bonded structure. Lou et al. [10] studied esterification activity by using various solid acid catalysts made from glucose, starch, sucrose and cellulose. The catalyst was typically prepared by carbonizing at 400 °C for 15 h under N2, and then sulfonating at 150 °C with concentrated H2SO4 for 15 h. The catalytic performance of the starch-derived catalyst was better than concentrated sulfuric acid. Wang et al. [11] prepared a catalyst by the sulfonation of incompletely carbonized cassava stillage residue with concentrated sulfuric acid and explored the esterification of methanol and free fatty acids. The catalyst was prepared by carbonizing at 600 °C under N2 and sulfonation below 160 °C. The catalyst showed high catalytic activity for esterification, and the acid value for waste cooking oil was reduced to below 2 mg KOH/g after the reaction. Kastner et al. [12] prepared a sulfonated carbon-based solid acid catalyst from peanut hulls, pine logging residues and wood chips. Catalysts were synthesized by sulfonating the biochar and wood-derived activated carbon using concentrated H2SO4 at 100, 150 and 200 °C (12 h) and gaseous SO3 (23 °C). The catalyst showed high activity for the esterification of palmitic acid and the conversion of palmitic acid was approximately 100%. The majority of these catalysts were typically prepared from biomass by carbonizing at high temperature under N2 and then sulfonating at 150 °C with concentrated H2SO4, fuming H2SO4 or gaseous SO3. However, the carbonization process is time-consuming under the high temperature and produces a lot of waste gas, while the sulfonation process is in extreme conditions and a low utilization ratio of sulfonating agent. Our group proposed a one-step method to prepare a solid acid catalyst by simultaneous carbonization and sulfonation with concentrated sulfuric acid. The preparation method has the advantages of easy operation, mild reaction conditions, low cost, and short reaction time, while prepared catalyst has a good catalytic performance.
Distillers’ grain (DG) is the main co-product of the Chinese liquor industry. With the growth of Chinese liquor production, about 100 million tons of DG is output every year [13]. So far, most DG has been used as a livestock feed [14]. DG is a very worthwhile material in the hemical industry, because it is a rich, unhydrolyzed and unfermented polymeric sugar, which can be converted to biochar, so the DG can be used to prepare a catalyst. However, there are few reports about using DG to prepare catalysts. In addition, the preparation of a catalyst from DG can enhance the profitability of the Chinese liquor industry.
In the present work, response surface methodology was adopted to determine the optimal preparation conditions for producing a solid catalyst by simultaneous carbonization and sulfonation of DG with concentrated H2SO4, and the catalytic performance was evaluated under a certain condition in the esterification of acetic acid and n-butanol as a probe reaction. Meanwhile, characterization was carried out by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), thermogravimetric analysis (TG), X-ray potoelectron spectroscopy (XPS), N2 adsorption/desorption, elemental analysis and acid-base titration methods. Finally, the stability of the catalyst was also investigated.
Materials and methods
Experimental materials and chemicals
Distillers’ grain (DG) collected at Anhui Shuanlun Vino was used in this study. Those test samples were dried in an oven for 24 h at 105 °C before the experiments and ground into particles (particle size was less than 0.28 mm). Sulfuric acid (98.0 wt%), acetic acid (99.5 wt%) and n-butanol (99.5 wt%) were purchased from Sinopharm Chemical Reagent as analytical grade and used without any further treatment.
Catalyst preparation
The catalyst was prepared from DG in one step. Simultaneous carbonization and sulfonation with concentrated sulfuric acid was used to introduce sulfonic groups onto the biochar with a ratio of DG to H2SO4 equal to 1 g:6 mL. Then, 5.0 g of DG and 30 mL sulfuric acid (85 wt%) were combined in a 100-mL round-bottom flask and heated in a water bath at 20 °C under air atmosphere. The mixture was kept mechanical stirred for 1.4 h. Finally, the mixture was diluted with distilled water. The product was collected by filtration using a water-circulating vacuum pump and washed with hot distilled water until the washwater showed a neutral pH value. The black solid acid catalys was dried at 80 °C in an air oven for 5 h.
