Abstract
The aim of this work is to introduce a novel and attractive protic acidic ionic liquid as catalyst for organic synthesis. To achieve this aim, N,N-diethyl-N-sulfoethanaminium hydrogen sulfate {[Et3N-SO3H]HSO4} was prepared by reaction of NEt3 with ClSO3H and then with H2SO4. The novel acidic ionic liquid was identified by Fourier-transform infrared (FT-IR), 1H nuclear magnetic resonance (NMR), 13C NMR, and mass spectroscopies. Its catalytic activity was then examined in the cross-aldol condensation reaction of arylaldehydes with cycloalkanones under solvent-free conditions, obtaining α,α′-bis(arylidene)cycloalkanones in high yield after short reaction time.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ionic liquids have been applied as useful alternatives to conventional solvents, catalysts or reagents in organic synthesis, due to their particular properties including: (1) chemical and thermal stability, (2) low vapor pressure, (3) controlled miscibility and polarity, (4) high boiling point, (5) nonflammability, (6) excellent solvent power for organic and inorganic compounds, and (7) ability to catalyze a wide range of organic reactions. Among them, protic acidic ionic liquids (AILs) have been extensively utilized as catalysts and reagents in organic synthesis [1–15]. This class of ionic liquids combine the advantages of both liquid and solid acids. Consequently, introduction of novel AILs to progress organic reactions is attractive and highly important.
It is now generally recognized that chemistry is one of the key sciences able to impact the environment in both a positive and negative fashion. Thus, there is growing awareness that design of synthetic or chemical processes should follow the basic principles of green chemistry to reduce risks to humans and the environment. Among several aspects of green chemistry, removal of volatile organic solvents from the reaction medium (i.e., use of solvent-free conditions) is of utmost importance. Furthermore, in many cases, solvent-free technique offers considerable synthetic advantages in terms of yield, selectivity, and simplicity of reaction procedure [16–21].
α,α′-Bis(arylidene)cycloalkanone derivatives have attracted much attention because of their use as precursors for synthesis of pyrimidine derivatives [22], interesting biological activities such as cholesterol-lowering [23], quinine reductase inducer [24], and antiparasitic [25] properties, and application as new organic materials in nonlinear optical systems [26], units of liquid-crystalline polymers [27], and cytotoxic analogues [28]. Cross-aldol condensation reaction between cycloalkanones and arylaldehydes has been used as the best synthetic route toward α,α′-bis(arylidene)cycloalkanones [29–37]. Several catalysts have been used to promote this transformation so far [29–37]. However, most of these methods are associated with one or more of the following disadvantages: (1) unwanted side-reactions, (2) need for application of sealed ampoules or tubes, (3) moderate yield, (4) long reaction time, (5) use of expensive, unavailable, and toxic catalysts, (6) application of additional energy (microwaves), (7) use of volatile organic solvents, (8) harsh conditions, and (9) poor agreement with green chemistry principles. Thus, the search for highly efficient catalysts for preparation of the mentioned compounds without the above-mentioned drawbacks remains important.
In this work, we synthesized a novel protic acidic ionic liquid, namely N,N-diethyl-N-sulfoethanaminium hydrogen sulfate {[Et3N-SO3H]HSO4}, and characterized it by studying its FT-IR, 1H NMR, 13C NMR, and mass spectra. We then applied the ionic liquid as a highly efficient, green, and homogeneous catalyst for cross-aldol condensation reaction between cycloalkanones and arylaldehydes in absence of solvent to afford α,α′-bis(arylidene)cycloalkanones.
