Abstract
FK506, a widely used immunosuppressant, is produced by industrial fermentation using Streptomyces tsukubaensis. In this study, a novel precursor, salicyl alcohol, was added and provided significant improvement in FK506 production. Furthermore, differences of the expression of the structural genes between the two media were also investigated. The results showed that the expression of key genes in enriched medium was much higher than that in the control. Compared with the non-enriched medium, the enriched medium caused 52.25-% enhancement of FK506 production. Salicyl alcohol could play a crucial role in the transcription of FK506 structural genes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
FK506 (tacrolimus), produced by Streptomyces tsukubaensis, is a 23-member macrolide with a hemiketal-masked α,β-diketoamide function (Fig. 1a) [1, 2]. It is a potent immunosuppressant widely used for preventing rejection of transplanted organs, Rh hemolytic disease of newborns and the treatment of autoimmune diseases, such as inflammatory skin diseases and eczema [3]. It has already served as neuroprotective and neuroregenerative agents [4]. FK506 and cyclosporine have shown similar mechanisms of action via inhibiting calcineurin phosphatase and, thereby, reducing T cell-mediated immune response by decreasing the production of interleukin-2 [5]. However, in clinical settings, the immunosuppressant FK506 is advantageous over cyclosporine because the effect of FK506 was 10–100 times more potent than cyclosporine, and FK506 is less toxic [6–8].
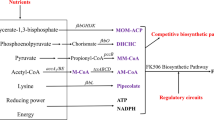
FK506 was first isolated by Fujisawa Healthcare Co. from fermentation of S. tsukubaensis in Japanese soil sample [1, 9]. There is minimal value in synthetic production of FK506 due to its low efficiency, high cost, unprofitability and other reasons. Therefore, scientists have great interested in improving FK506 production through development of the bioprocess. The biosynthetic pathway of FK506 production involves a hybrid polyketide synthase (PKS)-nonribosomal peptide synthetase (NRPS) system. The gene fkbO encoding a chorismate hydrolase is responsible for the biosynthesis of 4,5-dihydroxycyclohex-1-enecarboxylic acid (DHCHC), the starter unit for biosynthesizing FK506. Then DHCHC and ten extender units, named two malonyl-CoA, two methoxymalonyl-ACP, five methylmalonyl-CoA, and a specific allylmalonyl-CoA are incorporated into an incipient polyketide chain [10]. Then, the next steps, resulting in the nascent macrolactone intermediate of FK506 (preFK506), were the incorporation of lysine-derived pipecolic acid and a cyclization step, mediated by the NRPS and FK506-binding protein (fkbP). Eventually, post-PKS processing reactions were catalyzed by a specific methyl transferase and oxido-reductase leading to FK506 [11–13]. Increasing the production of FK506 is mainly focused on optimization of the fermentation conditions [14]. On one hand, changing the media and culture conditions to find out the significant effects on enhancing the biosynthesis of FK506 [15, 16]. On the other hand, construction of efficient engineered strains by genetic engineering are also applied to improve the yield of FK506 [17–19]. Furthermore, adding precursors of FK506 biosynthesis seems to be another way to enhance the output of FK506. Turlo and Gajzlerska found that it was an effective way to boost FK506 production by adding piperidinecarboxylic moiety analogues [2]. It is also reported that adding vinyl propionate, which is used as a precursor for converting propionyl-CoA to methylmalonyl-CoA, can significantly increased the production of FK506 of S. sp. RM7011 [20].
The aim of our research was to optimize the culture medium composition for the purpose of enhancing FK506 production in S. tsukubaensis No. 9993. In this study, we found a novel and effective precursor, o-hydroxybenzyl alcohol (salicyl alcohol; Fig. 1b), which could effectively boost the FK506 biosynthesis. In addition, the expression of fkbO and fkbP genes in enriched medium were higher than those in non-enriched medium.
Materials and methods
Microorganism, medium and cultivation conditions
The strain S. tsukubaensis No. 9993, which produced FK506, was taken from our laboratory and was deposited as No. FERM BP-927. The strain was grown on an agar plate containing 20 g/L of oatmeal, 2 g/L of sucrose and 10 g/L of agar at 28 °C in an incubator for 10 days. The seed medium (g/L) was: glucose 10, yeast extract 5, glycerol 10, corn steep liquor 5, soluble starch 15, CaCO3 2, pH 7.0. The fermentation medium (g/L) was: glucose 6, yeast extract 1.5, soybean powder 5, peanut meal 12, corn starch 30, K2HPO4 5. Fermentation was processed at 28 °C and 220 r/min for 7 days. In a 250 mL flask, 100 µL of spore suspension was inoculated into 30 mL of seed medium and cultured for 24 h. Then, 500 µL of seed solution was transferred into 25 mL of fermentation medium in a 250-mL flask and cultured for 7 days. Each condition was carried out for three replicates.