Experimental design
The parameters for preparing the catalyst were studied with a standard response surface methodology design called a Box–Behnken design. In this study, the catalytic performance was determined by three factors: sulfuric acid concentration (X 1), preparation temperature (X 2), and preparation time (X 3). X 1 varied from 70 to 90 wt% at 3 levels (70, 80 and 90 wt%), X 2 from 20 to 80 °C at 3 levels (20, 50 and 80 °C), and X 3 at 3 levels (1, 2 and 3 h). These three factors were found to be the important parameters. Experimental range and levels of the independent variables are shown in Table 1.
The response of interest in the study was the conversion of acetic acid and each response value was the average of triplicates. A second-order regression model was used to predict the response as described in Eq. (1) [15].
where R i is the response, b 0 is the constant coefficient, b i , b ii and b ij are the linear coefficients, quadratic coefficients and interaction coefficients, respectively, and X i , and X j are the factors, respectively.
Statistical analysis
ANOVA (analysis of variance) was used to determine the significant second-order model that fit the experimental response. The coefficient of determination, the R 2 value, was used to describe the fitting degree of the polynomial model equation. The significances of the regression coefficient were checked by the F test and p value. Model terms were evaluated by the p value with 95% confidence interval. The optimal values of the critical parameters were carried out using the numerical technique of the Design-Expert software v.8.0.5 for selected response. The representation of the response by second-order quadratic polynomials can be visualized as 3D surface plots and 2D contour plots.
Catalyst characterization
An XRD pattern was obtained from an X-ray Diffractometer (DX-2700; Rigaku, Japan) using Cu Kα radiation at 2θ of 10° to 80°, at a scanning speed of 2°/min. Infrared spectra were recorded on a FTIR spectrometer (Nicolet6700; WI, USA) in a KBr matrix in the range of 4000–500 cm−1. XPS measurement was performed on an electron spectrometer (ESCALAB250; Thermo, USA) with a monochromatic aluminum Kα X-ray source. Thermal stability of the catalyst was investigated through thermogravimetric (TG) measurements, and the furnace temperature for TG was increased from room temperature to 800 °C at a heating rate of 10 °C/min. The surface area of the catalyst was determined by nitrogen adsorption/desorption using an automated surface area and pore size analyzer (Autosorb-1, NOVA2200e). The acid density of SO3H in the catalyst was examined by elemental analysis using a Vario EL Elemental analyzer instrument (Elementar, Germany). The total acid density was determined by the acid-base titration method [16]. Finally, 0.1 g of catalyst was dispersed in 25 mL NaCl solution (2 mol/L) under ultrasonic vibration to be equilibrated. After filtration, the filtrate was titrated by NaOH solution (0.01 mol/L) with phenolphthalein as an indicator.
Performance of catalyst
The esterification reaction was carried out in a three-necked glass flask equipped with a mechanical stirrer and a reflux condenser. A certain amount of acetic acid, n-butanol and catalyst samples were heated up to a selected reaction temperature with continuous stirring. In this reaction, the experimental conditions were as follows: the molar ratio of n-butanol to acetic acid was 1.5:1, the reaction temperature of 117 °C, and the catalyst/total amount mass ratio was 0.6 wt%. After the esterification reaction had completed, the catalyst was separated by filtration and regenerated by washing with ethanol, then dried in air at 85 °C to recuperate its catalytic activity. The acetic acid concentration was determined according to GB/T 1668–2008. The conversion of acetic acid was calculated by the molar content of the acid using Eq. (2):
where A in and A out are the molar content of the original and the esterified acetic acid, respectively.
Results and discussion
Catalyst characterization
The XRD patterns of DG and catalyst (prepared under optimal preparation conditions) are shown in Fig. 1. Different from DG, the characteristic peak (2θ = 16°, silica) disappeared in the spectrum of the catalyst. The spectrum of this catalyst had a broad diffraction peak with 2θ ranged in 20°–30°, attributable to the characteristic peak of C (002). This result indicated that the catalyst was composed of amorphous carbon with a low degree of graphitization [17].
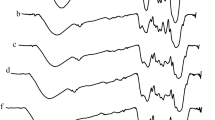
The FT-IR specta of DG and the catalyst are shown in Fig. 2. The vibration peak at 3390 cm−1 can be assigned to O–H, while that at 2890 cm−1 was the vibration peak of C–H. The band at 1640 cm−1 can be attributed to C=C aromatic stretching vibration [18]. After treatment with sulfuric acid, the stretching vibration peak of these functional groups dramatically decreased, which means that great changes have taken place in the structure of DG, and the decrease of the intensity of the stretching vibration peaks of the functional groups could be the result of bond formation with the sulfuric groups. Bands at 1180 cm−1 (for –SO3H) and 1070 cm−1 (for S=O), which represent the sulfonic acid group stretching modes, also confirm the successful incorporation of sulfonic acid groups into the solid acid catalyst [19].