Experimental
General
All chemicals were purchased from Merck or Fluka chemical companies. All known compounds were identified by comparison of their melting points and spectral data with those reported in literature. Reaction progress was monitored by thin-layer chromatography (TLC) using silica gel SIL G/UV 254 plates. 1H NMR (250 or 500 MHz) and 13C NMR (62.5 or 125 MHz) spectra were run on a Bruker Avance DPX FT-NMR spectrometer. Mass spectra were obtained with a Shimadzu GC–MS-QP 1100 EX model. Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
Procedure for preparation of ionic liquid [Et3N-SO3H]HSO4
A solution of triethylamine (0.50 g, 5 mmol) in CH2Cl2 (40 mL) was added dropwise to a stirring solution of chlorosulfonic acid (0.58 g, 5 mmol) in dry CH2Cl2 (40 mL) over a period of 10 min at 10 °C. Afterward, the reaction mixture was allowed to heat to room temperature (accompanied with stirring), and stirred for another 4 h. The solvent was evaporated, and the liquid residue was triturated with t-butyl methyl ether (3 × 10 mL) and dried under powerful vacuum at 90 °C to give [Et3N-SO3H]Cl as a viscous pale-yellow oil in 93 % yield [7]. Then, sulfuric acid (99.99 %, 0.49 g, 5 mmol) was added dropwise to [Et3N-SO3H]Cl (1.089 g, 5 mmol) over a period of 5 min at room temperature under pressure of nitrogen gas (to remove HCl produced during the reaction). The resulting mixture was stirred for 10 h at 60 °C under continuous flow of nitrogen gas to give [Et3N-SO3H]HSO4 as a viscous pale-yellow liquid in 99 % yield.
Spectral data of [Et3N-SO3H]HSO4
IR (Nujol): 583, 880, 1059, 1167, 1207, 2950, 2500–3500 cm−1. 1H NMR (250 MHz, DMSO-d6): δ (ppm) 1.42 (t, J = 7.5 Hz, 9H), 3.30 (q, J = 7.5 Hz, 6H), 8.43 (br, 1H), 11.59 (br, 1H). 13C NMR (62.5 MHz, DMSO-d6): δ (ppm) 8.3, 45.7. MS: m/z 280 (M++1), 279 (M+), 262 (M+–OH), 182 (M+–HSO −4 ), 101 (M+–[SO3H,HSO −4 ]).
General procedure for production of α,α′-bis(arylidene)cycloalkanones
A mixture of cycloalkanone (1 mmol), aldehyde (2.1 mmol), and [Et3N-SO3H]HSO4 (0.042 g, 0.15 mmol) in a test tube connected to a reflux condenser was stirred at 90 °C for the appropriate time (Table 2). After completion of the reaction, as monitored by TLC, the reaction mixture was cooled to room temperature, and recrystallized from EtOH (95 %) or by column chromatography on silica gel eluted with n-hexane/EtOAc (1/5) to give the pure product.
Selected spectral data of products
2,6-Bis(3-chlorobenzylidene)cyclohexanone (4)
1H NMR (500 MHz, CDCl3): δ (ppm) 1.69 (quintet, J = 5.7 Hz, 2H), 2.78 (t, J = 5.7 Hz, 4H), 7.30–7.37 (m, 6H), 7.41 (s, 2H), 7.62 (t, J = 1.2 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ (ppm) 23.7, 27.9, 126.3, 127.9, 129.3, 130.4, 130.9, 133.2, 135.8, 137.8, 188.9.
2,6-Bis(2-chlorobenzylidene)cyclohexanone (6)
1H NMR (500 MHz, CDCl3): δ (ppm) 1.75 (quintet, J = 5.8 Hz, 2H), 2.78 (t, J = 5.8 Hz, 4H), 7.28 (m, 4H), 7.34 (d, J = 3.4, 2H), 7.45 (d, J = 3.3 Hz, 2H), 7.89 (t, J = 1.2 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ (ppm) 23.2, 28.7, 126.8, 130.8, 130.9, 131.0, 134.5, 135.0, 135.5, 138.3, 189.2.
2,5-Bis(3-chlorobenzylidene)cyclopentanone (12)
1H NMR (500 MHz, CDCl3): δ (ppm) 3.01 (s, 4H), 7.29–7.33 (m, 4H), 7.39–7.42 (m, 2H), 7.43 (s, 2H), 7.51 (t, J = 1.2 Hz, 2H). 13C NMR (125 MHz, CDCl3): δ (ppm) 27.0, 128.6, 129.6, 130.5, 130.7, 132.5, 135.3, 137.1, 138.6, 196.0.
