Abstract
In this study, N-vinylcaprolactam, metacrylic acid sodium salt and itaconic acid sodium salt-based copolymeric and terpolymeric microgels were synthesized by precipitation polymerization method with 2,2′-azobis(2-methylpropioamidine) dihydrochloride as initiator. Then these microgels were characterized by SEM technique, cloud points and colloidal properties determinations. Volume phase transitions of copolymeric and terpolymeric N-vinylcaprolactam-based microgels are determined at an interval of 32–37 °C. Rhodamine B (model drug) and Nadalol (beta-blocker drug) were used to investigate the drug loading and release behavior of microgels. It is concluded that model drug loading capacity and release amount changed with the presence and amount of itaconic acid sodium salt in the microgel structure. In addition, the maximum drug release amount of microgels was found to be 58 and 55 % for Rhodamine B and Nadolol, respectively. As a result, we can say that the microgels obtained in this study are suitable for drug delivery applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are three-dimensional polymeric networks that swell in aqueous solutions but don’t dissolve in water [1, 2]. Stimuli-responsive hydrogels undergo a volume phase transition in response to environmental changes such as pH, temperature, light, electric field, and ions [3, 4]. These materials have attracted very strong interest in the past 25 years. The major disadvantage of macroscopic hydrogels is the slow response to external stimuli. In recent years, this problem has been overcome by the use of microgels [4]. Average diameter range of microgels can be assumed to be between 50 nm and 5 µm [5]. A microgel particle is cross-linked polymer colloid particle that swells in a suitable solvent [6, 7]. The choice of the solvent is determined by the chemical structure of the microgel network. For example, aqueous microgel particles are hydrophilic polymers [8]. Aqueous microgels can be used in different areas, such as coatings, agriculture, medicines, cosmetics, foods, etc. [5, 9]. Stimuli-responsive microgels are widely used in biomedical and biotechnological fields. The swelling and shrinking behavior of a microgel is affected by various external stimuli such as temperature, pH, ionic strength, magnetic field, electric field and light [10].
Thermo-responsive microgels are widely used drug delivery systems [11]. In recent years, many researchers have studied poly(N-isopropylacrylamide) (PNIPAM) microgels and copolymeric N-isopropylacrylamide (NIPAM) microgels as thermo-responsive polymer for drug delivery systems [12, 13]. However, the usage of NIPAM-based microgels is limited due to the toxic properties of these microgels [11]. Another polymeric microgel used in drug delivery systems is poly(N-vinyl-caprolactam) (PVCL). PVCL-based microgels show thermo-responsive behavior in aqueous solutions, and PVCL is a biocompatible polymer that has a volume phase transition temperature (VPTT) of 32–34 °C [8, 14, 15]. VPTT values of N-vinyl-caprolactam (VCL) copolymers shift to higher values depending on co-monomer ratio [15], and these microgels exhibit VPTT values close to human body temperature.
In recent years, extensive interest in VCL-based copolymeric microgels has been displayed, because of their potential use for drug delivery systems. Wang and his co-workers reported the synthesis of VCL-based biodegradable copolymeric microgels for drug delivery applications. In this study, biodegradable microgels were prepared via precipitation polymerization technique, where N,N-bis(acrylol) cystamine was used as crosslinking agent, and methacrylic acid (MAA) and polyethylene glycol methyl ether methacrylate were used as comonomers. Doxorubicin (DOX) was chosen as a model drug to investigate the drug loading and release behavior of copolymeric microgels [11]. Shah and co-workers described the preparation of temperature-sensitive copolymeric nanoparticles of poly(methyl metacrylate co-N-vinylcaprolactam). In this work, new copolymeric nanoparticles were synthesized by microemulsion polymerization [16]. Rejinold et al. [17] reported the preparation of curcimin-loaded thermo-responsive chitosan-graft-PVCL nanoparticles for cancer drug delivery. Berger et al. were synthesized poly(N-vinyl caprolactam-co-acetoacetoxyethyl metacrylate) microgel/clay nanohybrides in their study. The clay used in this research was synthetic laponite, and microgel-clay composites containing different amount of clay were synthesized. The microgel-clay hybride particles were used for the uptake of methylene blue for the aqueous solutions [9]. Boyko and co-workers investigated the synthesis and characterization of poly(N-vinylcaprolactam-co-acetoacetoxyethyl methacrylate) microgels. In this work, polymeric microgels were prepared by surfactant-free emulsion polymerization of VCL and acetoacetoxyethyl methacrylate in water with 2,2′-azobis(2-methylpropioamidine) dihydrochloride (AMPA) as azo-initiator [18].
Thorne and co-workers summarized microgel syntheses and applications up to 2011 in a review study [19]. NIPAM/BuA/AA microgels and l-Lactic acid/d-lactic acid microgels were used in pH-controlled oral insulin drug delivery [20] and protein drug delivery [21] applications, respectively.