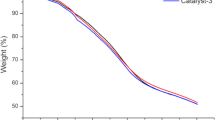
The TG curves of the raw material and catalyst in the air atmosphere are shown in Fig. 3. TG curve (a) for the DG is divided into four stages. The first stage from the initial temperature to 150 °C involves evaporation of the free water and physical adsorbed water. The second stage from 150 to 350 °C is assigned to the thermal decomposition of monosaccharides and hemicellulose. In the range of 350–500 °C, this is mainly attributed to the thermal decomposition of hemicellulose, cellulose and partial lignin. Mass loss of the four stages from 500 to 800 °C are for the further decomposition of lignin and the oxidation of non-graphitic and graphitic carbon. The TG curve (b) displays a slight weight loss from 30 to 110 °C because of the evaporation of free water and physically adsorbed water. The weight loss in the region of 150–310 °C could be attributed to the rupture of chemical bonds, such as –SO3H, –COOH, C–OH, etc. A rapid weight loss is observed from 310 to 500 °C, possibly because the thermal decomposition of hemicellulose, cellulose and partial lignin. In the range of 500–800 °C, this is mainly attributed to the further decomposition of lignin and the oxidation of non-graphitic and graphitic carbon. This result confirms that the catalyst has good thermal stability under esterification condition, especially when the temperature is below 150 °C [20].
XPS was conducted to survey the surface element composition of the catalyst. Figure 4 shows that the single O1s, C1s and S2p peaks were observed in their XPS survey spectra. The peak of 533 eV was assigned to the C–OH or C=O band, and a strong S 2p peak appeared at about 168 eV, which was assigned to the SO3H groups, indicating that S has been successfully introduced into this catalyst after sulfonation [21].
As Table 2 shows, acid density was determined by both acid-base titration (sum of –SO3H, –OH and –COOH) and elemental analysis (–SO3H). Raw material treated with concentrated H2SO4 had higher S contents than DG, and the density of the sulfonic groups increased to 0.89 mmol/g, while the total acid density also increased from 0.06 to 1.73 mmol/g snf the surface area of this catalyst also increased to 26.25 m2/g. Thus, treating with H2SO4 not only increased the acid density of catalyst, especially the acid density of –SO3H, but also increased the additional surface area [18].
Development of regression model equation
Box–Behnken design was used to develop the correlation between the solid acid catalyst preparation factors and the catalytic performance. As shown in Table 3, 17 experimental runs were generated by the Box–Behnken design, 5 of them repeated at the center of the design used to evaluate the pure error sum of squares. The minimum conversion of acetic acid is 55.0%, and it can be reached when using 70.0 wt% sulfuric acid at a preparation temperature of 80 °C for 2 h (experimental run no. 10). The maximum conversion of acetic acid is 98.5%, and it can be reached when using 90.0 wt% sulfuric acid at a preparation temperature of 20 °C and preparation time of 2 h (experimental run no. 11).
A regression analysis was carried out to fit the mathematical model to the experimental data aiming at an optimal region for the responses studied. Predicted response R for the conversion of acetic acid could be expressed by the second-order polynomial equation shown in Eq. (3).
The significance of each coefficient and the interaction strength between each independent variable can be reflected by p values. The model F value of 90.84 and low p value (p < 0.01%) indicated that the model was significant. The positive sign of the regression coefficients indicates asynergetic effect, whereas a negative sign indicates an antagonistic effect [22]. When p < 0.05, it is significant. From Table 3, the linear model terms [sulfuric acid concentration (X 1), preparation temperature (X 2) and preparation time (X 3)] and the quadratic model term (X 21 , X 22 ) were highly significant to the response, whereas the interaction terms (X 1 X 2 and X 1 X 3) and the square term (X 23 ) were nonsignificant.