Results and discussion
Catalyst characterization
As mentioned in the “Introduction,” the introduction of novel protic acidic ionic liquids (AILs) to promote organic reactions is important. Thus, we decided to prepare a novel AIL, namely N,N-diethyl-N-sulfoethanaminium hydrogen sulfate {[Et3N-SO3H]HSO4}. To achieve this aim, NEt3 (1 eq.) was reacted with ClSO3H (1 eq.) to afford [Et3N-SO3H]Cl [7]. Then, [Et3N-SO3H]Cl (1 eq.) was reacted with H2SO4 (1 eq.) to provide [Et3N-SO3H] HSO4 as a viscous pale-yellow liquid (Scheme 1).
After production of the novel ionic liquid, it was characterized by FT-IR, 1H NMR, 13C NMR, and mass spectra.
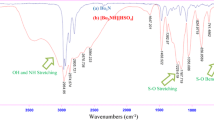
In the FT-IR spectrum of N,N-diethyl-N-sulfoethanaminium hydrogen sulfate, a broad peak observed at 2500–3500 cm−1 is related to OH of SO3H groups. The band at 1059 cm−1 was assigned to S–OH bending. The strong absorptions at 1207, 1167, and 583 cm−1 are related to asymmetric and symmetric stretching and bending S–O vibrations of SO3H and HSO −4 . Symmetric N–S stretching vibration was also observed at 880 cm−1. The peak at about 2950 cm−1 is assigned to stretching of hydrogens bonded to carbons.
In the 1H NMR spectrum of [Et3N-SO3H]HSO4 (Fig. 1), a triplet peak appearing at 1.42 ppm is related to nine hydrogens of three methyl groups. The quartet peak at 3.30 ppm corresponds to six hydrogens of three methylene groups. The two acidic hydrogens (HSO −4 and SO3H) of the AIL were observed at 8.43 and 11.59 ppm.
In the 13C NMR spectrum (Fig. 2), two peaks appearing at 8.3 and 45.7 ppm are related to carbons of methyl and methylene groups.
In the mass spectrum of the AIL (Fig. 3), its molecular mass (M+) and M++1 were observed at m/z = 279 and 280. The other peaks which assist in identification of [Et3N-SO3H]HSO4 are seen at 262 (M+–OH), 182 (M+-HSO −4 ), and 101 (M+-SO3H, HSO −4 ).
In another study, to prove that [Et3N-SO3H]Cl was completely converted to [Et3N-SO3H]HSO4, a solution of AgNO3 in distilled water was added to a solution of the AIL in distilled water. The absence of AgCl precipitate indicated complete conversion of [Et3N-SO3H]Cl to [Et3N-SO3H]HSO4.
Examination of catalytic activity of [Et3N-SO3H]HSO4 for synthesis of α,α′-bis(arylidene)cycloalkanones
After the AIL had been fully characterized, its catalytic activity to promote a significant organic transformation, i.e., synthesis of α,α′-bis(arylidene)cycloalkanones by condensation of arylaldehydes with cycloalkanones, was checked. First, the reaction of 3-nitrobenzaldehyde with cyclohexanone was carried out using different molar ratios of [Et3N-SO3H]HSO4 in the range of 80–100 °C; the corresponding results are summarized in Scheme 2 and Table 1, indicating that the best results were obtained when the reaction was carried out in the presence of 15 mol % AIL at 90 °C.
After the reaction had been optimized, the generality and effectiveness of the catalyst were explored by studying reactions of different arylaldehydes and cycloalkanones; the respective results are displayed in Table 2. As can be seen from this table, all the arylaldehydes (bearing halogens, and electron-withdrawing and electron-releasing substituents on their aromatic rings) afforded the desired α,α′-bis(arylidene)cycloalkanones in high to excellent yield and short reaction time. Thus, the ionic liquid is highly efficient and general for preparation of α,α′-bis(arylidene)cycloalkanone derivatives.