In this study, we synthesized poly(N-vinylcaprolactam-co-metacrylic acid sodium salt) [p(VCL-co-MMANa)], poly(N-vinylcaprolactam-co-itaconic acid sodium salt) [p(VCL-co-IANa)] copolymeric microgels and poly(N-vinylcaprolactam-co-metacrylic acid sodium salt-co-itaconic acid sodium salt) [p(VCL-co-MMANa-co-IANa)] terpolymeric microgel using precipitation polymerization method with AMPA as initiator. Rhodamine B (RhB) (model drug) and Nadalol (beta-blocker drug) were used to investigate the drug loading and release behavior of the microgels.
Experimental
Materials
VCL was purchased from Aldrich (USA) and recrystallized from hexane. The comonomers, itaconic acid (IA) and methacrylic acid (MAA), were obtained from Merck (Germany). IA and MAA were treated with 1 mol L−1 NaOH solution for preparing IA sodium salt (IANa) and MAA sodium salt (MAANa). The crosslinking agent, N,N′-methylenebisacrylamide (NMBA); and the water soluble initiator, AMPA, were purchased from Merck (Germany). RhB and Nadolol were obtained from Merck (Germany) and Sigma (USA), respectively. Their chemical structures are shown in Fig. 1. All solutions and standards were prepared using deionized water.
Synthesis of microgels
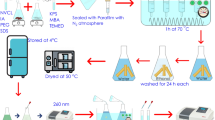
The synthesis of microgels was carried out under nitrogen atmosphere in a 250-mL round bottom glass reactor equipped with stirrer and reflux condenser. Appropriate amounts of VCL, IANa and MAANa monomers, NMBA as crosslinking agent and deionized water (total concentration 0.1 mol L−1) were added to the reactor. The reaction mixture was stirred for 45 min at 80 °C under nitrogen atmosphere. After that, AMPA was added as initiator and the reaction was carried out for 10 h at 80 °C. Symbols and synthesis conditions of VCL-based copolymeric and terpolymeric microgels were given in Table 1. Microgels were cleaned by dialysis method against water using a dialysis tube (sigma-aldrich D04105) to remove unreacted monomers and impurities. Dialysis of microgel dispersions was carried out for 7 days. The product was then dried by Telstar Lyo Quest freeze-dryer.
Particle size analysis
The zeta potential, particle size and polydispersity index (PDI) analyses of microgels were measured in deionized water at 25 °C with a Zetasizer Nano Series (Malvern Instruments, England) instrument.
Cloud point measurements
The transmittance of microgel dispersions was measured UV–visible spectrophotometer (PG Instruments, T80) at 672 nm. The intensity of the transmitted light decreased sharply when temperature passed through the cloud point. Turbidimetric measurements were performed from 26 to 40 °C with 1 °C increments.
SEM analysis
The scanning electron microscopy (SEM) micrographs of the microgels were obtained using a Quanta FEG 450 Scanning Electron Microscope (USA).
Drug loading release experiments
RhB and nadolol were chosen as a model drug and as a drug to investigate the drug loading and drug release behavior of microgels, respectively. The model drug loading experiments were carried out at different pH values (2.1, 5.5, 7.2) at room temperature using 500 mg L−1 RhB aqueous solution during a 48-h period. The drug loading experiments were performed in pH 7.2 buffer solution under the same conditions. The concentration of microgel-drug dispersions used in drug loading experiments was 0.01 mg microgel per mL RhB or Nadolol. The drug release study was also performed in pH 7.2 buffer solution at 37 °C. For this aim, model drug RhB and beta blocker drug (nadolol) loaded microgels were dispersed in buffer solution and placed in a centrifuge tube. These samples were centrifuged at 12,000 rpm for 20 min using Hettich Universal 320 R centrifuge. Then the amounts of released Nadolol and RhB in the supernatant were determined with PG Instruments T80 UV–Vis spectrophotometer at 271 and 554 nm, respectively.
Results and discussion
VCL-based microgels were synthesized by precipitation polymerization method with AMPA as initiator in this study. Synthesis conditions of these microgels are given in Table 1. SEM analyses, cloud point and colloidal properties (zeta potential and particle size) determinations were used for characterization of microgels. Drug loading and release behavior of microgels were investigated by using RhB (model drug) and Nadalol (beta-blocker drug).