The coefficient of determination R 2 can evaluate the accuracy and variability of this model. A low value of the coefficient of variation (C.V.) (2.55%) indicated better precision and reliability of the experiments. A high R 2-adj. value is necessary to ensure a satisfactory adjustment of the predicted values and the experimental values. As shown in Fig. 5, the predicted values obtained from the model were close to the experimental values. The p value of the lack-of-fit was 0.0022, indicating that it is significant. This means that the model cannot explain the data well. However, the R 2 adj. value of this model is 98.06% (nearly 1), indicating a high degree of correlation between the predicted and observed data.
Analysis of response surface
The response surface plots and contour plots provide a method to visualize the relationship between the responses and the experimental values of each factor and the type of interactions between the two factors [23]. The effect on the response was higher along with decreasing p value parameter and increasing the F value coefficient. Based on Table 4, the most to least significant parameters were: X 1 > X 21 > X 2 > X 22 > X 3. Figures 6, 7, and 8 show the response surface and contour plots of the conversion of acetic acid, wihch represents a function between the conversion of acetic acid with sulfuric acid concentration, preparation temperature and preparation time.
As can be seen from Figs. 6 and 7, sulfuric acid concentration plays an important role in the conversion of acetic acid, with it being rapidly enhanced with the increase of sulfuric acid concentration and then decreasing; the best sulfuric acid concentration was 85%. The higher the sulfuric acid concentration, the more conducive to the sulfonation reaction; however, concentrated sulfuric acid might bring negative side effects of sulfone, which also decreases the introduction of sulfonic acid groups and reduces the catalyst activity. The result conforms to the conclusion worked by Groggins, who reported that, as the concentration of water decreases, the rate of sulfonation increases steadily [24].
Figures 6 and 8 show that, with the decrease of preparation temperature, the conversion of acetic acid increased steadily. The highest conversion of acetic acid was obtained when the temperature was at its minimum point within the range studied. The same result was obtained by Groggins. Like other chemical reactions, the rate of sulfonation is approximately doubled for each increase of 10 °C in temperature, but the rate of the hydrolysis reaction increased more quickly.
Figures 7 and 8 show that introducing –SO3H groups into carbon materials with chemical bonds to concentrate sulfuric acid needs a certain preparation time. The conversion of acetic acid increased with the increasing of the preparation time and then decreased; the best preparation time was 1.4 h. This indicated that the carbonization and sulfonation process is relatively fast and could be completed within 1.4 h. By further prolonging the preparation time to 3 h, the rate of hydrolysis reaction increased more quickly, which also decreased the introduction of sulfonic acid groups and subsequently reduced the catalyst activity.
The model during the present study was used to determine the optimal preparation conditions, and under this conditions the conversion of acetic acid was at its highest. The experimental results of three experiments under the same reaction conditions and the predicted values are given in Table 5.
Catalyst reusability
Catalyst reusability is an important factor in measuring the performance of a catalyst. In order to investigate the reusability of this catalyst, it has been recycled 5 times. The used catalyst was collected by filtration after the completion of the reaction, then washed with ethanol three times and dried at 80 °C for 5 h. It was then was used the next time in identical conditions. The catalytic activity decreased with more recycling. The conversion of acetic acid was reduced to 91.8% after the first recycling experiment and then drastically decreased from 91.8% for the second usage to 58.3% for the third usage. After the fifth usage of this catalyst, the conversion of acetic acid had decreased to 50.7%, thus the catalyst has a poor reusability. The sulfur content of the recycled catalyst was determined by elemental analysis to assess the acid density of the –SO3H, which can be seen in Table 6. The result shows the density of –SO3H which decreased from 0.89 to 0.29 mmol/g after recycling five times. The decrease in –SO3H observed here shows that the route of deactivation is through leaching of the –SO3H bearing small polycyclic compounds; a similar behavior was observed by Fraile et al. [25]. Nevertheless, the five times reused catalyst can be easily regenerated again by sulfonation, in which the acid density of –SO3H was 1.37 mmol /g and the conversion of acetic acid can reach 97.9%. The detailed process is as follows: with a ratio of catalyst to H2SO4 equal to 1 g:3 mL, 2.0 g of the deactivated catalyst and 6 mL sulfuric acid (85 wt%) were combined in a 50-mL round-bottom flask and heated in a water bath at 20 °C under air atmosphere. The mixture was kept mechanically stirred for 1.4 h.