In the series of experiments, the culture mediums were enriched with salicyl alcohol, which acted as an FK506 precursor. The concentrations of the compound were 0, 0.0375, 0.0750, 0.1500, 0.2250, and 0.3000 g/L. Media were sterilized at 121 °C for 30 min, cooled to room temperature, then inoculated with 2 % (v/v) of the seed culture.
Analytical methods
Biomass was determined as reported [21]. The mycelia were centrifuged at 12,000 rpm for 10 min, washed three times by deionized water, centrifuged and dried at 80 °C for 24 h in desiccators to measure the dry weight. 3 mL of fermentation was transferred into a 10-mL centrifugal tube containing 2-mL methanol. After 20 min shaking at room temperature, the methanol extract was centrifuged for 20 min at 3000 rpm, and then the supernatant was collected for high-performance liquid chromatography (HPLC) equipped with a Zorbax SB-C18 packing (4.6 × 150 nm, 5 µm; Agilent, USA). The mobile phase involved 0.1-% H3PO4:acetonitrile = 32:68 (v/v) with a flow rate of 1 mL/min. The column temperature was 50 °C, and the detection wavelength was 205 nm [22]. The total sugar was determined by the dinitrosalicylic acid (DNS) method [23].
Real time-quantitative polymerase chain reaction (RT-PCR) analysis
The transcription level of fkbO and fkbP were assayed by RT-qPCR; Bio-Rad CFX 96) using SYBR Green Two-Step RT-qPCR SuperMix (Takara). The samples were isolated from the enriched medium and non-enriched medium at 72, 96, 120, 144 and 156 h. Total RNA was extracted by an RNAprep Pure Cell/Bacteria Kit (Tiangen Biotech, Beijing); then, cDNA was synthesized via reverse transcription. The sequences of primers are listed in Table 1.
Statistical analysis
The data are presented as the mean ± standard deviation (SD). The significance of paired data was determined by a student’s t-test. Data with more than two groups were analyzed by an analysis of variance (ANOVA) followed by the post-hoc Dunnett’s multiple comparison test. Differences were considered significant at *p < 0.05, **p < 0.01.
Results and discussion
Effect of salicyl alcohol on FK506 production
The production of FK506 in the medium was detected after 7 days of cultivation in the medium cultivated with enriched medium. The results were compared with the non-enriched culture medium (control). The output of FK506 in the medium containing salicyl alcohol was significantly higher than the control and the yield was enhanced by increasing the concentration of salicyl alcohol. The optimum concentration was 0.225 g/L, which provided a 49-% increase compared to that of the control (Fig. 2a). Under the optimum concentration, 0.225 g/L of salicyl alcohol, the yield of FK506 (394.02 µg/mL) was significantly improved when salicyl alcohol tested as a precursor of FK506 was added during early fermentation (0 h), higher than any other periods (Fig. 2b).
Effect of enriched medium on cell metabolism and FK506 production
The biomass accumulation in the non-enriched medium exceeded the enriched medium during any fermentation period, indicating that salicyl alcohol could inhibit cell growth (Fig. 3a). The rate of total sugar consumption was faster than that of the non-enriched medium in the prophase of fermentation. Subsequently, both of these two conditions had the similar sugar consumption rate. However, the consumption rate with enriched medium accelerated at the end of the stationary phase (144–168 h). In the prophase stage of fermentation, the strain growth in the enriched medium was slower than that of the control, but the total sugar consumption rate was faster (Fig. 3b); this indicated salicyl alcohol might act as a precursor for FK506 biosynthesis instead of a growth promoter. This was also observed in other studies on the abduction of erythromycin by n-propanol [24]. The results also showed that salicyl alcohol prolonged the stationary phase of S. tsukubaensis, which induced intermediate metabolites to synthesize FK506 during a later period of fermentation. The differences in pH are showed in Fig. 3c; the pH in the enriched medium was lower with more stabilization than that of the control, reflecting that FK506 biosynthesis might be favorable in low-pH conditions. In addition, pH stabilization is also of benefit for FK506 biosynthesis [25].