Cloud points of microgels
PVCL is a thermo-responsive and biocompatible polymer that has a VPTT of 32–34 °C [14]. In this study, we synthesized VCL-based copolymeric and terpolymeric microgels. Turbidimetric method (cloud point measurement) was used to determine the VPTT of microgels. The cloud points of microgels were determined using transmittance (%) versus temperature plots. As shown in Figs. 2, 3 and 4, volume phase transitions of M-1, M-2 and M-3 microgels were determined in range of 32–36, 32–37 and 34–37 °C, respectively. As is seen, the use of hydrophilic co-monomers such as MAANa and IANa in the synthesis of PVCL-based microgels caused a small increase of the VPTT value of PVCL. The hydrophilicity of polymer chain increased when MAANa and IANa contents increased; thus, the balance of repulsive and hydrophobic force would be broken at higher temperature, resulting in a higher VPTT [11, 22]. Depending on these results, we can say that VPTT values of copolymeric and terpolymeric VCL-based microgels are in the region of physiological temperature (32–38 °C) [8].
Colloidal (particle size, surface charge, colloidal stability) and morphologic properties of microgels
Microgels are polymeric colloidal particles with a particle size range of 1 nm–1 µm [12]. The ionic microgels are prepared using at least one comonomer such as AA, MAA, IA [6]. In this study, colloidal properties and morphology of the microgels were determined with a Zetasizer Nano Series instrument and Scanning Electron Microscopy, respectively. Zeta potential, particle size and PDI values of microgels are presented in Table 2. The zeta potential changed from −5.1 mV for M-1 copolymeric microgel to −10.7 mV for M-2 copolymeric microgel. The zeta potential of M-3 terpolymeric microgel is very close to the zeta potential of M-2. This situation can be explained as the use of IANa instead of MAANa has increased negative charge density on the microgel. PDI values of microgels are between 0.28 and 0.38. Particle size of microgels changed from 612 to 255 nm. The microgels were also investigated with SEM technique and the micrographs were taken at different magnifications. Since the micrographs of all microgels were almost the same, only the micrograph of M-1 was given in the manuscript (Fig. 5). As a result, particle sizes and SEM micrographs of microgels that are compatible with microgels have been given in the literature in terms of size and shape.
Drug loading and release
In this section, both model drug and drug loading and release experiment results are presented. In vitro model drug loading and release experiments used RhB as a model drug. These experiments were performed at different pH values (7.2, 5.5 and 2.1) in order to determine the effect of pH on drug loading and release. The results of RhB loading and release experiments are given in Figs. 6 and 7, respectively. As seen from the figures, the highest loading capacity and release amount were obtained at pH 7.2. At this pH value, maximum model drug loading and release amounts for M-1 were found to be 92 and 60 mg g−1, respectively. Eventually, all in vitro model drug loading and release experiments were performed at pH 7.2.
Time-dependent model drug loading and release graphics for all microgels are given in Figs. 8 and 9, respectively. The order of loading and release values of the microgels was found to be: M-2 > M-3 > M-1. Model drug loading capacity and release amount changed with the presence and amount of IANa in the microgel structure. RhB loading capacities of M-1 (MAANa/IANa: 2/0), M-2 (MAANa/IANa: 0/2), M-3 (MAANa/IANa: 1/1), were found to be 92, 163 and 145 mg g−1, respectively. Similarly, RhB release amounts of M-1, M-2 and M-3 were also found to be 60, 94 and 87 mg g−1, respectively. In the case of using only IANa (M-2) in microgel synthesis, the highest model drug loading capacities and release amounts were obtained. In the case of same feeding compositions of MAANa and IANa (M-3), these values decreased in the range of 8–10 %. In the case of absence of IANa (M-1), the lowest model drug loading capacities and release amounts were obtained. This change can be originating from the presence of two carboxyl group in IANa structure. Electrostatic interaction between RhB and microgel network increased with an increase in the hydrophilic group amount of microgels. Therefore, this situation caused an increase in model drug loading capacities and release amounts.
The beta- blocker Nadolol was used as the drug in in vitro drug loading and release experiments. These experiments were carried out for the M-2 sample. Sample selection was determined according to the highest model drug loading and release experiment results. Drug loading capacity of M-2 microgel was found to be 70 mg g−1. A time-dependent model drug release graphic for M-2 microgel is given in Fig. 10. As seen from figure, the maximum drug release amount of M-2 microgel was 38 mg g−1.
For the purpose of comparison, the results of our study and reported drug release in the literature for different polymeric microgels for drugs/model drugs are given in Table 3. As can be seen from the Table, drug release experiments were carried out depending on the type of drug and microgel. The highest observed values in the drug release experiments for each study are presented in Table 3. These data can be interpreted as that the drug release values of VCL-based copolymeric microgels synthesized in this study were acceptable, compatible, and comparable with the results of other microgels that have been given in the literature [11, 22–25].