In another study, the regenerability and reusability of the catalyst were studied. For this purpose, the reaction of 3-nitrobenzaldehyde with cyclohexanone using [Et3N-SO3H]HSO4 was carried out several times, and the reaction mixtures were combined. Afterward, H2O was added to the combined reaction mixtures, followed by stirring for 3 min and filtering (the catalyst is soluble in H2O, whereas the reaction mixture is not). The filtrate (containing the catalyst) was basified by NaOH; under these conditions, [Et3N-SO3H]HSO4 was completely converted to Et3N and Na2SO4. Then, the solution was extracted by CH2Cl2, washed by H2O, and dried over Na2SO4. The recovered NEt3 in CH2Cl2 was reacted with chlorosulfonic acid, and then with H2SO4 according to the mentioned procedure to give [Et3N-SO3H]HSO4. The catalytic activity of the reproduced catalyst was the same as the first one.
Conclusions
We introduce a novel protic acidic ionic liquid as a highly efficient catalyst in organic synthesis by preparing [Et3N-SO3H]HSO4 and applying it as catalyst for synthesis of α,α′-bis(arylidene)cycloalkanones. The benefits of our new catalyst in this reaction include: (1) low cost of starting materials for catalyst preparation, (2) easy preparation of catalyst, (3) generality, effectiveness, novelty, and nontoxicity of catalyst, and (4) performing the reaction in high yield and short time under solvent-free conditions.
References
B. Kirchner (ed.), Ionic Liquids (Topics in Current Chemistry) (Springer, Berlin, 2010)
P. Wasserscheid, T. Welton, Ionic Liquids in Synthesis, 2nd edn. (Wiley, Weinheim, 2008)
H. Eshghi, M. Bakavoli, M. Ghasemzadeh, Res. Chem. Intermed. 41, 3999 (2015)
A. Hasaninejad, A. Zare, M. Shekouhy, J. Ameri Rad, J. Comb. Chem. 12, 844 (2010)
A.S. Kucherenko, D.E. Siyutkin, O.V. Maltsev, S.V. Kochetkov, S.G. Zlotin, Russ. Chem. Bull. 61, 313 (2012)
K. Zhuo, Q. Du, G. Bai, C. Wang, Y. Chen, J. Wang, Carbohydr. Polym. 115, 49 (2015)
A. Zare, A.R. Moosavi-Zare, M. Merajoddin, M.A. Zolfigol, T. Hekmat-Zadeh, A. Hasaninejad, A. Khazaei, M. Mokhlesi, V. Khakyzadeh, F. Derakhshan-Panah, M.H. Beyzavi, E. Rostami, A. Arghoon, R. Roohandeh, J. Mol. Liq. 167, 69 (2012)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, H.G. Kruger, Z. Asgari, V. Khakyzadeh, M. Kazem-Rostami, J. Org. Chem. 77, 3640 (2012)
S. Salahi, M.T. Maghsoodlou, N. Hazeri, F. Movahedifar, R. Doostmohammadi, M. Lashkari, Res. Chem. Intermed. 41, 6477 (2015)
A. Jamalian, B. Rathman, G.L. Borosky, K.K. Laali, Appl. Catal. A Gen. 486, 1 (2014)
A. Zare, T. Yousofi, A.R. Moosavi-Zare, RSC Adv. 2, 7988 (2012)
H. Naeimi, Z.S. Nazifi, C. R. Chim. 7, 41 (2014)
A.R. Hajipour, M. Karimzadeh, H. Tavallaei, J. Iran. Chem. Soc. 12, 987 (2015)