Conclusion
In summary, a carbon-based solid acid catalyst derived from DG was prepared through simultaneous carbonization and sulfonation with concentrated sulfuric acid. A Box–Behnken design was implemented to optimize the sulfuric acid concentration, preparation temperature and preparation time. The optimal preparation conditions are as follows: sulfuric acid concentration of 85 wt%, preparation temperature of 20 °C, and preparation time of 1.4 h. Under these optimal conditions, the catalyst had a high density of SO3H (0.89 mmol/g), and the total acid density was highest (1.73 mmol/g). The catalyst prepared in the given conditions showed good catalytic performance in which the conversion of acetic acid can reach 97.6%. During the probe reaction, it can be found that the activity of this catalyst will be gradually lost along with recycling, but it can be easily regenerated by H2SO4.
References
M.J. Ramos, A. Casas, L. Rodríguez, R. Romero, Á. Pérez, Appl. Catal. A-Gen. 346, 79 (2008)
S. Wang, C. Li, Y. Wen, H. Wei, B. Li, X. Wang, Res. Chem. Intermed. 42, 8131 (2016)
Y.M. Park, J.Y. Lee, S.H. Chung, I.S. Park, S.Y. Lee, D.K. Kim, J.S. Lee, K.Y. Lee, Bioresour. Technol. 101, S59 (2010)
J.M. Hidalgo, D. Kaucký, O. Bortnovsky, Z. Sobalík, R. Černý, Res. Chem. Intermed. 41, 9425 (2015)
K. Suwannakarn, E. Lotero, J.G. Goodwin, C. Lu, J. Catal. 255, 279 (2008)
M.A. Harmer, Q. Sun, Appl. Catal. A-Gen. 221, 45 (2001)
M.R. Talukder, J.C. Wu, S.K. Lau, L.C. Shimin, A. Lim, Energ. Fuel. 23, 1 (2008)
A. Alsalme, E.F. Kozhevnikova, I.V. Kozhevnikov, Appl. Catal. A-Gen. 349, 170 (2008)
M. Toda, A. Takagaki, M. Okamura, J.N. Kondo, S. Hayashi, K. Doment, M. Hara, Nature 438, 178 (2005)
W.Y. Lou, M.H. Zong, Z.Q. Duan, Bioresour. Technol. 99, 8752 (2008)
L. Wang, X. Dong, H. Jiang, G. Li, M. Zhang, Bioresour. Technol. 158, 392 (2014)
J.R. Kastner, J. Miller, D.P. Geller, J. Locklin, L.H. Keith, T. Johnson, Catal. Today 190, 122 (2012)
X. Zhou, P. Zheng, Biotechnol. Lett. 35, 679 (2013)
M. Fardisi, L.J. Mason, K.E. Ileleji, D.S. Richmond, J. Stored Prod. Res. 66, 25 (2016)
A.K. Singh, K.P. Singh, Res. Chem. Intermed. 42, 2247 (2016)
M. Li, D. Chen, X. Zhu, Chin. J. Catal. 34, 1674 (2013)
Z. Hoseinabadi, S.A. Pourmousavi, M. Zamani, Res. Chem. Intermed. 42, 6105 (2016)
Y. Zhou, S. Niu, J. Li, Energ. Convers. Manag. 114, 188 (2016)
H.H. Mardhiah, H.C. Ong, H.H. Masjuki, S. Lim, Y.L. Pang, Energ. Convers. Manag. 144, 10 (2017)
X.L. Song, X.B. Fu, C.W. Zhang, W.Y. Huang, Y. Zhu, J. Yang, Y.M. Zhang, Catal. Lett. 142, 869 (2012)
Y.Y. Bai, L.P. Xiao, R.C. Sun, Cellulose 21, 2327 (2014)
A. Shokri, K. Mahanpoor, D. Soodbar, J. Environ. Chem. Eng. 4, 585 (2016)
O.D. Mante, F.A. Agblevor, R. McClung, Fuel 108, 451 (2013)
P.H. Groggins, Unit Processes in Organic Synthesis, 5th edn. (McGraw-Hill, New York, 1958), p. 350
J.M. Fraile, E. García-Bordejé, L. Roldán, J. Catal. 289, 73 (2012)
Acknowledgement
Financial support from National Natural Science Foundation of China (21277039) is acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Zhang, L., Sun, W. et al. One-step preparation of sulfonated carbon-based solid acid from distillers’ grain for esterification. Res Chem Intermed 43, 5917–5932 (2017). https://doi.org/10.1007/s11164-017-2971-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-2971-y