Response to structural gene transcription in the FK506 biosynthesis pathway
The transcription levels of FK506 biosynthesis structural genes, tcsABCD, fkbGHIK, fkbL, fkbO, fkbP, fkbABC, and fkbDM, were investigated in this section. Chorismatase (FkbO) was encoded by fkbO, which was the rate-limiting enzyme in the first step of FK506 biosynthesis [26]. Therefore, transcription of the key gene fkbO could be regarded as a signal of FK506 overproduction. The NRPS FkbP was responsible for extending the set linear polyketide intermediate by an unnatural amino acid residue, L-pipecolate, then generated 9-deoxo-31-O-demethyl-FK506 (preFK506) through intramolecular lactonization for further tailoring [12, 13]. To calculate the relative gene expression, RT-qPCR analysis was performed. The results showed that salicyl alcohol had a remarkable effect on gene transcription related to FK506 biosynthesis. In this study, the transcription level of fkbO was higher than the control before 120 h, which indicated a large number of precursors were supplied for FK506 biosynthesis during the fermentation process. Generally, the response of production was later than that of transcription [26]. Research found the maximum fkbO transcriptional level was reached at 96 h, while the yield of FK506 was increasing (Figs. 3d, 4). It was found that enhancement of fkbP expression occurred during secondary metabolite stimulation, which corresponded to FK506 production in the stationary phase. In early fermentation, the main goal was to accumulate the precursors for FK506 biosynthesis; hence, fkbP, which for biosynthesized FK506, was higher after 96 h. The more the expression of fkbP, the more the linear polyketide chain is cyclized; additionally, fkbP expression can enhance secondary metabolite synthesis, thereby increasing FK506 production.
Conclusions
In this study, we covered an approach to enhance FK506 production by adding salicyl alcohol as a precursor. The biosynthesis of FK506 was improved significantly, and reached yields 52.2 % higher (423.8 µg/mL) than that of non-enriched medium (278.5 µg/mL). The results suggest that salicyl alcohol could play an important role in the transcriptions of fkbP and fkbO. It has great potential for industrial fermentation of FK506, increasing production to meet market demands.
References
T. Kino, H. Hatanaka, M. Hashimoto, M. Nishiyama, T. Goto, M. Okuhara et al., FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antibiot. (Tokyo) 40(9), 1249–1255 (1987)
J. Turło, W. Gajzlerska, M. Klimaszewska, M. Król, M. Dawidowski et al., Enhancement of tacrolimus productivity in Streptomyces tsukubaensis by the use of novel precursors for biosynthesis. Enzyme Microb. Technol. 51(6), 388–395 (2012)
A.W. Thomson, P.B. Carroll, J. McCauley, J. Woo, K. Abu-Elmagd, T.E. Starzl et al., FK 506: a novel immunosuppressant for treatment of autoimmune disease. Springer Semin. Immunopathol. 14(4), 323–344 (1993)
B.G. Gold, Neuroimmunophilin ligands: evaluation of their therapeutic potential for the treatment of neurological disorders. Expert Opin. Investig. Drugs 9(10), 2331–2342 (2000)
J. Liu, J.D. Farmer Jr, W.S. Lane, J. Friedman, I. Weissman, S.L. Schreiber, Calcineurin is a common target of cyclophilin-cyclosporin a and FKBP-FK506 complexes. Cell 66(4), 807–815 (1991)
J.D. Pirsch, J. Miller, M.H. Deierhoi, F. Vincenti, R.S. Filo, A comparison of tacrolimus (FK-506) and cyclosporine for immunosuppression after cadaveric renal transplantation. FK506 kidney transplant study group. Transplant. 63(7), 977–983 (1997)
H. Jiang, M. Kobayashi, Differences between cyclosporin A and tacrolimus in organ transplantation. Transplant. Proc. 31(5), 1978–1980 (1999)
D. Kelly, P. Jara, B. Rodeck, R. Reding, O. Bernard, M. Burdelski et al., Tacrolimus dual therapy versus cyclosporin-microemulsion triple therapy in pediatric liver transplantation: results from a multicentre randomized trial. Am. J. Transplant. 2(Suppl 3), 351 (2002)