M.A. Zolfigol, A. Khazaei, A.R. Moosavi-Zare, A. Zare, V. Khakyzadeh, Appl. Catal. A Gen. 400, 70 (2011)
A.K. Rawat, S. Bhattacharya, S.M.S. Chauhan, Tetrahedron Lett. 55, 4537 (2014)
K. Tanaka, Solvent-Free Organic Synthesis (Wiley, Weinheim, 2009)
E.S. Putilova, G.V. Kryshtal, G.M. Zhdankina, N.A. Troitskii, S.G. Zlotin, Russ. J. Org. Chem. 41, 512 (2005)
A. Khazaei, M. Khazaei, S. Rahmati, J. Mol. Catal. A Chem. 398, 241 (2015)
H. Moghanian, A. Mobinikhaledi, M. Deinavizadeh, Res. Chem. Intermed. 41, 4387 (2015)
A. Zare, M. Merajoddin, A.R. Moosavi-Zare, M. Zarei, M.H. Beyzavi, M.A. Zolfigol, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2154-7
A.R. Moosavi-Zare, M.A. Zolfigol, S. Farahmand, A. Zare, A.R. Pourali, R. Ayazi-Nasrabadi, Synlett 25, 193 (2014)
J. Deli, Pharmazie 39, 539 (1984)
C. Piantadosi, I.H. Hall, J.L. Irvine, G.L. Carlson, J. Med. Chem. 16, 770 (1973)
A.T. Dinkova-Kostova, C. Abeygunawardana, P. Talalay, J. Med. Chem. 41, 5287 (1998)
S.F.P. Braga, É.V.P. Alves, R.S. Ferreira, J.R.B. Fradico, P.S. Lage, M.C. Duarte, T.G. Ribeiro, P.A.S. Júnior, A.J. Romanha, M.L. Tonini, M. Steindel, E.F. Coelho, R.B. de Oliveira, Eur. J. Med. Chem. 71, 282 (2014)
J. Kawamata, K. Inoue, T. Inabe, M. Kiguchi, M. Kato, Y. Taniguchi, Chem. Phys. Lett. 249, 29 (1996)
K. Gangadhara, K. Kaushal, Polymer 36, 1903 (1995)
J.R.A. Dimmock, M.P. Padmanilayam, G. Zello, K.H. Nienaber, T.M. Allen, C.L. Santos, E. De Clercq, J. Balzarini, E.K. Manavathu, J.P. Stables, Eur. J. Med. Chem. 38, 169 (2003)
A. Amoozadeh, E. Tabrizian, S. Rahmani, C. R. Chim. 18, 848 (2015)
E. Tabrizian, A. Amoozadeh, S. Rahmani, M. Salehi, M. Kubicki, Res. Chem. Intermed. (2015). doi:10.1007/s11164-015-2039-9
A. Zare, M. Merajoddin, A. Hasaninejad, A.R. Moosavi-Zare, V. Khakyzadeh, C. R. Chim. 16, 380 (2013)
A. Solhy, W. Amer, M. Karkouri, R. Tahir, A. El Bouari, A. Fihri, M. Bousmina, M. Zahouily, J. Mol. Catal. A Chem. 336, 8 (2011)
B. Das, P. Thirupathi, I. Mahender, K.R. Reddy, J. Mol. Catal. A Chem. 247, 182 (2006)
A. Lahyani, M. Chtourou, M.H. Frikha, M. Trabelsi, Ultrason. Sonochem. 20, 1296 (2013)
N. Iranpoor, F. Kazemi, Tetrahedron 54, 9475 (1998)
M.A. Bigdeli, G.H. Mahdavinia, S. Jafari, H. Hazarkhani, Catal. Commun. 8, 2229 (2007)
L.-T. An, J.-P. Zou, L.-L. Zhang, Catal. Commun. 9, 349 (2008)
Acknowledgments
The authors thank the Research Council of Payame Noor University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ahmadi, S., Zare, A., Aali-Hosaini, M. et al. Design, characterization, and use of N,N-diethyl-N-sulfoethanaminium hydrogen sulfate {[Et3N-SO3H]HSO4} as a novel and highly efficient catalyst for preparation of α,α′-bis(arylidene)cycloalkanones. Res Chem Intermed 42, 6245–6253 (2016). https://doi.org/10.1007/s11164-016-2458-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2458-2