Conclusion
In this study, p(VCL-co-MMANa), p(VCL-co-IANa), p(VCL-co-MMANa-co-IANa) copolymeric and terpolymeric microgels were synthesized using precipitation polymerization method. Then, these microgels were characterized by cloud points determination, colloidal properties determination and SEM technique. In addition, Rhodamine B (RhB) (model drug) and Nadolol (beta-blocker, drug) were used to investigate the drug loading and release behaviors of the microgels. The following conclusions can be drawn from the obtained results: volume phase transitions of copolymeric and terpolymeric VCL-based microgels are determined at an interval of 32–37 °C according to cloud point experiments. Particle size analysis indicated that stable colloidal dispersions were successfully obtained. SEM analysis has also shown the formation of microgel. The order of RhB loading and release values of the microgels was found to be in the following order: M-2 > M-3 > M-1. Increase of model drug loading capacities and release amounts can arise from electrostatic interactions between RhB and microgel network increasing with increase in hydrophilic group amount of microgels. In addition, maximum nadolol release amount of M-2 microgel was 38 mg g−1. As a result, we can say that the microgels obtained in this study are suitable for drug delivery applications.
References
S. Chaterji, I.K. Kwon, K. Park, Prog. Polym. Sci. 32, 1083–1122 (2007)
J. Kopecek, Nature 417, 388–391 (2002)
S. Zhou, B. Chu, J. Phys. Chem. B 102, 1364–1371 (1998)
I. Varga, T. Gilanyi, R. Meszaros, G. Filipcsei, M. Zrinyi, J. Phys. Chem. B 105, 9071–9076 (2001)
R. Pelton, Adv. Colloid Interface Sci. 85, 1–33 (2000)
B.R. Saunders, N. Laajam, E. Daly, S. Teow, X. Hu, R. Stepto, Adv. Colloid Interface Sci. 147–148, 251–262 (2009)
B.R. Saunders, B. Vincent, Adv. Colloid Interface Sci. 80, 1–25 (1999)
A. Imaz, J. Forcada, J. Polym. Sci., Part A: Polym. Chem. 46, 2766–2772 (2008)
S. Berger, R. Singh, J.D. Sudha, H.J. Adler, A. Pich, Polymer 51, 3829–3835 (2010)
X.J. Ju, L. Liu, R. Xie, C.H. Niu, L.Y. Chu, Polymer 50, 922–929 (2009)
Y. Wang, J. Nie, B. Chang, Y. Sun, W. Yang, Biomacromolecules 14, 3034–3046 (2013)
V.C. Lopez, S.L. Raghavan, M.J. Snowden, React. Funct. Polym. 58, 175–185 (2004)
V.C. Lopez, J. Hadgraft, M.J. Snowden, Int. J. Pharm. 292, 137–147 (2005)
D. Crespy, R.M. Rossi, Polym. Int. 56, 1461–1468 (2007)
N.I. Shtanko, W. Lequieu, E.J. Goethals, F.E. Prez, Polym. Int. 52, 1605–1610 (2003)
S. Shah, A. Pal, R. Gude, S. Devi, Eur. Polym. J. 46, 958–967 (2010)
N.S. Rejinold, M. Muthunarayanan, V.V. Divyarani, P.R. Sreerekha, K.P. Chennazhi, S.V. Nair, J. Colloid Interface Sci. 360, 39–51 (2011)
V. Boyko, A. Pich, Y. Lu, S. Richter, K.F. Arndt, H.J.P. Adler, Polymer 44, 7821–7827 (2003)
J.B. Thorne, G.J. Vine, M.J. Snowden, Colloid Polym. Sci. 289, 625–646 (2011)
C. Ramkissoon-Ganorkar, F. Liu, M. Baudys, S.W. Kim, J. Controlled Release 59, 287–298 (1999)
Y. Zhang, W. Zhu, B. Wang, J. Ding, J Control Release 105, 260–268 (2005)
F. Meeussen, E. Nies, H. Berghmans, S. Verbrugghe, E. Guethals, F. Du Prez, Polymer 41, 8597–8602 (2000)
Z. Nart, N. Kayaman Apohan, J. Polym. Res. 18, 869–874 (2011)
J.P.K. Tan, C.H. Goh, K.C. Tam, Eur. J. Pharm. Sci. 32, 340–348 (2007)
S. Lou, S. Gao, W. Wang, M. Zhang, Q. Zhang, C. Wang, C. Li, D. Kong, J. Appl. Polym. Sci. 131, 1–7 (2014). doi:10.1002/app.41146
H. Vihola, A. Laukkanen, J. Hirvonen, H. Tenhu, Eur. J. Pharm. Sci. 16, 69–74 (2002)
Acknowledgments
This work is a part of the Ph.D. thesis titled “Usage of Polymeric Hydrogels and Microgels in Drug Release Applications”, prepared at Istanbul University, Institute of Science, and supported by the Research Fund of the Istanbul University, Project Number: 29693.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Özkahraman, B., Acar, I., Gök, M.K. et al. N-vinylcaprolactam-based microgels: synthesis, characterization and drug release applications. Res Chem Intermed 42, 6013–6024 (2016). https://doi.org/10.1007/s11164-016-2422-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2422-1