T. Kino, H. Hatanaka, S. Miyata, N. Inamura, M. Nishiyama, T. Yajima et al., FK-506, a novel immunosuppressant isolated from a Streptomyces. II. Immunosuppressive effect of FK-506 in vitro. J Antibiot. 40(9), 1256–1265 (1987)
J.N. Andexer, S.G. Kendrew, M. Nur-e-Alam, O. Lazos, T.A. Foster, A. Zimmermann et al., Biosynthesis of the immunosuppressants FK506, FK520, and rapamycin involves a previously undescribed family of enzymes acting on chorismate. Proc. Natl. Acad. Sci. 108(12), 4776–4781 (2011)
D. Goranovič, G. Kosec, P. Mrak, S. Fujs, J. Horvat, E. Kuščer et al., Origin of the allyl group in FK506 biosynthesis. J. Biol. Chem. 285(19), 14292–14300 (2010)
M.A. Gregory, H. Hong, R.E. Lill, S. Gaisser, H. Petkovic, L. Low et al., Rapamycin biosynthesis: elucidation of gene product function. Org. Biomol. Chem. 4(19), 3565–3568 (2006)
R. McDaniel, M. Welch, C.R. Hutchinson, Genetic approaches to polyketide antibiotics. 1. Chem. Rev. 105(2), 543–558 (2005)
K. Wu, L. Chung, W.P. Revill, L. Katz, C.D. Reeves, The FK520 gene cluster of Streptomyces hygroscopicus var. ascomyceticus (ATCC 14891) contains genes for biosynthesis of unusual polyketide extender units. Gene 251(1), 81–90 (2000)
H. Motamedi, S. Cai, A. Shafiee, K.O. Elliston, Structural organization of a multifunctional polyketide synthase involved in the biosynthesis of the macrolide immunosuppressant FK506. Eur. J. Biochem. 244(1), 74–80 (1997)
B.P. Singh, B.K. Behera, Regulation of tacrolimus production by altering primary source of carbons and amino acids. Lett. Appl. Microbiol. 49(2), 254–259 (2009)
D. Abbanat, W. Maiese, M. Greenstein, Biosynthesis of the pyrroindomycins by Streptomyces rugosporus LL-42D005; characterization of nutrient requirements. J. Antibiot. 52(2), 117–126 (1999)
B. Gust, G.L. Challis, K. Fowler, T. Kieser, K.F. Chater, PCR-targeted Streptomyces gene replacement identifies a protein domain needed for biosynthesis of the sesquiterpene soil odor geosmin. Proc. Natl. Acad. Sci. 100(4), 1541–1546 (2003)
B. Gust, G. Chandra, D. Jakimowicz, T. Yuqing, C.J. Bruton, K.F. Chater, λ Red-mediated genetic manipulation of antibiotic-producing Streptomyces. Adv. Appl. Microbiol. 54, 107–128 (2004)
S.J. Mo, S.K. Lee, Y.Y. Jin et al., Application of a combined approach involving classical random mutagenesis and metabolic engineering to enhance FK506 production in Streptomyces sp. RM7011. Appl. Microbiol. Biotechnol. 97, 3053–3062 (2013)
R. Ye, Q. Wang, X. Zhou, Lincomycin, rational selection of high producing strain and improved fermentation by amino acids supplementation. Bioprocess Biosyst. Eng. 32(4), 521–529 (2009)
Y. Zhao, R. Ye, L. Zheng, Studies on quantitative determination methods of tacrolimus in the fermentation broth. Chin. J. Pharm. Anal. 26(10), 1417–1420 (2006)
K. Tasun, P. Chose, K. Ghen, Sugar determination of DNS method. Biotechnol. Bioeng. 12, 921 (1970)
H.A. El-Enshasy, N.A. Mohamed, M.A. Farid, A.I. El-Diwany, Improvement of erythromycin production by Saccharopolyspora erythraea in molasses based medium through cultivation medium optimization. Bioresour. Technol. 99(10), 4263–4268 (2008)
Z. Wei, L. Bai, Z. Deng, J. Zhong, Impact of nitrogen concentration on validamycin A production and related gene transcription in fermentation of Streptomyces hygroscopicus 5008. Bioprocess Biosyst. Eng. 35(7), 1201–1208 (2012)
W. Du, D. Huang, M. Xia, J. Wen, M. Huang, Improved FK506 production by the precursors and product-tolerant mutant of Streptomyces tsukubaensis based on genome shuffling and dynamic fed-batch strategies. J. Ind. Microbiol. Biotechnol. 41(7), 1131–1143 (2014)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sun, Y., Ye, R. Impact of a novel precursor on FK506 production and key gene transcription in Streptomyces tsukubaensis No. 9993. Res Chem Intermed 42, 3351–3358 (2016). https://doi.org/10.1007/s11164-015-2215-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2215-y